A EAACI drug allergy interest group survey on how European allergy specialists deal with β-lactam allergy
Abstract
An accurate diagnosis of β-lactam (BL) allergy can reduce patient morbidity and mortality. Our aim was to investigate the availability of BL reagents, their use and test procedures in different parts of Europe, as well as any differences in the diagnostic workups for evaluating subjects with BL hypersensitivity. A survey was emailed to all members of the EAACI Drug Allergy Interest Group (DAIG) between February and April 2016, and the questionnaire was meant to study the management of suspected BL hypersensitivity. The questionnaire was emailed to 82 DAIG centres and answered by 57. Amoxicillin alone or combined to clavulanic acid were the most commonly involved BL except in the Danish centre, where penicillin V was the most frequently suspected BL. All centres performed an allergy workup in subjects with histories of hypersensitivity to BL: 53 centres (93%) followed DAIG guidelines, two national guidelines and two local guidelines. However, there were deviations from DAIG recommendations concerning allergy tests, especially drug provocation tests. A significant heterogeneity exists in current practice not only among countries, but also among centres within the same country. This suggests the need to re-evaluate, update and standardize protocols on the management of patients with suspected BL allergy.
Graphical Abstract
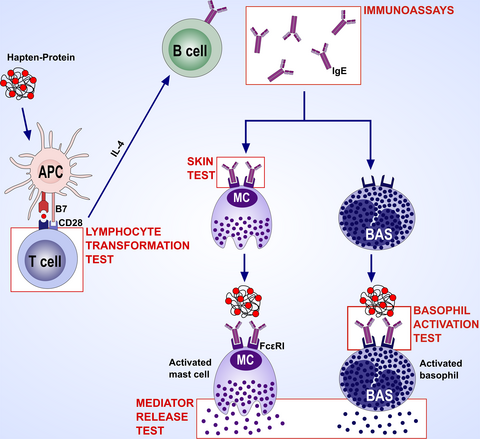
Allergy testing in immediate and nonimmediate reactors to betalactams. The combination of amoxicillin plus clavulanic acid and amoxicillin alone was the most commonly involved BL, although differences among countries concerning the most common penicillin involved exist. All centres performed an allergy workup in subjects with histories of hypersensitivity to BL although there were heterogeneity and deviations from DAIG recommendations concerning allergy tests. Data from this survey suggest the need to re-evaluate, update and standardize protocols on the management of patients with suspected BL allergy.

Abbreviations
-
- AX
-
- amoxicillin
-
- BAT
-
- basophil activation tests
-
- BL
-
- β-lactam
-
- BP-OL
-
- benzylpenicilloyl-octa-L-lysine
-
- CLV
-
- clavulanic acid
-
- DAIG
-
- Drug Allergy Interest Group
-
- DPT
-
- drug provocation tests
-
- EAACI
-
- European Academy of Allergology and Clinical Immunology
-
- ELISpot
-
- enzyme-linked immunospot
-
- ENDA
-
- European Network on Drug Allergy
-
- IDT
-
- intradermal tests
-
- LAT
-
- lymphocyte activation tests
-
- LTT
-
- lymphocyte transformation tests
-
- MD
-
- minor determinant
-
- MDM
-
- minor determinant mixture
-
- NSP
-
- narrow-spectrum penicillins
-
- PT
-
- patch tests
-
- RC
-
- respondent centres
-
- SPT
-
- skin prick tests
-
- SsIgE
-
- serum-specific IgE
-
- ST
-
- skin tests
-
- U.S.
-
- United States of America
-
- UK
-
- United Kingdom
1 INTRODUCTION
β-Lactam (BL) antibiotics are highly effective against bacteria and among the most prescribed drugs globally. Unfortunately, they can provoke hypersensitivity reactions. These reactions are either immediate (ie occurring within 1-6 hours after the last drug administration) or nonimmediate (ie occurring at least 1 hour after the initial drug administration in sensitized patients, but usually after several hours or even days).1, 2
It is important to assess subjects with histories of hypersensitivity reactions to BL, as up to 70% of such subjects are indeed not allergic based on diagnostic test results.3 A recent large retrospective U.S. cohort study,4 which compared patients with and without histories of penicillin allergy, has shown that an unverified history of penicillin allergy is associated with longer hospital stays and higher rates of Clostridium difficile, methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus infections compared with patients with no history of penicillin allergy. Therefore, an accurate allergy assessment in order to prevent the mislabelling of patients as penicillin allergic can reduce patient morbidity and mortality, microbial resistance to antibiotics and the economic costs associated with prolonged hospital stays and expensive non-BL antibiotic use.5-10
In this regard, the European Academy of Allergology and Clinical Immunology (EAACI) Drug Allergy Interest Group (DAIG) published guidelines for evaluating both immediate11 and nonimmediate12 reactions to BL in 2003 and 2004, respectively. In 2009, these guidelines were updated.13 These guidelines11-13 suggest performing skin tests (ST) first and then drug provocation tests (DPT) if allergy tests are negative and no contraindications exist. However, changes in BL prescription patterns, differences in the organization of allergy services and variations in BL reagent availability across Europe might have influenced the implementation of these guidelines in daily allergy practice. Up-to-date information evaluating approaches to BL allergy testing across Europe, and specifically the adherence to the aforementioned guidelines,11-13 is lacking and thus necessary. Therefore, this study was designed to investigate the availability of BL reagents, their use and test procedures in different parts of Europe, as well as any differences in the diagnostic protocols for evaluating subjects with BL hypersensitivity and how closely European practice adheres to the DAIG guidelines. This is a prerequisite before further attempts to harmonize the diagnostic protocols throughout Europe can be made (Figure 1).
2 METHODS
A survey was emailed to all DAIG members between 8 February and 6 April 2016. This survey was developed based on the contents of the DAIG guidelines on assessment of subjects with histories of hypersensitivity reactions to BL11-13 and consisted of several sections, each of which aimed to document a specific topic (Table 1).
| I. General data |
|
| II. Allergy workup data |
|
- BL, β-Lactam; DAIG, European Academy of Allergology and Clinical Immunology (EAACI) Drug Allergy Interest Group.
In the first part of the questionnaire, information was collected on the specializations of the physicians who usually perform BL testing, existence of national or local guidelines, number and type (ie adults and children) of subjects with histories of hypersensitivity reactions evaluated annually by each centre, BL most frequently involved and main clinical manifestations of hypersensitivity reactions.
The information collected in the second part regarded the diagnostic workup, specifically the use of the classification of hypersensitivity reactions as immediate and nonimmediate, use of different workups according to the reaction type (immediate or nonimmediate), use of guidelines (eg DAIG, national and local), in vivo and in vitro tests carried out, sequence in performing them and setting of the allergy workup (eg outpatient clinic, day hospital and emergency care).
Participants were asked whether they performed the full allergy workup according to the DAIG guidelines,11-13 and, if not, they were asked to specify any differences. Additional questions regarded the reagents for ST and concentrations used.
As far as in vitro tests are concerned, participants were asked whether they routinely undertook serum-specific IgE (SsIgE) assays and basophil activation tests (BAT) in immediate reactors, or lymphocyte transformation tests (LTT), lymphocyte activation tests (LAT) and enzyme-linked immunospot (ELISpot) assays in nonimmediate ones. In case of positive responses, participants were asked in which situations they carried out these tests. Questions were asked to determine which cut-off was used in the ImmunoCAP® (Thermo Fisher Scientific, Uppsala, Sweden; ie 0.1 or 0.35 kU/L) and whether the ratio specific/ total IgE was calculated, as well as to determine which activation markers were used in the BAT. Participants were also asked whether they performed ST in subjects displaying positive responses to in vitro tests.
With regard to DPT, information was requested on whether participants performed such tests, and, in case of positive answers, whether they followed the DAIG guidelines,11-13 applying different protocols in immediate and nonimmediate reactors, or used other protocols.14, 15 In the latter case, questions were asked to determine in how many steps the participants reached the maximum single unit dose and whether nonimmediate reactors underwent a therapeutic course at home and for how many days. Participants were asked whether they re-evaluated immediate reactors presenting negative results in allergy workups, including DPT, and after which time interval between the last reaction and allergy testing they retested such patients.
Finally, participants gave information on whether they recommended alternative antibiotics in subjects diagnosed as allergic. In case of positive responses, they were asked which antibiotics they recommended (eg BL belonging to classes other than those of the responsible ones, quinolones and macrolides) and whether they prescribed them after negative ST followed by DPT, only after a negative ST or DPT, or without performing ST and/or DPT.
Answers were not obligatory, while multiple answers and free-text comments were permitted to capture the likely diversity of BL testing in Europe. This meant that there were different total (absolute) numbers in some answers, and therefore, results have been presented as number and percentage of respondents to ensure clarity.
3 RESULTS
The questionnaire was emailed to 82 DAIG centres and answered by 57 (response rate 69.5%): 12 from Italy and Turkey, seven from Portugal and Spain, five from Switzerland, two from France, Netherlands and United Kingdom (UK), and one from Austria, Belgium, Denmark, Germany, Greece, Lithuania, Romania and Serbia.
3.1 General data
According to the survey, in Denmark, Greece, Lithuania, Portugal, Romania, Spain and UK, patients were assessed exclusively by allergists; in France, Netherlands and Switzerland, by both allergists and dermatologists; in Germany and Italy, by allergists, dermatologists and paediatricians, but in Italy, mostly by allergists; in Turkey and Serbia, by both allergists and paediatricians, but in Turkey, mostly by allergists; in Austria, by both dermatologists and paediatricians; and in Belgium, by internists with allergy subspecialization.
There were national guidelines on the diagnosis of drug allergy in France, Germany, Italy, Netherlands, Portugal, Spain, Turkey and UK. Overall, 53 (93%) of the 57 participants followed the DAIG guidelines, two followed national guidelines, and two followed local guidelines: one (Danish) because there were no national guidelines and the other (Swiss) because testing based on local experience was preferred.
Thirty-two (56%) of the 57 participants reported that they evaluate more than 100 patients with a suspicion of BL allergy per year, whereas 15 participants between 50 and 100 patients, and 10 < 50. Five centres evaluated only children and the remaining 52 both adults and children, but mainly adults.
Penicillins were responsible for 70% or more of all hypersensitivity reactions in 42 (91%) of the 46 respondent centres (RC: ones which answered this question). The combination of amoxicillin (AX) plus clavulanic acid (CLV) and AX alone was the most commonly involved BL in 53 (98%) of the 54 RC, whereas in the Danish centre, penicillin V was the most frequently suspected BL. In 41 (93.1%) of the 44 RC, cephalosporins were responsible for 10%-40% of all BL hypersensitivity reactions. The most commonly involved cephalosporins were ceftriaxone, cefaclor and cefuroxime (Table 2).
| RCa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate of penicillins involved in HR | 46 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| RCb | 0 | 0 | 0 | 3 | 0 | 1 | 17 | 14 | 9 | 2 | |
| Rate of immediate reactions to penicillins | 41 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| RCb | 3 | 3 | 4 | 3 | 2 | 5 | 12 | 3 | 4 | 0 | |
| Rate of nonimmediate reactions to penicillins | 41 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| RCb | 4 | 4 | 8 | 5 | 3 | 5 | 5 | 3 | 4 | 0 | |
| Rate of cephalosporins involved in HR | 44 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| RCb | 9 | 14 | 17 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | |
| Rate of immediate reactions to cephalosporins | 39 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| RCb | 2 | 5 | 7 | 2 | 2 | 2 | 9 | 5 | 5 | 0 | |
| Rate of nonimmediate reactions to cephalosporins | 39 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| RCb | 6 | 7 | 9 | 4 | 3 | 2 | 4 | 2 | 2 | 0 | |
| Most common penicillin involved in HR | 54 | AX/CLV | AX | PV | AM | BC | PG | Other | |||
| RCc | 41 | 12 | 1 | 0 | 0 | 0 | 0 | ||||
| 2nd most common penicillin involved in HR | 46 | AX | AM | AX/CLV | PG | BC | PV | Other | |||
| RCc | 25 | 7 | 5 | 5 | 2 | 1 | 1 | ||||
| Most common cephalosporin involved in HR | 34 | CT | CU | CE | CZ | CX | |||||
| RCc | 19 | 8 | 5 | 2 | 0 | ||||||
| 2nd most common cephalosporin involved in HR | 30 | CE | CT | CU | CZ | CX | |||||
| RCc | 11 | 8 | 6 | 4 | 1 | ||||||
| Most common manifestation of HR | 54 | MP | UA | U | An | E | |||||
| RCd | 22 | 15 | 14 | 3 | 0 | ||||||
| 2nd most common manifestation of HR | 37 | MP | UA | U | An | E | |||||
| RCd | 14 | 1 | 11 | 8 | 3 |
- AM, ampicillin; AX, amoxicillin; AX/CLV, amoxicillin + clavulanic acid; BC, bacampicillin; PG, penicillin G; PV, penicillin V; CE, cefaclor; CT, ceftriaxone; CU, cefuroxime; CX, cephalexin; CZ, cefazolin; An, anaphylaxis; E, erythema; MP, maculopapular exanthema; U, urticaria; UA, urticaria-angioedema.
- a Total number (No) of respondent centres (which answered the question concerned).
- b No of respondent centres which found the above rate.
- c No of respondent centres which found the above involved β-lactam.
- d No of respondent centres which found the above manifestations of hypersensitivity reactions.
The most frequent clinical manifestations were urticaria and maculopapular rash (Table 2).
3.2 Allergy workup data
All 57 centres performed an allergy workup in subjects with histories of hypersensitivity reactions to BL. Fifty-five (98%) of the 56 RC classified BL hypersensitivity reactions as immediate and nonimmediate. Table 2 shows the rate of immediate and nonimmediate reactions to penicillins and cephalosporins reported by the RC.
Forty-nine (87.5%) of the 56 RC applied different protocols for evaluating immediate and nonimmediate reactions. Overall, 46 (93.9%) of the 49 RC applied the DAIG protocols for assessing either immediate or nonimmediate reactors, two (Italian and Swiss) applied local protocols and one (Turkish) a national guideline. All centres except the Danish centre (98.2%) performed ST for both immediate (Table 3) and nonimmediate reactions, if there was an indication. However, 2 centres (Portuguese) did not perform ST in subjects with severe reactions and 1 centre (Portuguese) in children. Moreover, nine of the 54 RC did not perform ST in subjects with clear histories of nonimmediate reactions.
| RCa | Yes | No | |||
|---|---|---|---|---|---|
| Test sequenceb | |||||
| In vitro tests → ST→ DPT | 54 | 40 | 74.1% | 14 | 25.9% |
| ST→ in vitro tests → DPT | 54 | 7 | 13% | 47 | 87% |
| ST→ DPT | 54 | 6 | 11.1% | 48 | 88.9% |
| In vitro tests → DPT | 54 | 1 | 1.9% | 53 | 98.1% |
| Skin tests | 57 | 56 | 98.3% | 1 | 1.7% |
| On subjects with clear histories | 54 | 49 | 90.7% | 5 | 9.3% |
| On subjects with ambiguous histories | 54 | 52 | 96.3% | 2 | 3.7% |
| On subjects with no historiesc | 54 | 8 | 14.8% | 46 | 85.2% |
| Reagents | |||||
| All those of the DAIG protocol | 57 | 39 | 68.4% | 18 | 31.6% |
| Those of DAIG protocol, no benzylpenicillin | 57 | 12 | 21.1% | 45 | 78.9% |
| Those of DAIG protocol, no BP-OL and MD | 57 | 4 | 7% | 53 | 93% |
| Other panel reagents | 57 | 2 | 3.5% | 55 | 96.5% |
| Positivity criteria and timing for reading | |||||
| DAIG criteria | 52 | 45 | 86.5% | 7 | 13.5% |
| Other criteria | 52 | 7 | 13.5% | 45 | 86.5% |
| In vitro tests | 57 | 54 | 94.7% | 3 | 5.3% |
| Performed routinely | 50 | 35 | 70% | 15 | 30% |
| If performed routinely | |||||
| Before ST | 34 | 22 | 64.7% | 12 | 35.3% |
| Before ST in severe reactors | 34 | 19 | 55.9% | 15 | 44.1% |
| After negative ST | 34 | 4 | 11.8% | 30 | 88.2% |
| Methods: | |||||
| SsIgE-CAP | 53 | 33 | 62.3% | 20 | 37.7% |
| SsIgE-CAP and BAT | 53 | 14 | 26.4% | 39 | 73.6% |
| SsIgE-CAP and BAT and other SsIgE | 53 | 4 | 7.5% | 49 | 92.5% |
| Other SsIgE | 53 | 1 | 1.9% | 52 | 98.1% |
| Other methods | 53 | 1 | 1.9% | 52 | 98.1% |
| SsIgE-CAP | |||||
| SsIgE-CAP cut-off: 0.1 kU/L | 52 | 20 | 38.5% | 32 | 61.5% |
| SsIgE-CAP cut-off: 0.35 kU/L | 52 | 32 | 61.5% | 20 | 38.5% |
| SsIgE-CAP ratio total/specific IgE | 43 | 8 | 18.6% | 35 | 81.4% |
| BAT markers | |||||
| CD63 | 24 | 8 | 33.3% | 16 | 66.7% |
| CD63 and CD203c | 24 | 15 | 62.5% | 9 | 37.5% |
| Other markers | 24 | 1 | 4.2% | 23 | 95.8% |
| Setting of allergy workup | |||||
| Only in DH | 55 | 21 | 38.2% | 34 | 61.8% |
| Only in OPC | 55 | 13 | 23.6% | 42 | 76.4% |
| In both DH and OPC | 55 | 17 | 30.9% | 38 | 69.1% |
| In DH, OPC and ECS | 55 | 3 | 5.5% | 52 | 94.5% |
| In both OPC and ECS | 55 | 1 | 1.8% | 54 | 98.2% |
- BAT, basophil activation test; BP-OL, benzylpenicilloyl-octa-L-lysine; DAIG, European Academy of Allergology and Clinical Immunology (EAACI) Drug Allergy Interest Group; DH, day hospital; DPT, drug provocation tests; ECS, emergency care setting; MD, minor determinant; OPC, outpatient clinic; SsIgE, serum-specific IgE assay with the ImmunoCAP; ST, skin tests.
- a No of respondent centres (which answered this question).
- b Order in which tests were carried out in the respondent centres.
- c On subjects with no histories of hypersensitivity reactions to β-lactams, but with histories of hypersensitivity reactions to other drugs.
3.2.1 Skin test reagents
In 47 centres (82.4%), benzylpenicilloyl-octa-L-lysine (BP-OL, 0.04 mg/mL, DAP®, Diater, Leganés, Spain) and sodium benzylpenilloate (0.5 mg/mL, DAP®) were available and used as major and minor determinant (MD) of benzylpenicillin, at a concentration up to 8.64 × 10−5 mol/L (ie undiluted) and 1.5 × 10−3 mol/L (ie undiluted), respectively. Three centres (two Italian and one British) used homemade benzylpenicillin determinants, whereas seven centres (two French, two Turkish, one Danish, one Lithuanian and one Romanian) did not use benzylpenicillin determinants because they were unavailable. Injectable AX and CLV (Diater) were available and used in 33 (57.8%) and 24 (42.1%) centres, respectively.
3.2.2 Immediate reactions
For evaluating immediate reactors, all tests (ie ST, in vitro tests and DPT) were performed in 50 centres (87.7%), only ST and DPT in 6 centres (10.5%) (three Turkish, two French and one Dutch), and ST and in vitro tests in 1 centre (Austrian).
Table 3 provides information on allergy testing in immediate reactors, particularly on the tests used, order in which they were performed, characteristics of patients who underwent ST, reagents used, positivity criteria and timing of reading, as well as methods of in vitro tests and setting of allergy workup.
In case of positive in vitro tests, 22 (44%) of the 50 RC did not perform ST.
In case of negative results in both ST and in vitro tests, 53 (98.1%) of the 54 RC performed DPT and one (Spanish) did not; 44 (84.6%) of the 52 RC applied the DAIG protocol.
Twenty-two (39.3%) of the 56 RC retested patients after a negative allergy workup, including DPT; 12 of the 18 RC retested after a time interval of 6 months between the last reaction and allergy testing and six after a time interval of 1 year.
3.2.3 Nonimmediate reactions
For evaluating nonimmediate reactors, patch tests (PT), ST, in vitro tests and DPT (ie all tests) were performed in 18 centres (31.5%), PT, ST and DPT in 34 centres (59.6%), only PT and ST in 4 centres (three Turkish and one British) and only DPT in 1 (Turkish, paediatric).
Table 4 provides information on allergy testing in nonimmediate reactors, particularly on the tests used, order in which they were performed, methods of in vitro tests and setting of allergy workup.
| RCa | Yes | No | |||
|---|---|---|---|---|---|
| Test sequenceb | |||||
| In vitro tests→PT/ST→DPT | 49 | 14 | 28.6% | 35 | 71.4% |
| PT/ST→DPT | 49 | 32 | 65.3% | 17 | 34.7% |
| PT/ST→in vitro tests→DPT | 49 | 3 | 6.1% | 46 | 93.9% |
| In vitro tests | |||||
| Performed routinely | 56 | 6 | 10.7% | 50 | 89.3% |
| Performed in selected cases | 56 | 10 | 17.9% | 46 | 82.1% |
| If performed in selected cases: | |||||
| Before PT and/or ST in severe reactors | 10 | 7 | 70% | 3 | 30% |
| After negative PT and/or ST | 10 | 3 | 30% | 7 | 70% |
| Methods | |||||
| Lymphocyte transformation test | 16 | 11 | 68.8% | 5 | 31.2% |
| Lymphocyte activation test | 16 | 3 | 18.7% | 13 | 81.3% |
| Other unspecified | 16 | 2 | 12.5% | 14 | 87.5% |
| Setting of allergy workup | |||||
| Only in DH | 54 | 18 | 33.3% | 36 | 66.7% |
| Only in OPC | 54 | 16 | 29.6% | 38 | 70.4% |
| In both DH and OPC | 54 | 18 | 33.3% | 36 | 66.7% |
| In both DH and ECS | 54 | 1 | 1.9% | 53 | 98.1% |
| In both OPC and ECS | 54 | 1 | 1.9% | 53 | 98.1% |
- DAIG, European Academy of Allergology and Clinical Immunology (EAACI) Drug Allergy Interest Group; DH, day hospital; DPT, drug provocation tests; ECS, emergency care setting; OPC, outpatient clinic; PT, patch tests; ST, skin tests.
- a No of respondent centres (which answered this question).
- b Order in which tests were carried out in the respondent centres.
In both children and adults with histories of mild nonimmediate reactions, 47 (85.4%) of the 55 RC performed the full allergy workup according to the DAIG guidelines,12, 13 whereas 8 centres did not; in particular, 1 centre (Danish) did not use PT and another (Turkish) used a different protocol.
In subjects with negative results in allergy tests, 49 (89.1%) of the 55 RC carried out DPT routinely; 34 (70.8%) of the 48 RC applied the DAIG protocol for DPT and 14 did not. Specifically, 19 of the 30 RC reached the maximum single unit dose in 3 steps, 10 centres in more than 3 steps and 1 centre (Swiss, paediatric) in only one step. Moreover, subjects with negative DPT underwent therapeutic courses at home for 2 days in 3 centres, 3 days in 9 centres, 4-7 days in 6 centres (two Turkish, one Belgian, one Portuguese, one Spanish and one Swiss) and 3-10 days in 1 (Danish).
In the management of patients with histories of severe non-immediate reactions (eg Stevens-Johnson syndrome, toxic epidermal necrolysis and acute generalized exanthematous pustulosis), 25 (52.1%) of the 48 RC performed the full allergy workup, according to the DAIG algorithm,12, 13 which includes PT and, in case of negative results, delayed-reading ST. Twenty-three centres (47.9%) did not perform the full allergy workup; specifically, 15 centres (31.2%) performed only PT. In subjects with severe nonimmediate reactions, according to the aforesaid algorithm,12, 13 all centres did not perform DPT.
3.3 Identification and/or recommendation of alternative antibiotics in BL-allergic subjects
In subjects diagnosed as allergic, 53 (94.6%) of the 56 RC recommended alternative antibiotics; 48 (90.6%) of these 53 centres specified the antibiotics concerned. Thirteen centres recommended only non-BL antibiotics (8 centres, quinolones and macrolides; 3 centres, quinolones, macrolides and other non-BL antibiotics; 1 centre, quinolones and other non-BL antibiotics; and 1 centre, macrolides); 10 centres recommended only alternative BL (7 centres all alternative BL: cephalosporins, aztreonam and carbapenems in penicillin-allergic subjects; penicillins, aztreonam and carbapenems in cephalosporin-allergic subjects; and three only cephalosporins in penicillin-allergic subjects); and 25 centres recommended both non-BL antibiotics and alternative BL.
Forty-seven (95.9%) of the 49 RC prescribed alternative BL after negative ST and DPT with the BL concerned, whereas 4 centres prescribed them after negative ST only.
4 DISCUSSION
Our survey indicates that subjects with BL hypersensitivity reactions are evaluated by allergists in most European countries, together with dermatologists and/or paediatricians in several countries such as France, Germany, Netherlands, Serbia, Switzerland and Turkey. However, dermatologists and paediatricians care for such patients in Austria and internists with allergy subspecialization in Belgium. This diversity might be caused by different public healthcare systems. The great majority (93%) of physicians followed the DAIG guidelines, even though there were national guidelines in many countries.
The differences among countries concerning the most common penicillin involved in hypersensitivity reactions could be explained by a different BL consumption pattern. In fact, in southern Europe, combinations of BL, particularly AX/CLV, are used extensively, especially in Italy and Spain. In Spain, the percentage of reactions attributed to AX/CLV increased to 60% between 2011 and 2014, with a decrease in reactions to AX alone.16 Also in Spain, an increasing number of patients with selective hypersensitivity to CLV have been reported.17-19 Moreover, many publications about cephalosporin hypersensitivity come from Italy,20-25 the country with the highest consumption of 3rd and 4th generation cephalosporins.26 Cephalosporins represented 11.4% of all antibiotic outpatient prescriptions in Europe.26
With regard to the allergy workup, this survey demonstrates that there is a heterogeneity in practice and deviations from the DAIG recommendations, as observed in a UK national survey of investigations for BL hypersensitivity.27 The DAIG guidelines11-13 suggest performing ST (and also PT in nonimmediate reactors) first and then DPT if allergy tests are negative and no contraindications exist. In immediate reactions, in vitro tests, specifically SsIgE assays and BAT, are also suggested.11-13, 28 The choice of performing ST and/or PT is based on the suspected pathogenic mechanisms: skin prick tests (SPT) followed by intradermal tests (IDT) are recommended for immediate reactions and PT, as well as delayed-reading SPT and IDT, for nonimmediate ones.11-13
Skin tests with the major and minor benzylpenicillin reagents have a significant impact on the diagnosis of immediate reactions to BL, particularly to benzylpenicillin (also called penicillin G), with very good negative predictive value.29-33 For this reason, the use of these reagents in the diagnosis of such reactions is recommended by the DAIG guidelines, as well as some national ones and/or practice parameters,11, 12, 34, 35 but unfortunately, these reagents are not available in all European countries.
Benzylpenicilloyl-poly-L-lysine (PPL, Pre-Pen®, AllerQuest LLC, Plainville, CT, USA) and BP-OL (DAP®, Diater) are the commercially available major determinants in use today. In Europe, the commercially available MD are benzylpenicillin and sodium benzylpenilloate (DAP®). In the past, there were minor determinant mixtures (MDM) available from Allergopharma (Hamburg, Germany: benzylpenicillin and sodium benzylpenicilloate) and Diater (benzylpenicillin, sodium benzylpenicilloate and benzylpenicilloic acid). In some European countries that participated in this survey (Austria, Belgium, France, Germany, Greece, Italy, Netherlands, Portugal, Spain, Switzerland, Turkey and UK), Diater sold DAP®, which since May 2011 has included BP-OL and sodium benzylpenilloate. However, seven participants (two from France and Turkey, and one from Denmark, Lithuania and Romania) did not use PPL/BP-OL and MD.
The highest concentrations recommended in both SPT and IDT are as follows: 5 × 10−5 mol/L for PPL (ie undiluted), 8.64 × 10−5 mol/L for BP-OL (ie undiluted), 1.5 × 10−3 mol/L for sodium benzylpenilloate (ie undiluted) and 10 000 IU/mL for benzylpenicillin. It should be noted that, in the European documents,11-13, 36 the correct values of reagent concentrations, which should have been expressed in mol/L, were changed incorrectly to mmol/L.
AX and ampicillin are recommended for ST by the DAIG protocols. However, injectable AX is not available in all European countries. For this reason, since 2013 in some European countries that participated in this survey (Austria, Germany, Italy, Netherlands, Portugal, Spain, Switzerland and UK), Diater has sold, as an additional MD, AX sodium, which has been shown to be equivalent to injectable AX in terms of ST responses, as well as in vitro immunochemical (RAST and RAST inhibition) and biological test (BAT) results.37
In some European countries that participated in this survey (Austria, Belgium, Germany, Netherlands, Portugal, Spain, Switzerland and UK), CLV is also available from the same company. In other European countries, these reagents are not registered, which in addition to their high costs may constitute an obstacle for a harmonized European approach. Reflecting this situation, even though the European guidelines11-13 recommend skin testing subjects with hypersensitivity reactions to BL not only with PPL/BP-OL and MDM/MD, but also with AX, and any suspected BL, including CLV, in order to detect side-chain-specific sensitizations, injectable AX and CLV were used only in 33 and 24 centres, respectively.
For evaluation of nonimmediate reactions, 56 centres (98.2%) performed delayed-reading ST and 47 of them carried out the full allergy workup according to the DAIG guidelines12, 13 even though IDT with PPL/BP-OL and MDM/MD are scarcely useful.38
As far as in vitro tests for immediate reactions are concerned, according to the European documents,11, 13, 28 both SsIgE assays and BAT are complementary to ST. The former determine SsIgE by immunoassay and the second basophil activation by flow cytometry. However, the SsIgE assay with ImmunoCAP® is suitable for a limited number of BL (penicillin G, penicillin V, AX, ampicillin and cefaclor). Moreover, its sensitivity is rather low and variable (0%-50%) and seems to correlate with the severity of the reaction.30, 39-41 Lowering the threshold from 0.35 to 0.1 kUA/L increases sensitivity, although it also reduces specificity, particularly for cases with total IgE>200 kU/L.42, 43 However, a recent study by Vultaggio et al 43 demonstrated that the use of the specific IgE/total IgE ratio increases the ImmunoCAP® specificity. In this study, specific IgE/total IgE ratio values ≥0.002 had a positive predictive value of 92.5%. According to our survey, 38.5% of the 52 RC used a cut-off of 0.1 kU/L and only 18.6% of the 43 RC calculated the specific/total IgE ratio.
The sensitivity of the BAT for penicillins ranges from 22% to 55% 40, 44 and for CLV can reach up to 52.7%. The BAT shows a good specificity, ranging from 79% to 96%.18, 40, 44, 45 Even though the BAT enables in vitro evaluation for BL, such as CLV and most cephalosporins, for which no immunoassays are available, only 26.3% of the centres performed this test, as it requires local laboratory facilities and fresh blood samples.
The European guidelines11, 13 suggest performing in vitro tests before ST in subjects with histories of severe anaphylaxis in order to reduce the risk of systemic reactions to ST. However, only 56% of the 34 RC performed in vitro tests before ST in such subjects.
According to the aforesaid DAIG position paper,28 LTT, LAT and ELISpot assays can be used for evaluating nonimmediate reactions to BL. LTT determine specific lymphocyte proliferation, LAT determine specific lymphocyte activation and ELISpot measures cytokine-producing cells. Nevertheless, our survey indicates that only 26.1% of the 23 RC performed LTT or LAT routinely and 73.9% in selected cases.
The European position paper on DPT46 considers that they are intended to establish a firm diagnosis in subjects with a suspected BL hypersensitivity, to exclude a hypersensitivity in nonsuggestive histories or to provide safe alternatives in allergic patients and thus prove tolerance. According to our survey, 98.1% of the 54 RC performed DPT in immediate reactors presenting negative ST and in vitro tests; however, one Danish centre performed DPT in patients with negative in vitro tests without ST47; 84.6% of the 52 RC followed the DAIG protocol,11, 13 but only 39.3% of the 56 RC retested patients after a negative allergy workup, including DPT, likely because they believed this was mostly unnecessary and not cost effective.
With regard to DPT in nonimmediate reactors, even though 70.8% of the 48 RC applied the DAIG protocol, actually there was a significant heterogeneity concerning the number of steps for reaching the maximum single unit dose and the duration of DPT (from a single therapeutic dose administered in one day to a therapeutic course of more than 7 days).
5 CONCLUSIONS
This survey documented that most drug allergy European reference centres belonging to the European Network on Drug Allergy (ENDA) followed the DAIG guidelines on the management of BL allergy. One of the limitations of the study is that it lacks information from countries in Northern Europe and that some countries were represented by only one or two centres, whereas others had more than five centres represented. In addition, only centres belonging to the ENDA have been included in the survey and the adherence to published guidelines may be lower in other, often smaller, centres in the same country. On the other hand, this study has demonstrated a significant heterogeneity in current practice not only among countries, but also among centres belonging to the same country. Overall, data from this survey suggest the need to re-evaluate, update and standardize protocols on the management of patients with suspected BL allergy.
The standardization of skin testing protocols with regard to the reagents used, their dilutions, the number of steps and positivity criteria, some of which varied widely in this survey, needs to be improved in order to ensure the diagnosis accuracy and patient safety. In this connection, there is to resolve the issue of unavailability of some reagents, such as BP-OL and MD, in several European countries.
The use of reliable in vitro tests should be increased, especially in evaluating subjects who experienced severe reactions. In these subjects, in vitro tests should be performed before ST or PT in order to reduce the risk of systemic reactions to the in vivo tests.
The standardization of DPT protocols concerning the number of steps for reaching the maximum single unit dose and the duration of DPT in nonimmediate reactors should be implemented. In any case, future guidelines should recommend that all centres undertaking BL testing have the resources to perform DPT, which remain the “gold standard” for diagnosing BL hypersensitivity due to limited sensitivity of both ST and in vitro tests even if optimal protocols are followed.
ACKNOWLEDGMENTS
The present study has been supported in part by Institute of Health “Carlos III” of the Ministry of Economy and Competitiveness (grants co?funded by European Regional Development Fund (ERDF): PI15/01206, PI18/00095 and ARADyAL RD16/0006/0001).
We thank all the centres participating in the questionnaire: Wohrl S: Floridsdorf Allergy Centre (FAZ), Vienna (Austria). Sabato V: University of Antwerp | UA—Departement Translationeel pathofysiologisch onderzoek, Antwerp (Belgium). Garvey LH and Mosbech H: Allergy Clinic, Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Copenhagen (Denmark). Barbaud A: Dermatology and Allergy Department, University Hospital of Nancy, Nancy, (France). Chiriac A: Department of Pulmonology, Division of Allergy, Hôpital Arnaud de Villeneuve, University Hospital of Montpellier, Montpellier (France). Brockow K: Department of Dermatology and Allergy Biederstein, Technische Universität München, Munich (Germany). Makris MP: Allergy Unit “D. Kalogeromitros,” 2nd Department of Dermatology and Venereology, Medical School, National and Kapodistrian University of Athens, Athens (Greece). Berti C: Private physician, Varese (Italy). Bommarito L: Allergology and Clinical Immunology, AOU Città della Salute e della Scienza di Torino, Molinette Hospital, Turin, Turin (Italy). Cortellini G: Allergologia, U.O. Medicina Interna e Reumatologia, Azienda Sanitaria della Romagna, Rimini (Italy). Del Bono A: Presidio Ospedaliero Centro Ospedale A. Fiorini—Terracina Servizio di Pneumologia e Allergologia, Terracina (Italy). Della Torre F: Milan, IRCCS San Raffaele Scientific Institute, Università Vita-Salute San Raffaele, Milan (Italy). Dolcher MP: Clinical Immunology Unit, University of Pisa, Italy, Pisa (Italy). Bonadonna P: Unità di Allergologia, Azienda Ospedaliera Universitaria Integrata, Verona (Italy). Cremonte L: Ospedale S. Giacomo, Novi Ligure (Italy). Romano A: Unità di Allergologia, Presidio Columbus, Rome (Italy). Roncallo C: Struttura Dipartimentale Centro Day Hospital, Allergologia e Immunologia Clinica—Azienda Socio Sanitaria Territoriale di Mantova, Mantova (Italy). Saretta F: Azienda per L'Assistenza Sanitaria numero 2 Bassa Friulana-Isontina, Gorizia (Italy). Testi S: Unità di Allergologia, Ospedale San Giovanni di Dio, Firenze (Italy). Kvedariene V: Vilnius University·Clinic of Infectious and Chest Diseases, Dermatovenereology and Allergology, Vilnius (Lithuania). Oude Elberink H: University of Groningen, Groningen (The Netherlands). Terreehorst I: Department of Otorhinolaryngology, Academic Medical Centre, Amsterdam (The Netherlands). Almeida E: Unidade de Imunoalergologia, Centro Hospitalar Tondela Viseu, Viseu (Portugal). Cadinha S: Serviço de Imunoalergologia, Centro Hospitalar Vila Nova de Gaia/ Espinho, Gaia (Portugal). Chambel M: Departamento de Imunoalergologia, Hospital CUF descobertas, Lisboa (Portugal). Faria E: Serviço de Imunoalergologia, Centro Hospitalar Universitário de Coimbra, Coimbra (Portugal). Geraldes L, Serviço de Imunoalergologia, Hospital Senhora da Oliveira, Guimarães (Portugal). Lopes A: Serviço de Imunoalergologia, Centro Hospitalar Lisboa Norte, Lisboa (Portugal). Spinola A: Serviço de Imunoalergologia, Centro Hospitalar Lisboa Norte, Lisboa (Portugal). Petrisor C: Department of Anaesthesia and Intensive Care, University of Medicine and Pharmacy “Iuliu Hatieganu” (CP, MM, NH), Cluj-Napoca (Romania). Atanaskovic-Markovic M: Medical Faculty, University Children's Hospital, University of Belgrade, Belgrade (Serbia). Torres MJ: Allergy Unit, Regional University Hospital of Malaga-IBIMA-UMA, Malaga (Spain). Cabañas-Moreno R: Allergy Unit, Hospital Universitario La Paz, Madrid (Spain). Ortega-Rodríguez N: Allergy Unit, Hospital Universitario de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria (Spain). Barranco Jiménez R: Allergy Unit, Hospital Universitario 12 de Octubre, Madrid (Spain). Moreno-Rodilla E: Allergy Unit, Hospital Universitario de Salamanca, Salamanca (Spain). Sanchez-Morillas L: Allergy Unit, Hospital Universitario Clínico San Carlos, Madrid (Spain). Muñoz-García E: Allergy Unit, Hospital Universitario de Getafe, Madrid (Spain). Ballmer-Weber B: Allergy Unit, Department of Dermatology, University Hospital, Zurich (Switzerland). Bircher A: Allergology, University Hospital Basel, Basel (Switzerland). Adult Allergist 2 (Switzerland). Caubet JC: Pediatric Allergy Unit, Geneva University Hospital, Geneva (Switzerland). Ped Allergist 2 (Switzerland). Bavbek S: Ankara University School of Medicine Department of Chest Diseases, Division of Immunology and Allergy, Ankara (Turkey). Buyukozturk S: Istanbul University Istanbul School of Medicine, Department of Internal medicine, Division of Immunology and Allergy, Istanbul (Turkey). Celik G: Ankara University School of Medicine Department of Chest Diseases, Division of Immunology and Allergy, Ankara (Turkey). Demirel Y: Ankara University School of Medicine Department of Chest Diseases, Division of Immunology and Allergy, Ankara (Turkey). Dursun B: Recep Tayyip Erdoğan University School of Medicine, Department of Internal Medicine, Division of Immunology and Allergy Rize (Turkey). Erkocoglu M: Department of Pediatric Allergy and Immunology, Abant İzzet Baysal Üniversitesi, Bolu (Turkey). Isik R: Koç University School of Medicine, Department of Pulmonology Division of Immunology and Allergy, Istambul (Turkey). Misirlioglu ED: University of Health Sciences, School of Medicine, Ankara (Turkey). Oner F: Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital, Department of Immunology and Allergy, Ankara (Turkey). Sekerel B: Hacettepe University School of Medicine, Department of Pediatrics, Division of Immunology and Allergy, Ankara (Turkey). Sin B: Ankara University School of Medicine Department of Chest Diseases, Division of Immunology and Allergy, Ankara (Turkey). Soyer O: Hacettepe University School of Medicine, Department of Pediatrics, Division of Immunology and Allergy, Ankara (Turkey). Shrimpton A: Clinical Immunology and Allergy Unit and Department of Immunology and Protein Reference Unit, Sheffield Teaching Hospitals NHS Trust, Sheffield (UK). Nakonechna A: Allergy and Immunology Clinic, Royal Liverpool and Broadgreen University Hospitals, Liverpool (UK).
CONFLICTS OF INTEREST
None of the authors have any conflict of interest, nor have they received any money for this study. Research is part of their daily activities. All authors had full access to all data and take responsibility for the integrity and accuracy of the data analysis.
AUTHORS CONTRIBUTION
AR and MJT are the chair and secretary of the EAACI TF on betalactam allergy. They have prepared the survey, analysed results, drafted the introduction and compiled the entire manuscript. GC, PW, MAM, AB, ABi, MB, KB, JCC, JC, AC, PD, LHG, HM, HM and AM are members of the EAACI TF on betalactam allergy and have worked on the development of the survey, on the analysis of results, and have written specific sections of the manuscript. All authors reviewed the entire final manuscript.