Stability of regulatory T cells in T helper 2–biased allergic airway diseases
Abstract
Regulatory T (Treg) cells potentially suppress the deleterious activities of effector T cells and maintain a state of tolerance against antigens in the airway mucosa. A decrease in the number and function of Treg cells is observed in T helper 2 (Th2)–biased allergic airway diseases. However, adoptive transfer of naturally occurring Treg (tTreg) cells or peripherally derived Treg (pTreg) cells in asthmatic mouse models did not yield satisfactory results in any previous studies. Here, we review the recent progress in the identification and plasticity of tTreg and pTreg cells in Th2–biased airway diseases and summarize the factors affecting the stability and function of Treg cells. This review may serve as foundation for understanding the molecular mechanisms underlying the stability of tTreg and pTreg cells and development of effective strategies for treating allergic airway diseases.
1 INTRODUCTION
The airway mucosa is constantly exposed to airborne allergens and exogenous particles, and the immune system appropriately responds to these exposures and finally forms a state of immune tolerance. To achieve immunological tolerance, Treg cells play a crucial role in the airway mucosa by suppressing effector cells, including T helper 1 (Th1), Th2, and Th17 cells; suppressing eosinophils, mast cells, and basophils; inhibiting inflammatory cell infiltration in local tissues; and inducing immunoglobulin isotype E (IgE) class switching to IgG4.1, 2 Initially, the defect in regulatory T (Treg) cells was found in scurfy mice with severe autoimmunity and in humans with immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome.3 In airways, a decrease or dysfunction in Treg cells has a detrimental role in allergic airway inflammatory diseases with a skewed Th2 response.4-6 In such cases, increasing the number, improving the suppressive function, and maintaining the stability of Treg cells are considered reasonable strategies for regulating the Th2 response.
In this review, we describe the identification markers of Treg cell subsets, discuss the stability of Treg cell subsets in Th2–biased airway diseases, and summarize the factors affecting the stability and function of Treg cells, which will aid in the development of effective strategies for treating allergic airway diseases.
2 tTreg CELL AND pTreg CELL IDENTIFICATIONS
Treg cells can be classified into the following two main subsets: thymus-derived Treg (tTreg) and peripherally derived Treg (pTreg) cells. T-cell receptor (TCR) on tTregs predominantly recognizes self-antigens, which is important for maintaining self-tolerance and preventing autoimmunity7; in contrast, pTreg cells are thought to primarily govern tolerogenic responses against foreign antigens and microbes.8 Typically, CD4+, CD25+, CD127low, and forkhead box P3 (FOXP3)high are markers required to identify both tTreg and pTreg cells in mouse and humans.8 High expression of neuropilin 1(Nrp1), programmed cell death-1(PD-1), Helios, the guanine exchange factor Swap70, and low expression of CD6 are used to distinguish tTreg cells from pTreg cells.9-12 Conflicting data shed doubt on their specific roles13; however, TCR repertoire analysis in mouse indicated very little overlap between FOXP3+Helios− pTreg cells and FOXP3+Helios+ tTreg cells, suggesting Helios can be taken as a specific marker for tTreg cells.14 Treg cells, regardless of tTreg cells and pTreg cells, with selectively expressed markers such as glucocorticoid-induced tumor necrosis factor receptor (GITR) and cytotoxic T lymphocyte–associated antigen-4 (CTLA-4), are regarded as active form in humans and mouse.15 Critically, the expression of CD137+CD154- on human FOXP3+ Treg cells displays antigen-specific activation and a stable suppressive signature, allowing for rapid identification and purification after prior expansion.16 However, CD137 is a marker, which distinguishes the activated form of tTreg cells after antigen stimulation, but cannot be used directly for determination of antigen/allergen-specific tTreg cells. In addition to CD4+CD25+FOXP3+pTreg cells, biomarkers to identify different types of pTreg cells such as type 1 regulatory T (Tr1) cells, Foxp3+ invariant natural killer T (iNKT) cells, and Foxp3+ gammadelta (γδ) T cells in allergic airway inflammatory diseases are listed in Table 1.
| Cell subsets | Identification markers | Ref. |
|---|---|---|
| tTreg cell | Humans and mouse: CD4+, CD25+, CD127low, FOXP3high, Nrp1+, PD-1+, Helios+, Swap70+, CD6-, | 9-15 |
| CD4+CD25+FOXP3+pTreg cell | Humans and mouse: CD4+, CD25+, CD127low, FOXP3high, Nrp1-, PD-1-, Helios-, Swap70-, | 9-15 |
| Tr1 cell | Humans and mouse: IL-10+, FOXP3-, ICOS+, PD-1+, CD49b+, LAG3+, CD226+ | 19-21 |
| FOXP3+ iNKT cell |
Humans:CD25+, FOXP3+, CD1d+, invariable TCR(CD1d+Vα24Jα18Vβ11), CTLA4- or GITR- Mouse: CD25+, FOXP3+, CD1d+, invariable TCR ( CD1d+Vα14Jα18 with Vβ8, or Vβ7 or Vβ2), CTLA4- or GITR- |
26-28 |
| FOXP3+ γδT cells |
Humans: TCRγδ+, CD25+, FOXP3+, CD27+, CD45RA+ or TCRγδ+, CD25+, FOXP3+, CD27+, CD45RA- Mouse: TCRγδ+, CD25+, FOXP3+, CD27+, or TCRγδ+, CD25+, FOXP3-, CD27+, CD39+, CD122+ |
30-33 |
Note
- tTreg, thymus-derived regulatory T cell; pTreg, peripheral derived regulatory T cell; γδ, gammadelta; Tr1 cell, type 1 regulatory T cell; FOXP3, fork head box 3; CTLA-4, cytotoxic T lymphocyte–associated antigen-4; GITR, glucocorticoid-induced tumor necrosis factor receptor; TCR, T-cell receptor; LAG-3, lymphocyte activation gene 3; ICOS, inducible T-cell costimulator; PD-1, programmed cell death 1; Nrp1, neuropilin 1.
Type 1 regulatory T cells are an inducible regulatory T-cell population in human chronic rhinosinusitis with nasal polyps (CRSwNP) tissue and mouse lung tissue, which is characterized by the abundant production of IL-10 and absence of FOXP3 expression.17, 18 Inducible T-cell costimulator (ICOS), PD-1, CD49b, LAG-3, and CD226 are expressed on Tr1 cells; however, none can be regarded as a specific marker.19, 20 At present, coexpression of CD49b, CD226, and LAG3 is still used for both human and mouse Tr1 cell purification and isolation.21 Type 1 iNKT cells and CD1d-restricted innate-like T cells, which share the properties of both T cells and natural killing cells, can produce Th1, Th2, or Th17 cytokines in allergic mice models or from allergic airway patients.22-25 When FOXP3 is induced, FOXP3+iNKT cells obtain immunosuppressive properties, with the expression of CD25 but not necessarily CTLA4 or GITR.26 Thus, combined markers CD25+, FOXP3+, CD1d+, and invariable TCRs were used for human and mouse FOXP3+iNKT cell identification.27, 28 In contrast to classical αβ T cells, γδT cells directly recognize antigens independent of MHC/peptide complexes. An increased number of γδT cells with IL-17 secretion is observed in the peripheral blood of patients with moderate/severe allergic rhinitis.29 Surprisingly, inflammatory γδT cells gain a suppressive function when FOXP3 is induced. Human FOXP3+Vδ2 T cells express CD25+CD27+CD45RA+, while human FOXP3+Vδ1 T cells exhibit CD25+CD27+CD45RA-expression.30, 31 Thus, γδTCR and CD25 are also used to define mouse FOXP3+γδT cell.32 Recently, a novel mouse regulatory γδ T cell CD39+γδ T cell expressing CD25, CD27, CD39, and CD122 but not FOXP3 was identified both in vitro and in vivo.33 Taken together, γδTCR, CD25, and FOXP3 are common markers to identify human and mouse FOXP3+γδ T cell.
3 tTreg CELLS AND Th2 MILIEU
tTreg cells are Treg cells arise from the thymus and constitutively express the FOXP3 protein. The suppressive function of tTreg cells in allergic airway diseases has been widely reported.5, 34 Administration of ovalbumin (OVA) peptide-specific CD4+CD25+ T cells in an allergic mouse model alleviated the Th2 response in the airways.35 On contrary, the same adoptive transfer of OVA-specific tTreg cells in mouse some time did not yield satisfactory results.36 We assume that the pro-inflammatory cytokine interleukin-6 (IL-6) in allergic airway diseases leads to the loss of FOXP3 expression in tTreg cells, affecting the stability of tTreg cells and resulting in conversion of tTreg cells to Th17 cells.37 Pro-inflammatory Treg cells such as IL-4–producing FOXP3+ cells, IL-17–producing FOXP3+ cells, and interferon-γ (IFN-γ)-producing FOXP3+ cells were noticed in patients with asthma, and IL-4–producing FOXP3+ cells were strongly correlated with the severity of asthma.38, 39 Therefore, the instability and plasticity of tTreg cells in the inflammatory environment markedly affects their suppressive functions.
In Th2–biased allergic airway diseases, the GATA-binding protein 3 (GATA3) transcription factor is essential for the development and proliferation of Th2 cells. Thus, therapeutic targeting of GATA3 can significantly attenuate both late and early asthmatic responses after allergen provocation in patients with allergic asthma.40 The intrinsic expression of GATA3 in tTreg cells limited tTreg polarization toward an effector T-cell phenotype in mouse inflamed small and large intestinal lamina propria by binding and promoting the activity of the conserved noncoding sequence 2 (CNS2) of Foxp3.41 Complete GATA3 depletion in murine Treg cells resulted in the formation of tTreg cells that were defective in peripheral homeostasis and suppressive function and gained the Th17 cell phenotypes.42 Ubiquitin-specific peptidase 21 (USP21), an E3 deubiquitinase, reportedly prevented the loss of Foxp3 and controlled mouse tTreg cell stability in vivo by upregulating GATA3.43 These findings highlight the essential regulatory role of GATA3 in tTreg cell stability (Figure 1). However, further studies are needed to investigate whether the intrinsic role of GATA3 in tTreg cell stability can be exploited via therapeutic targeting of GATA3 or Th2 cytokines in human airway diseases.
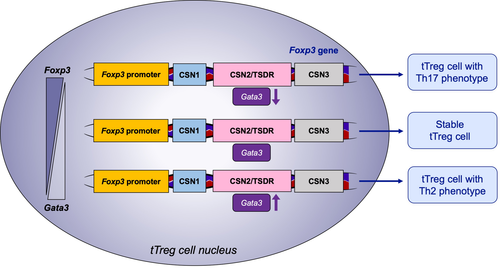
In contrast, enhanced GATA3 and FOXP3 expression was observed in IL-4+ Treg cells in the peripheral blood of patients with asthma.39 Mice with conditional knockout of the Wiskott-Aldrich syndrome protein (WASP) phenotypically exhibited an increase in GATA3 expression in effector memory Foxp3+Treg cells,44 which failed to limit the Th2 response mediated by food allergy.44 Epithelial cell-derived cytokines such as IL-33 have been described to propagate the Th2 response in human CRSwNP tissue by binding to its receptors ST2 on CD4+ T cells45; ST2+CD45RO+CD4+ cells, known as pathogenic Th2 subset in allergic diseases, are related to sublingual immunotherapy efficacy.46 GATA3 drives the expression of ST2 on Treg cells, and ST2+ Treg cells represent as active form in mice with increased suppressive function by secreting IL-10 and transforming growth factor-β (TGF-β).47, 48 However, airway administration of OVA with IL-33 impaired murine immunologic tolerance in the lungs because of dysregulated tTreg cell with secretion of Th2 cytokines and upregulated expression of GATA3 and ST2.49, 50 Recently, significant hypermethylation of the Gata3 promoter and hypomethylation of Foxp3 promoter were detected in sustained epicutaneous immunotherapy in mice.51 Collectively, these findings suggest that the balance between FOXP3 and GATA3 ensures the suppressive function of tTreg cells.
Besides the type 2 response-related molecules, other molecules synthesized by the tTreg cell also affect the stability of tTreg cells for allergic response suppression. Cyclic adenosine monophosphate (cAMP), an important second messenger regulating various cellular function, is synthesized within the cell from adenosine triphosphate (ATP) by the enzyme adenylyl cyclase.52 Generally, tTreg cells directly utilize cAMP to suppress the effector T cells via gap junction-based cell contact-dependent intercellular communication.53 Prevention of cAMP degradation strongly increased the suppressive potency of tTreg cells for Th2 cells in vitro and in vivo and may partially ameliorate the poor result from adoptive transfer of tTreg cells in mice.53 Notably, decreased cAMP in dendritic cells was recently reported to provoke the Th2 immune response in mouse asthma models.54 Consequently, inhibition of cAMP degradation by a PKA-selective cAMP agonist in dendritic cells favored the Th2 response elimination in these mice.55 The kinase PIM1, constitutively active serine/threonine protein kinases, negatively regulates human Foxp3 chromatin binding activity by specifically phosphorylating Foxp3 at Ser422.56 The concentration of PIM1 kinase was upregulated in the lungs of mice with OVA sensitization and challenge or in human tTreg cells with increasing intensity of TCR stimulation and in the presence of IL-6.56, 57 Herein, IL-6 mediated the conversion of human tTreg cells to Th17 cells, which was at least partially inhibited by using PIM1 inhibitor to prevent the phosphorylation of human Foxp3 at its Ser422 residue.
Epigenetic modifications influence tTreg function. The mechanistic target of rapamycin (mTOR) pathway is an important regulator of T-cell responses, and signaling is achieved via two different complexes, mTORC1 and mTORC2, to regulate T-cell responses. mTORC1-dependent glucose metabolism initiates Th2 cell differentiation,58 but Treg cells are less reliant on glycolysis.59 Genetic deletion of Raptor, a scaffold protein which recruits substrates of mTORC1, has been shown to restrict mTOR signaling pathway and metabolically maintain tTreg cell stability and function in mice.60 Furthermore, mTORC1 in lysosome has been shown to be responsible for the instability of TRAF3IP3-deficient murine tTreg cells, indicating that the overexpression of transmembrane protein TRAF3IP3 promotes the stability of tTreg cells via the inhibition of mTORC1 signaling.61 An increased percentage of FOXP3+ Treg cells has been observed in CRSwNP tissue with the usage of mTOR inhibitor rapamycin.62 Using a nonobese diabetic/severe combined immunodeficiency (NOD/scid) mouse model, Tresoldi and colleagues59 have demonstrated that FOXP3+tTreg cells expanded by rapamycin treatment were stable even upon in vivo transfer in the absence of further rapamycin treatment. The authors proposed that this demonstration may justify the clinical use of these cells in future cell therapy–based trials.
Although Treg cells are CD4+ cells, their suppressive function can be facilitated by the interaction between MHC I and CD8+ cells via signaling through the GITR.63, 64 Herein, adoptive transfer of tTreg cells in CD8−/− mice has been shown to enhance the lung allergic responses in the mouse model, due to the conversion of tTreg cells into pathogenic IL-13–producing effector T cells in vivo.65 Thymic dendritic cells (DCs) assist the induction of tTreg cells to mediate central tolerance. Peripherally, classic phenotype DCs (CD103−CD11b+) promote the differentiation of pTreg cells from naïve CD4+ T cells in vitro in a TGF-β-dependent and retinoic acid-dependent manner in allergic rhinitis patients receiving sublingual immunotherapy.66, 67 However, the effect of activated DCs in generation functional Treg cells is impaired by using a widely used adjuvant in allergen-specific immunotherapy, due to low expression of programmed death-ligand 1 (PD-L1) and inhibition of mTOR activation in DCs.68 A recent study has indicated that autophagy in DCs is required to stabilize Treg expression signatures, particularly as autophagy-deficient DCs with downregulated ICOS ligand consistently failed to maintain pTreg cell expansion, stability, and function.69 These findings highlight the role of DCs to generate and maintain the stability of Treg cells (Figure 2). However, not all phenotypes of DCs possess tolerogenic properties. Another recent study demonstrated that in type 2 airway disease, IL-33 induces the conversion of Treg cells to Th17 cells through an antigen-pulsed mature dendritic cell–mediated pathway, whereas IL-33 alone had no direct effect.70

4 pTreg CELLS, Treg-SPECIFIC DEMETHYLATED REGION (TSDR), AND THE Th2 MILIEU
The TSDR in Foxp3 is highly conserved and fully demethylated in tTreg cells, whereas the CpG island of CNS2 in Foxp3 is partially demethylated in pTreg cells.71 Although the induction of Foxp3 in vitro by using TGF-β is independent of the TSDR demethylation status,72 the methylated TSDR in murine pTreg cells is closely related to the instability of FOXP3 expression and loss of pTreg cell function under certain conditions.73, 74 Adoptive transfer of OVA-specific pTreg cells during allergen challenge effectively attenuated airway inflammation and improved airway function.75 In such case, knowing the regulation of TSDR demethylation in pTreg cells is important for maintaining the stability of pTreg cells in Th2–biased diseases. The regulation of TSDR is summarized in Figure 3.
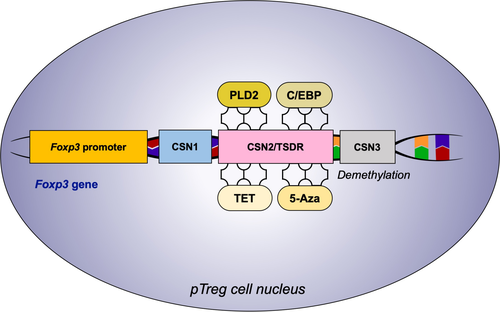
Recently, a reduction in TSDR methylation using the common DNA methyltransferase inhibitor 5-azacytidine (5-Aza) promoted human pTreg cell generation with a combination of suboptimal T-cell receptor (TCR) stimulation.76 Compared to untreated pTreg cells, cells treated with 5-Aza produced higher levels of IFN-γ, TGF-β, and IL-2, which are essential for pTreg cell differentiation.76 The Th2 cytokine IL-4 was reported as an antagonist of Treg cell conversion.77 CCAAT/enhancer-binding protein (C/EBP) binds to the methyl-CRE sequence in the Foxp3 TSDR, thus enhancing Treg cell generation by reducing the inhibitory effect of IL-4 on FOXP3 expression in mouse asthmatic models.78 Recombinant human phospholipase D2 (rhPLD2) also inhibited the IL-5–induced response in chronic asthmatic guinea pig models, functioning as a regulator for the nuclear factor kappaB (NF-κB) and protein kinase C (PKC) signaling pathways.79 Consequently, administration of rhPLD2-treated pTreg cells in asthmatic mouse models markedly improved the quality and quantity of Treg cells, eventually alleviating lung inflammation significantly by reducing Foxp3 TSDR methylation.80 Moreover, the CNS2 DNA demethylation and stabilization of FOXP3 expression were facilitated via ten-eleven translocation 2 (TET2) and TET3 enzymes.81 The influence of vitamin C treatment on pTreg cell stability was also associated with the TET enzyme activity, and the effect of vitamin C disappeared in mouse TET2(-/-) pTreg cells.82 The mitochondrial respiratory chain is required for stable T cell–suppressive function but not for the survival of Treg cells. Mice lacking the mitochondrial complex III specifically in Treg cells affected the TSDR region by inhibiting TET family of DNA demethylases.83 Methyl-CpG binding protein 2 (MeCP2) was recruited to the CNS2 region of the Foxp3 locus to promote local histone H3 acetylation, thereby counteracting inflammation-induced epigenetic silencing of Foxp3 in mouse.84 Unexpectedly, histone H3 acetylation at the Foxp3 locus was higher in children with allergic asthma compared to healthy controls.85 This indirectly demonstrates no association between Foxp3 H3 acetylation and Treg cell function in patients with asthma.86 Collectively, TSDR is an important methylation-sensitive element regulating Foxp3 expression and closely related to pTreg cell stability. Generally, the methylation or demethylation of TSDR in Foxp3 is considered to occur in both strands. However, a recent study reported a strand-biased hemimethylation pattern in the human Foxp3 promoter and TSDR; this hemimethylation was characterized by complete methylation of the coding (reverse) strand and significant demethylation of its complementary template strand.87 The strand-bias methylation mentioned in this literature may be consistent with the partial demethylation of TSDR in pTreg cells.
In addition to TSDR-based regulation, direct regulation of Foxp3 results in stable pTreg cells. Loss of sentrin/SUMO-specific protease 3 (SENP3) in mouse Treg cells impaired the transcription of Foxp3 and Pdcd1. Thus, SENP3-deficient Treg cells showed inflammatory characteristics with much lower FOXP3 expression and aberrant IFN-γ and IL-17 release.88 STAT5 is a key positive regulator of FOXP3 expression,89 and STAT3 binds to a silencer element within the Foxp3 locus, thereby limiting the expression of FOXP3 by reducing the binding of STAT3 on Smad3. Our group also demonstrated that phosphorylated STAT3 positively regulated FOXP3 expression in the Th2–biased upper airway disease CRSwNP.90
Unlike tTreg cell expression of high-affinity TCRs, which are predominantly specific for autoantigens, a broad TCR repertoire on pTreg cells mainly recognizes exogenous antigens. pTreg cells mediate suppression by depleting peptide-MHC class II from DCs in an antigen-specific manner,91 in that OVA-specific pTreg cells were found to be more tolerogenic in comparison with polyclonal tTreg cells in OVA-asthmatic mouse model.36 The upregulation of Der p 1-specific FOXP3+Helios+ tTreg and Der p 1-specific IL-10+T cell detection by tetramers has also been observed in allergic rhinitis patients undergoing 30 weeks subcutaneous house dust mite specific immunotherapy.92 However, not all induced antigen-specific Treg cells are functional. A study by Pellerin and colleagues 93 has demonstrated that although peanut-specific Tr1 cells could be induced in vitro from PBMCs of both healthy controls and peanut-allergic individuals upon stimulation with the main peanut allergens of Arachis hypogaea (Ara h 1 and 2), only Tr1 cells from healthy controls were anergic and efficiently inhibited Th2 cytokine production upon peanut-specific restimulation. While there is clearly much to understand about antigen-specific responses of Treg cells in allergic airway disease, this quest is hindered by limited availability of appropriate peptide-MHC multimers, which restricts identification of the specific antigens.
5 CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Currently, novel treatments targeting the GATA3 transcription factor and Th2 cytokines are used to ameliorate the different types of allergic diseases. However, GATA3 and some Th2 response mediators play indispensable roles in FOXP3 expression and tTreg cell stability. In fact, the balance between GATA3 and FOXP3 is a possible strategy for ensuring tTreg cell stability. Manipulation of the TSDR methylation status in vitro affects the activity of pTreg cells and stability of FOXP3 expression. Therefore, we further propose the regulation of Foxp3 TSDR to stabilize pTreg cells in Th2–biased diseases. However, the molecular mechanisms involved in regulating FOXP3 expression remain unclear and further studies are needed to exploit their applicability in the clinical setting. Moreover, antigen-specific response of Treg cells should not be ignored in the further studies, as these might help to better explain their specific roles in pathogenesis of allergic airway diseases.
ACKNOWLEDGMENTS
This work was supported by grants from the National Nature Science Foundation of China (81630023, 81700887, and 81970851), National Key R&D Program of China (2016YFC20160905200), Program for Changjiang Scholars and Innovative Research Team (IRT13082), Beijing Municipal Administration of Hospital's Youth Programme (QML20180201), China International Medical Foundation (2017-N-01-03), and Scientific Research Foundation for the Researcher in Beijing Tongren Hospital (2017-YJJ-GGL-008, 2018-YJJ-ZZL-038).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




