What did we learn from multiple omics studies in asthma?
Abstract
More than a decade has passed since the finalization of the Human Genome Project. Omics technologies made a huge leap from trendy and very expensive to routinely executed and relatively cheap assays. Simultaneously, we understood that omics is not a panacea for every problem in the area of human health and personalized medicine. Whilst in some areas of research omics showed immediate results, in other fields, including asthma, it only allowed us to identify the incredibly complicated molecular processes. Along with their possibilities, omics technologies also bring many issues connected to sample collection, analyses and interpretation. It is often impossible to separate the intrinsic imperfection of omics from asthma heterogeneity. Still, many insights and directions from applied omics were acquired—presumable phenotypic clusters of patients, plausible biomarkers and potential pathways involved. Omics technologies develop rapidly, bringing improvements also to asthma research. These improvements, together with our growing understanding of asthma subphenotypes and underlying cellular processes, will likely play a role in asthma management strategies.
1 INTRODUCTION
Asthma has been studied extensively for decades. It is one of the most common respiratory diseases affecting 300 million of people worldwide, and about 346,000 patients die from the disease yearly.1 Incidence rates of asthma grow constantly, and the underlying reasons for this are not fully understood. Current hypotheses range from reduced microbiome exposure in daily life, childhood infections to growing air pollution.2-4 Emerging technologies in investigating cellular processes and cellular environment bring possibilities for better asthma management, control and possibly cure.
Asthma was traditionally studied using questionnaires, measuring lung function and analysis of cells and individual mediators of various body fluids (blood, urine, sputum). While all these are still actively used in clinics, asthma research is progressing to the side of understanding the disease on molecular and cellular levels. A molecular world of asthma started to unfold just recently, due to our growing understanding of asthma complexity, drastically lowered price on omics-related equipment and analyses; and willingness of stakeholders to broaden indication criteria of available drugs to another diseases.
Starting with the Human Genome Project (HGP),5, 6 omics have flourished: tremendous amounts of data are available in open databases. Genomics, transcriptomics, proteomics, metabolomics, epigenomics, microbiomics—all technologies required are now available to improve medical care. However, the hype surrounding new technologies and methodologies in analysis of cellular information may lead to further disappointments due to inflated societal expectations and concerns regarding cost-effectiveness, privacy, ethical, legal and social implications (ELSI).7-9
Even before the advent of the omics era, we grasped that the classical definition of asthma implicates many conditions with similar clinical presentation (shortness of breath, wheezing, coughing) but different biological backgrounds. Asthma can easily be confused with other respiratory conditions such as COPD.10 Hence, the clinical diagnosis features restrictions and subjectivity, which could prevent effective treatment. Omics technologies helped start delineating molecular asthma fingerprints. We are still in the inception of discovering new interactions and newer ways of molecular communications inside the cell, but the discoveries we have made already changed the direction of clinical trials in asthma. However, there is still a huge gap between what we learn from omics analyses and clinical implementation of its results in asthma.
Omics-related terms and definitions
omics
A collective name for -ome studies in biology that investigate molecular information (eg genome, proteome) in a cell.
Genome
A comprehensive information on a DNA sequence of an organism.
SNP
Single nucleotide polymorphism (SNP) is a variation of a single nucleotide in a genome locus.
Transcriptome
A snapshot of all messenger RNA (mRNA) transcripts that can be found in a cell/tissue at a given time. Transcriptome echoes the dynamic environment inside the cell.
Epigenome
A complete set of nuclear information that is not coded in DNA and can affect gene expression. It includes DNA methylations, histones modifications and alterations in chromatin structure.
Proteome
All proteins and peptides identified in cells/tissues at a given time.
Metabolome
A snapshot of all low molecular weight metabolites—(small molecules such as amino acids, carbohydrates, etc)—that can be identified in cells/tissue at a given time. Metabolites are products of metabolic processes.
Microbiome
DNA information of microbiome (bacteria, fungi, viruses) identified in a given compartment/organ, for example gut, mouth, skin. Metagenomics studies microbiome usually by applying 16s RNA sequencing.
Exposome
All internal and external conditions that cumulatively influencing an organism thoughout its life.
Breathome
A profile of metabolites in exhaled air, identified by eNose ("electronic nose") device or analysed using mass spectrometry.
VOC
Volatile organic compounds are organic chemicals produced by the host's metabolism or microbiome situated in the lungs. Their volatility allows detection of these compounds in exhaled air in breathomics.
GWAS, EWAS
Genome- and Epigenome-Wide Association Study aim to find associations between genome and epigenome with phenotypes (disease, drug response, physiological characteristics).
QTL (eQTL, meQTL, pQTL, mQTL)
Quantitative trait loci (expression (eQTL), methylation (meQTL), protein (pQTL), metabolic (mQTL)) that are associated with expression level, methylation, protein abundance or metabolites, so functional relevance of loci can be inferred.
GWIS
Genome-Wide Interaction Studies are used to infer interactions between some environmental exposure (eg microbial) and phenotypic characteristics.
miRNA
microRNA is a type of noncoding RNA that may influence gene expression level, for example by degrading mRNA.
Histones modifications
Histones are the proteins involved in DNA packing and may influence gene expression. Histones can be modified by methylation/demethylation, acetylation/deacetylation, ubiquitination, etc
Systems biology
A study of interactions in molecular pathways that is based on computational methods and mathematical models.
Single-cell omics
Omics collected at a single-cell level.
2 GENOMICS, TRANSCRIPTOMICS AND EPIGENOMICS
Genomics and transcriptomics by far have the most developed technological and methodological advances amongst omics-related approaches, while epigenomics is more novel and less developed. The relative accessibility of genomics and transcriptomics was greatly increased in the last decade. For genomics, many high-throughput technologies are available, ranging highly in equipment and one-run price, accuracy and speed. For transcriptomics, RNA-seq and microarrays are broadly used nowadays. However, none of these technologies are ideal. The detailed excellent comparison of them can be found elsewhere.12-15
2.1 Genomewide association studies (GWAS) and candidate-gene studies failed to provide a “magic bullet” gene list that provoke asthma or explain treatment response
Genomics studies genetic variants of organisms in order to find associations and functional relevance of these variants with phenotypes. Since high-throughput technologies are becoming relatively cheap, efficient and widespread, there have been many discoveries in the field of genomics. These findings are predominantly correlational due to many factors involved in the phenotypic manifestations and our limited knowledge on cellular and molecular processes. Moreover, most of the studies are observational and in some cases underpowered.16, 17 Still, genetic association studies help gain insights into the underlying molecular mechanisms of the diseases (or subphenotypes).
Candidate-gene (CG) studies and genomewide association studies (GWASs) are the two main approaches to study genome-phenotype associations. GWAS represents an unbiased approach for finding single nucleotide polymorphism (SNP) associations with specific phenotypes and may result in novel genes to consider for a phenotype. In contrast, CG studies use a priori information from different sources to identify the association.18 GWAS and CG can organically be combined: the list of SNPs found in GWAS can serve as a prior in CG study. GWAS acquired popularity in asthma research over the last decade as it helped to overcome the lack of reproducibility of previous CG studies.19, 20 However, for complex human diseases, GWASs often result in nonintersecting outcomes and small effect size (typical OR is 1.1-1.520, 21). Some of the major critiques to GWAS are inability to take into account gene-gene interaction (ie epistatic effects), and the requirement of large patient numbers, which is not always possible. A recent GWAS with the largest number of patients to date featuring approximately 5000 patients (5135 cases and 25 675 nonasthmatics for stage 1; 5414 cases and 21 471 nonasthmatics for stage 2) found 21 signals (out of 24 in total) intersecting with previous studies.22
For complex human diseases like asthma, the expectation to find just one or several genes for disease susceptibility seems biologically and mathematically unrealistic23 (see Figure 1). Genome studies often explain only a fraction of heritability of complex human diseases.24-26 Despite the efforts to create a well-reproducible list of SNPs/genes for asthma susceptibility throughout the years, this goal was not reached.
Even though the results of GWAS in asthma often do not intersect, one prominent result demonstrated exception from the rule: the 17q12-21 locus. 17q12 was initially found to be associated with asthma in the first GWAS on asthma,27 was reproduced many times,22, 28-33 and further enlarged to 17q12-21. The expression of three genes (ORMDL3, GSDMB, PGAP3) was found to be connected to this locus,34 but their exact roles in asthma just started to be elucidated.35
A clinically relevant phenotype being investigated in GWAS studies is the response to medication: short-acting beta agonists (SABA), long-acting beta agonists (LABA), inhaled corticosteroids (ICS) or leukotriene modifiers (LTM). Most of the reported genetic variants do not reach the significance threshold and rarely intersect in their reported variants.36 Bronchodilator response in GWAS (measured in % of change in lung function) after SABA is estimated to be 10%-29% heritable,37, 38 with genes including ADRB2 (beta-2 agonists receptor).39 ADRB2 codes for a receptor which is the pharmacological target for short- and long-acting beta agonists (SABA and LABA, respectively). ADRB2 has mixed results across studies, some claiming that variants of ADRB2 are responsible for poor LABA response, some claiming no significant difference can be found.39-41 ADRB2 has a more stable association with LABA response in paediatric asthma,42 and the currently ongoing PUFFIN clinical trial43 aims to investigate whether prospective genotyping of ADRB2 variation can be used for a better treatment outcome.43 For ICS response, three loci within the genes GLCCI1, CRHR1 and FCER2 were replicated in at least one study.44 For leukotriene receptor agonist response, ALOX5 and MRP1 were found to be associated, and ALOX5 was replicated positively in independent populations.45 For a further detailed overview on GWAS studies in asthma, one may consult following references.36, 45-49
We emphasize that at the moment both genotyping of 17q12-21 and ADRB2 are not used in clinical practice; however, research on these continues, and this might lead to clinical implementation in the future. More information on these two discoveries can be found in the “Major milestones discoveries” panel.
To sum up, various genetic variants were identified to be associated with asthma and treatment response. The fact that associations are often not replicated might be due to low patient numbers, different inclusion criteria, differences in asthma definition, differences in disease severity, intrinsic heterogeneity and different outcomes measured. Classical asthma definition on the basis of clinical phenotypes is still being actively used in clinical trials. However, one could seriously question the present clinical gold standard. So, an ouroboros-like problem arises: heterogeneity in cohorts hampers delineation of the molecular subphenotypes, and as molecular subphenotypes are unknown in advance, we cannot improve inclusion criteria for enhancing subphenotype signals. These unresolved issues intercept us from using genomics in routine asthma care.
2.2 Transcriptomics provides a list of plausible biomarkers for asthma subphenotypes
Transcriptome is defined by all RNA transcripts in the cell at a given time and under a specific condition. Transcriptomic approaches are applied widely in asthma. Amongst available methods (RNA-seq, microarrays), microarrays were mostly utilized in asthma research due to low cost. Microarrays allow to detect a fraction of all mRNA transcripts that is predefined by probes; RNA-seq is a newer technology, and it can collect transcripts in an unbiased way with high accuracy.
Asthma subphenotypes on immune pathways (Th2-high/Th2-low), previously identified on the basis of cell counts in sputum,50 was also established in a seminal microarray study on bronchial brushings.51 As expected, Th2-high subphenotype is connected to atopic profile and airway remodelling and showed better corticosteroids response compared to Th2-low.
The U-BIOPRED52 consortium collected a large-scale data set on asthma patients. Analysis of transcriptomics in collected blood identified a vast amount (1693) of differentially expressed genes for asthma in comparison with control (nonasthmatics).53 Analysis of sputum transcriptomics from U-BIOPRED showed different pathobiological profiles in four clinical clusters (see “Major Discoveries” panel). Furthermore, it appeared that clinical phenotypes such as adult-onset asthma or asthma with fixed airflow limitation are characterized with distinct transcriptomic profiles.54, 55 However, such differential gene expression varied between sample sites (sputum, nasal brush, endobronchial brush and endobronchial biopsy), highlighting the role of local biology in asthma.
Unbiased sputum transcriptomics delineated three distinct gene expression profiles that were only partly associated with type 2 inflammation,56 suggesting that gene expression profiling provides complementary information to traditional biological concepts on asthma phenotyping. Interestingly, the results from sputum transcriptomics caused renaming Th2-high profile to type 2 gene mean (T2GM), characterized by three genes (IL-4, IL-5 and IL-13).57 Another study provided a confirmation for this in SARP cohort.58, 59 They also demonstrated that in severe asthma corticosteroids do not significantly decrease the T2GM profile in sputum; they suggested to add extra medication like inhibitors of type 2 cytokines for these patients. These findings demonstrate the potential of omics studies in personalized medicine approach.
Overall, transcriptomics provided confirmation for the necessity to subphenotype asthma. It has been demonstrated that transcriptomic level is not completely reflected on proteomics level.60, 61 This may signal that neither of these technologies provide highly accurate results or that more factors should be considered in our search of “missing heritability” of asthma and explaining disease processes.
2.3 Epigenomics may hold keys to a missing genotype-phenotype link
A combination of environmental factors including allergens (ie pet exposure), smoking and city pollution have been confirmed to influence asthma onset.62-64 This implies that genetic variants cannot serve as a diagnostic/prognostic tool by themselves; therefore, the combination of environmental exposures and genetic predisposition is important to study. An intricate question of how the environmental effect changes the genetics' manifestation can be answered by epigenetics.
Epigenetics is broadly defined as a nuclear information that complements genomics and can alter gene expression. Epigenomics includes information about DNA methylation, histone modifications and various forms of noncoding RNA. Epigenetics, especially methylation, can be inherited across generations. Methylation is the most popular type of epigenetics studied nowadays. Epigenetics depends on the exposome (all internal and external exposures throughout life65) and can be highly fluctuating; one may consider epigenetics as one of the adaptive cellular mechanisms, immune system in particular, in response to a changing environment. Epigenome-wide association studies (EWASs), similar to GWAS, investigate association of epigenomics with asthma, its severity and drug response.
Epigenomics shows a high potential for prenatal and perinatal asthma routes. Skewness of immune system to Th2 pathways has been one of the hypotheses for early-onset asthma,66 and studies established that epigenetics controls differentiation of T cells.67, 68 A meta-EWAS with 8 cohorts (668 cases) found many differentially methylated sites associated with asthma-related immune response.69 Also, early-onset asthma was connected to prenatal and perinatal exposome such as maternal smoking and diet.70, 71 In a large-scale EWAS, methylation in 14 CpG sites (connected to activation of eosinophils and cytotoxic T cells) was associated with childhood asthma.72
Microbiome can be considered as another exposure factor on epigenomic level as it is now believed to drastically influence a host organisms' health.73-80 The understanding of this influence is still in its infancy, but it shows a big potential. Huang et al81 demonstrated significant association between bacteria abundance and their diversity in airways with bronchial hyperresponsiveness. Also, specific microbiota in severe asthma patients were associated with clinical disease features.82 The fact that obesity sometimes co-occurs with asthma is another example of a possible association between asthma and the microbiome.83-86 Obesity is potentially connected to a different gut microbiome composition in comparison with normal weight subjects,87, 88 which might lead to a systemic changes and occurrence or aggravation of asthma symptoms.84-86
Epigenomics acquired some scepticism in asthma research, mainly due to the expected limited effect, lack of reproducibility, similar to genomics.89-92 EWASs are also criticized for merely reflecting cell counts, since epigenetics highly depends on a tissue type. In addition, methylation assays currently cover only a small part of all CpG islands (1.6%-2.9%93). Finally, the reported differentially methylation sites often do not show any connectivity to asthma-related genes.94
A question of tissue and cell type relevance has major importance for epigenomics, since epigenomics signatures greatly vary in different tissue types.95 Until now, most of the studies in asthma analysed epigenome in peripheral blood cells, while some used nasal epithelium and lung tissue.96 Signals from eosinophils and regulatory T cells (Treg) were found to be associated with asthma in EWAS.72, 97, 98 For allergic asthma, immune blood cells were found to be more relevant than for the nonallergic counterpart.96 Nasal epithelium has been found to be a good proxy for bronchial tissue in a recent study.99, 100 A novel approach—single-cell omics—is rising in popularity now and would likely help clarify cell types importance.101, 102 Epigenomic alterations at single-cell level will likely enable studying the cell-specific signal connected to disease such as asthma.103-106
Overall, epigenomics may be a missing link between the genotype and phenotype. To use epigenomics widely, better technology should be developed and sample collections and analyses have to be standardized.107, 108 Translational perspectives on epigenomics include diagnostics and prognostics, subphenotypes determination, prevention and treatment.
3 PROTEOMICS, METABOLOMICS, BREATHOMICS
3.1 Promise of proteomics: subphenotype asthma patients
Proteomics is a research area that focusses on the large-scale study of proteins produced by cells, tissues and organisms. Analytical techniques used in this field of research identify, qualify and quantify proteins and their functions.109-113 Proteins play fundamental roles in fulfilment of biological functions in cells. Therefore, their expression levels can be considered as momentary reflections of the cellular state. Despite the valuable contribution of genomics and transcriptomics studies to asthma research, it should be noted that a single gene or mRNA strand can generate several different protein isoforms as a result of alternative splicing of RNA and post-translational modification of synthesized proteins. Accordingly, even though the human genome comprises approximately 30,000 genes, cells contain multiples of this amount in proteins.114-121
In respiratory research, proteomics can be used to identify biomarkers that determine disease in an early state, allow patient risk stratification, determination of disease progression, personalization of therapy regimens and/or assess therapeutic response.122, 123 Currently, FeNO is recommended in some guidelines as an indirect measurement of eosinophilic inflammation in patients124; however, classical asthma diagnosis according to the GINA guidelines1 does not consider any markers of inflammation or proteins. Proteomics may offer a more sensitive and specific diagnosis based on molecular and disease-specific protein pathways.
Different types of samples are suitable for proteomics analytical techniques. In respiratory research, proteomics have previously been applied on different biological samples, such as serum, circulating cells, bronchoalveolar lavage fluid (BALF), nasal lavage fluid (NLF), induced sputum, exhaled breath condensate (EBC), epithelial lining fluid (ELF) and, albeit sporadically, biopsies (see Table 1). However, data from a single sampling location most likely will provide insufficient information about the complete respiratory proteome. For example, BALF provides an impression of the proteome status of the epithelial lining fluid, but does not give complete insight into the changes of the proteome that occur in the walls of the airways.122
| Sample | Advantages | Disadvantages | References |
|---|---|---|---|
| Blood Serum | Minimally invasive and therefore collectible from virtually all patients. | Sample will also contain components originating from different compartments as a result of the circulation and exchange of interstitial fluids. | 125, 192, 195 |
| Contains large quantities of protein. | Serum samples generally feature a wide dynamic range of proteins and peptides as well as salts, making sample processing and proteomic analyses more difficult. | ||
| Induced sputum | Sample mainly consists of secretions by the airways and contains both immune cells and immune mediators. |
Not a suitable procedure for severe asthma patients. Not successful in a large minority of mild-to-moderate asthmatics. |
125, 190, 192, 247, 248 |
| Guidelines for sampling available. | Sputum samples are diluted. | ||
| Very accessible. | Samples are at risk for being contaminated. | ||
| The presence of highly charged mucins in sputum interferes with the gel-based separation procedures, resulting in a diminished resolution. | |||
| Glycoproteins MUC5AC and MUC5B increase the viscosity of mucin present in the sample, making sample preparation difficult. | |||
| Exhaled Breath Condensate (EBC) | Can be collected noninvasively. | Samples are quite often contaminated due to passage of the airways during collection. Contamination should therefore be assessed by quantifying the presence of salivary amylase. | 157, 249-254 |
| Technical standard available for the collection of EBC. | Mediators and cytokines often do not reach the lower limit of detection or quantification. | ||
| Previous studies suggest that inflammatory markers in EBC are indicative for bronchial inflammation. | |||
| Suitable procedure for children. | |||
| Bronchoalveolar lavage fluid (BALF) | Sample can be indicative of the inflammatory status of cells present on the airways and the influx of immune cells to this compartment. | More invasive than induced sputum, blood or exhaled breath condensate and therefore less applicable in more severe asthma patients. However, the procedure can be safely executed as long as adequate monitoring during the procedure and postprocedure recovery can be ensured. | 255-263 |
| The cellular profile of BALF appears to differ between several types of lung disease. | Samples are diluted due to the use of saline and salts during the procedure. | ||
| Sample contains soluble components of the apical bronchial and alveolar surfaces of the airways. | Samples may contain components due to irritation of the airways due to the procedure. | ||
| Samples are at risk of being contaminated during aspiration. | |||
| May contain components of plasma due to leakage through the airways. | |||
| Proteins in BALF may result from both endogenous and exogenous sources. | |||
| Epithelial lining fluid (ELF) | Contains large quantities of protein, although DNA and RNA contents are low. | Not a suitable procedure for more severe asthma patients. | 248, 264 |
| ELF samples are collected undiluted as a tip is used during bronchial microprobing. | May contain components of plasma due to leakage through the airways. | ||
| Biopsies | Sample consists of several cell types and allows assessment of several pathological aspects at once. | Invasive procedure and therefore unsuitable for a lot of patients. | 125, 192, 248, 263 |
| Cells harvested from biopsies can be used for the development of primary cell cultures. | Unsuitable procedure for children. | ||
| Very hard to find volunteers to act as healthy controls. |
Whereas in genomics and transcriptomics studies, we can sequence and measure expression level comprehensively, proteomic studies are hardly performed at a similar scale as result of the diverse biochemical properties amongst proteins. Different methods of detection are used in proteomics, which can roughly be divided into immunoassays (Western blot, immunohistochemistry and ELISA) and mass spectrometry (MS). The former appeared earlier and is suitable for detection of small portion of proteins,125 whereas the latter aims to collect fractions of the whole proteome.
Before detection can occur, a targeted and complex separation of the protein mixtures is required.126-131 Initially, proteomic analyses were performed using two-dimensional gel electrophoresis.132-135 Nonetheless, these methods are generally unsuitable for the analysis of diverse complex mixtures of proteins and are strongly influenced by differences in technical procedures.136-139 Hence, liquid chromatography (LC)-based separation methods have been developed, which are usually followed by mass spectrometry.140 Both techniques are combined in shotgun proteomics, wherein generated spectra of peptide fragments are used for the identification of proteins with the aid of spectral databases.122, 125 Another popular high-throughput technique is based on microarrays—an emerging tool in proteomics that will make it possible to analyse large quantities of different proteins.128, 129, 141, 142
Proteomics techniques are increasingly used in asthma research for mapping the respiratory proteome. Large-scale registers for asthma patients, in which clinical data and biological materials from large numbers of asthma patients have been collected, have delivered valuable findings for the asthma research field using proteomics. The SARP research group was able to classify patients on asthma severity based on protein expression levels of 18 cytokines in BALF samples.143 Moreover, this research group was able to demonstrate differences in inflammatory mediators in sputum between granulocytic inflammatory profiles of asthmatic patients and asthma severity.144-146 More recently, researchers from U-BIOPRED were able to form four clusters based on clinical characteristics of asthma patients. Subsequently, significant differences in the expression levels of ten proteins in sputum could be demonstrated amongst previously established clinical clusters.147 Furthermore, current-smoking and ex-smoking severe asthmatic patients could be distinguished from nonsmoking patients in U-BIOPRED at the sputum proteomic level. In addition, significant differences were demonstrated in gene expression profiles between bronchial epithelial cells from current-smoking severe asthmatics and nonsmoking severe asthma patients as determined by pathway analysis, gene set variation analysis and protein–protein interaction analysis.148 Moreover, multiple molecular subphenotypes of eosinophil- and neutrophil-mediated asthma have very recently been identified on the basis of sputum proteomic signatures.149
3.2 Metabolites are potential surrogates of current and (previous) cell processes. Breathomics tries to bridge the gap between bench and bedside
Metabolomics focusses on analysing low-molecular biochemical compounds (molecular weight < 1500 Da) that originate from the metabolism. Metabolites are strongly implicated in homeostasis and disorders where they help maintain the redox balance, participate in oxidative stress, cellular signalling, apoptosis and inflammatory processes, etc The metabolic state is the result of both gene expression and environmental factors and can therefore be informative for disorders of multifactorial nature, such as asthma.150, 151 The composition of metabolites and/or their fragments can possibly offer a pathophysiological reflection of the current state of the disorder. As inflammatory mediators usually have a short half-life, they are rapidly degraded to various metabolites. Potentially, the analysis of these metabolites allows determination of previous cellular responses involved in inflammatory processes. If a metabolic composition is specific to a particular disease, it could potentially be used as a biomarker or to broaden knowledge on the pathophysiology of a disorder.152-156
The development of metabolomics occurred approximately simultaneously with the development of the proteomic research field. Despite the enormous difference in size between the compounds of interest in these research disciplines, both fields share many of their analytical techniques: sample separation based on gas or liquid chromatography and analysis via MS Similar to the proteome, the metabolome is dynamic and is highly influenced by factors such as BMI, treatment, diet and smoking status.157-159 In addition to mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy is regularly applied in metabolomic analyses. However, this analytical method features much lower sensitivity and specificity compared to MS-based methods. Consequently, high concentrations of analytes would be required for valid analysis.152, 160-162 Metabolomic studies predominantly experience the same issues as proteomic studies, such as very limited sample sizes, lack of standardization for sample collection, handling and storage as well as a very wide dynamic range found in samples.122, 163
Most metabolomic studies to date have focussed on distinguishing asthma patients from healthy controls, COPD patients and amongst each other on the basis of phenotypes such as asthma severity. The main metabolites found in these studies are involved in tricarboxylic acid metabolism, hypermethylation, phospholipid regulation, hypoxia, oxidative stress and immune reactions.150, 152, 164-178 However, most studies were severely limited by low sample size, diagnostic heterogeneity and very limited number of metabolites that was studied. Furthermore, results are very rarely independently replicated and, due to a lack of standardization of the analytical methodology, technical inconsistencies between studies complicate comparisons of outcomes.
An emerging derivative of metabolomics is breathomics, wherein either gas chromatography/mass spectrometry (GC/MS) or electronic noses measure volatile organic compounds (VOC) in exhaled air.179 The first technology is based on the principles of standard analytical chemistry, whereas the second is based on cross-reactive sensor technology with the subsequent application of powerful pattern recognition algorithms.180 The first results with eNoses are promising; recent studies have demonstrated clusters of asthma and COPD patients with specific inflammatory phenotypes (eosinophilic or neutrophilic) on the basis of specific VOC profiles in exhaled air.181-185 With proper validation and proven clinical utility, molecular profiling of VOCs may provide a noninvasive alternative to blood and sputum measurements. Moreover, the speed of assessments, the small size of devices and the possibility to link some eNoses to existing spirometry equipment make it highly applicable in clinical practice.186 However, it should be emphasized that the detection method does not allow to delineate the contributing individual compounds in exhaled air. For gaining mechanistic insight, simultaneous analysis of breath samples by mass spectrometry is required.187
3.2.1 Challenges
The results achieved so far in proteomics and metabolomics have not led to a shift in paradigm in clinical practice, mainly due to the ambiguity and large variability in results, impeding interpretation and translation into clinical setting. First, the current lack of standardization of sampling and analytical methods in these studies makes it difficult to compare results between studies and often results in conflicting outcomes.122, 136, 188-190 Second, studies generally do not validate results using other analytical techniques or an external cohort.191, 192 Third, most of the studies incorporating high-throughput omics measures have been cross-sectional or distributed over a few data points. Complex diseases are extremely dynamic, and hence, cross-sectional measurements fail to capture the gamut of information associated. Fourth, the cohort size found in most studies is generally limited/inadequate, which can be attributed to the time-consuming methodology and costs. Finally, experimental shortcomings (eg separating low abundant compounds from the noise136, 193, 194) often lead to unreproducible results.195-198
Major Milestone Discoveries
17q12-21 locus
Identified and reproduced by many GWAS studies, the 17q12-21 locus is one of the most significant discovery in asthma omics-based research.27, 28, 31, 206, 207 The locus was associated with early-life asthma in non-African populations. Alterations in 2 SNPs in 17q12-21 lead to upregulation of three genes: ORMDL3 and GSDMB (direct regulation), and PGAP3 (indirect regulation). However, the identified odds ratio in a meta-GWAS study for the locus is modest (1.16, 95% CI, 1.13-1.19), but this may be due to heterogeneity in asthma and diverse cohorts used in the study.207 Moreover, the locus is found to be associated with other diseases, mostly autoimmune,208-211 which could suggest its involvement in immune development in general. The role of PGAP3 in asthma is unclear. GSDMA was connected to expression of TGF-beta 1 and ALOX5.35 For ORMDL3, plenty suggestions were made on the function of ORMDL3, such as IL-17 secretion and eosinophil trafficking.35
Even though the locus has been studied intensively during the last 10 years, this has not resulted in a shift in clinical practice. To the best of our knowledge, one clinical trial is ongoing in connection to 17q and asthma [NCT00856947].
5q31-q32, ADRB2
ADRB2 is a gene producing beta2-adrenergic receptor. Regularly prescribed for asthma patients—short- and long-acting beta agonist (SABA and LABA)—aim to target ADRB2 receptor; thus, variants of ADRB2 can significantly affect drug response. As expected, it was found to be associated with asthma drug response in candidate-gene studies and GWAS (for SABA).40, 212
However, a meta-analysis has not confirmed the importance of ADRB2 variants.41 For COPD, being also treated by SABA/LABA, 3 functional variants of ADRB2 have not been found to be significantly associated in a systematic review and meta-analyses.213 Many clinical trials are registered with assessment of ADRB2 and treatment response in asthma (eg NCT03654508, NCT02758873, NCT03493503; completed: NCT01786616, NCT02230332, NCT00350207, NCT00708227, NCT00200967). A recent systematic review that assessed 32 peer-reviewed publications on the ADRB2 and its connections to asthma concluded that rs1042713 variant is important for asthma in children.39 The PUFFIN clinical trial43 (randomized, placebo-controlled double-blind) addresses this particular SNP and treatment response in asthmatic children. More studies are needed to evaluate the effect of ADRB2-based treatment on better asthma control. Some ethnic groups (eg African American) can benefit from studies of rare variants in ADRB2, since rare variants are more frequent in these populations.
Large projects researching (severe) asthma
Asthma, although a widespread disease, has a relatively low amount of patients per facility. Larger-scale combined efforts are necessary to obtain enough statistical power and include unbiased representation of the population to studies. Recognizing this necessity, several large-scale projects were created. “Unbiased biomarkers for the prediction of respiratory disease outcome” (U-BIOPRED52),“Severe asthma research program” (SARP59) and “Mechanisms of the Development of ALLergy” (MeDALL,214) are remarkable projects, in which omics data were collected and used to cluster patients with asthma.
Clusters in U-BIOPRED data
The U-BIOPRED data set, one of the biggest data collections of asthma patients in the world, contains both clinical data as well as data originating from biological samples, such as sputum, breath, urine, etc The data set has previously been used to find clusters of patients on the basis of combined proteomics and transcriptomics data, obtained from patients sputum. Four clusters with clinically distinctive phenotypes were identified. One cluster contained well-controlled asthma patients with low-to-high ICS use and slightly reduced lung function, while 3 other clusters contained severe asthma patients, one of which was mostly characterized by smoking history, another—by OCS therapy, third—by obesity, exacerbations and consisting mostly of females. For transcriptomics, post hoc analysis also showed different biological pathways associated with these clusters. For further details, we refer readers to read.147 The authors concluded that this or similar studies will help develop a personalized medical care in asthma. Before these results can be translated to clinics, validation in independent cohorts is necessary. Stability of these clusters should also be verified by collecting and analysing omics data longitudinally.
4 DISCUSSION
Omics technologies have provided efficient ways to investigate cellular processes and to find causal relationships between them and observed phenotypes. Our view on human diseases changed in the last decade, as we started to grasp intricate pathophysiology and factors involved in diseases. Despite active omics employment in asthma research, it has not yet been possible to fully comprehend asthma on a molecular level. However, considerable progress has been made: starting with unbiased analyses, such as GWAS, to identify novel genes and finishing with the integration of several omics layers, that may result in the identification of clinically relevant disease subphenotypes.
The rational use of omics in asthma research could pave the way to more personalized asthma treatment regimens, wherein “one-size-fits-all” approach is substituted by treatment based on biological mechanisms and treatable traits.215 For instance, Th2-mediated endotypes were confirmed with omics, which improved our knowledge of pathophysiology and quality of asthma care via the development and selection of biomarkers and new therapeutics for T2-high asthma.216 Also, GWASs in asthma resulted in a few clinical trials that investigate genotype-guided treatment regimens on the basis of ADRB2 and 17q variants.
One of the main issues in omics analyses is “dimension hell”: the number of hypotheses (SNPs, genes, proteins, etc) being tested is large, in comparison with the number of patients. To find statistically significant results, application of dimensionality reduction methods is required in a combination with large amounts of patients.217, 218 Thus, research hospitals need to combine their efforts to include as many patients as possible. Large-scale projects similar to U-BIOPRED and SARP demonstrated the power of collective efforts directed towards the same goal—improving our understanding of asthma. U-BIOPRED, for instance, together with clinical data collected information on several omics layers. The group generated clusters of patients on the basis of clinical characteristics and demonstrated significant differences in biological pathways between them in sputum.56, 147 Another example is the Pharmacogenomics in Childhood Asthma (PiCA) consortium which studies pharmacogenomics of asthma in children. The consortium resulted in acceleration of research due to active knowledge exchange.42, 219 One more example of large-scale collaborations is AsthmaMap project220 that developed pathway-based representation of asthma mechanisms with input from experts in the respiratory field, information from literature and databases. This resource is meant to be regularly updated and promotes collaboration between experts from different fields.
Considering the dynamic nature of complex diseases, it is unreasonable to assume that single time point measurements will capture the entire complexity for such processes. Hence, studies designed to encompass the temporal fluctuations from omics platforms are crucial to make accurate predictions about the disease outcome. Such longitudinal monitoring studies addressing temporal dynamics using computational modelling approaches are lacking and needs immediate attention.
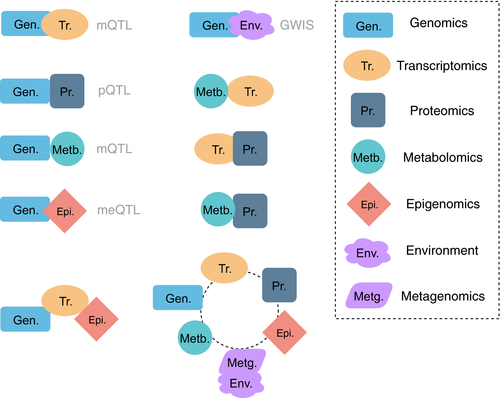
Lack of statistical power and missing heritability may also be addressed by omics layers integration (see Figure 2). One layer does not allow us to draw phenotypic traits accurately, in addition to the batch effects, large variability and lack of standardization are observed in omics studies. A combination of information from several layers holds potential to compensate for this. This approach is relatively novel and acquiring more attention in the recent years. The U-BIOPRED group has recently established its analytical approach for integrating their omics data sets, but the final results are yet to be published.221-223 Kelly et al224 integrated metabolomics and proteomics for 325 asthmatic children, demonstrating that ORMDL3 miRNA and sphingolipid metabolism can be connected. Further, employment of computational models of biological pathways (so-called executable biology225) can complement the integrative approach in a journey to understand molecular mechanisms in asthma.226
Bioinformatics software packages that are used in omics analyses have their own limitations. The “zoo” of various tools proves it difficult to choose amongst them since the differences and similarities are not always clearly described. Scientists are rewarded for new publications rather than stable and robust software. Because of that, many tools are not actively supported leading to inevitable errors propagation in new research projects. In clinical research, the lack of statistical knowledge and deep understanding of underlying methods also leads to improper applications and interpretations of the results. Examples of misuse of bioinformatics tools include incorrect understanding of tools' parameters,227 excel-related gene names errors,228, 229 internal bugs,230 incompatibility of the software packages that can be missed.231 Bioinformaticians and systems biologists with statistics, computer science or molecular biology backgrounds, thus, should be a part of the research team to control for these issues.
None of the omics signatures have been translated into clinical practice. The findings from omics in asthma research should first prove their clinical value, cost-effectiveness and applicability as effective biomarkers in large studies of asthma. Furthermore, the benefits of these findings should be clearly communicated; this requires a bridge between researchers and clinical practitioners. For that, cross-field experts (bioinformaticians, systems biologists, immunologists, (pharmaco)geneticists) should be engaged to translate between two environments.
Future Research Perspectives
Integrative systems approaches will provide a cleaner signal for subphenotyping.
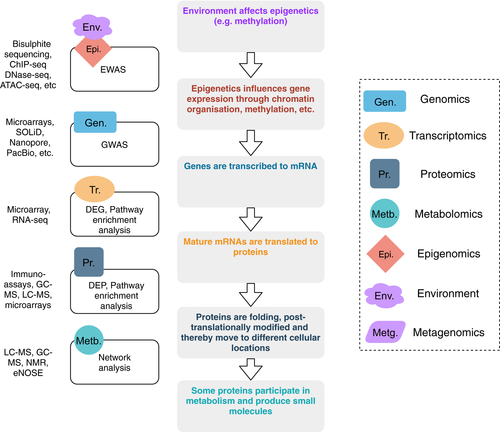
Relationships in cellular networks are much more complicated than we hoped: gene expression and corresponding functional protein can be altered in many ways and on multiple levels, starting from translation and up to post-translational modifications of the protein and its transport to the functional location (see Figure 1). We measure genomics and transcriptomics comprehensively, while epigenomics, proteomics, metabolomics extract only fractions of the molecular data on these levels for now. As single layer omics data are noisy and provide partial information on each layer, we would highly benefit from a combination of various layers together (see Figure 2). This approach is relatively novel and has attracted more attention over recent years. Mostly, two layers are combined together; integration of more than two layers is rarely possible due to costs involved and computational challenges. Methods are already available for such an approach, with examples of SNF and iCluster.232 It was recently implemented with promising outcomes for cancer, COPD and immune response.221, 233-236
Single-cell omics helps to identify the signal from asthma-specific cells.
Functioning of the proteins manifests differently in different cell types. Until recently, it was impossible to sort and collect omics of one cell type effectively. Single-cell omics has already proven to be a productive technique in cancer studies,237-239 and it is anticipated that it will produce effective results in asthma as well. A particular importance of separating the signal from different cells is implied for transcriptomics, proteomics and epigenomics. The main critique for these studies is that they may merely measure the difference in cell composition between disease and control patients. The importance of tissue for asthma can thus be re-evaluated by single-cell omics analysis.
Identification of cross-connectivity at molecular level with other diseases.
Traditional diagnoses may no longer suffice as gold standard for biological phenotyping. Looking for differences and commonalities of one disease from another would help identify the best treatment scheme and to repurpose drugs. For example, atopic asthma shares some molecular components with allergy; asthma and COPD also share some molecular pathways. Obesity has associations with asthma, but the exact shared components between them remain to be discovered.
Components like inflammation or ageing can be shared between many diseases, leading to a thought that the “one size fits all” treatment will soon be outdated in favour of pathway-based personalized treatment. This represents more of a systematic approach (systems medicine) and invites for a large shift in paradigm in medical care practices: patients would be treated not on the level of specific disease manifestations, but rather as complex systems. For example, asthma treatment might take into account comorbidities involving a combined view of physicians, immunologists, nutrition experts, infectionists, dermatologists, etc, where they all decide together on a personalized basis what treatment scheme is the most beneficial for that patient.
The systems medicine approach may get a boost from collecting all information on molecular mechanisms for diseases in one place. The DiseaseMaps240, 241 project aims to build a comprehensive molecular representation of diseases. The project has developed maps for diseases such as cancer, Parkinson's disease, rheumatoid arthritis.242-245 Recently, AsthmaMap has been developed on two levels.220, 246 Such projects will allow actual implementation of the four pillars of modern medicine (P4: Predictive, Preventive, Participatory and Personalized).
CONFLICT OF INTEREST
AH Maitland-van der Zee has received grants from the Dutch Lung Foundation, ERACOSYSMED, UK CF Foundation and FP7; furthermore, she received research grants from GSK, Boehringer Ingelheim, Novartis and AstraZeneca. AH Maitland-van der Zee has received consultancy fees from AstraZeneca and Chiesi. Dr Peter Sterk reports grants from Public-Private grant by the Innovative Medicines Initiative (IMI), related to the topic of this manuscript; in addition, he reports Scientific Advisorship and an inconsiderable interest in Breathomix BV. The other authors have nothing to disclose.




