Chemokine receptors in allergic diseases
Abstract
Under homeostatic conditions, as well as in various diseases, leukocyte migration is a crucial issue for the immune system that is mainly organized through the activation of bone marrow-derived cells in various tissues. Immune cell trafficking is orchestrated by a family of small proteins called chemokines. Leukocytes express cell-surface receptors that bind to chemokines and trigger transendothelial migration. Most allergic diseases, such as asthma, rhinitis, food allergies, and atopic dermatitis, are generally classified by the tissue rather than the type of inflammation, making the chemokine/chemokine receptor system a key point of the immune response. Moreover, because small antagonists can easily block such receptors, various molecules have been developed to suppress the recruitment of immune cells during allergic reactions, representing potential new drugs for allergies. We review the chemokines and chemokine receptors that are important in asthma, food allergies, and atopic dermatitis and their respectively developed antagonists.
Abstract

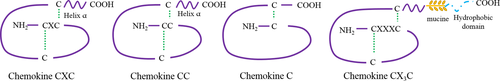
In homeostasis, as well as under inflammatory conditions, immune cells travel throughout the entire organism in the blood and lymphatic vessels. Homing of immune cells to inflamed tissues and organs is a crucial step for defense against pathogens and infections. Under inflammatory conditions, immune cells migrate to the site of inflammation owing to a common process orchestrated by small molecules of 15 kDa called chemokines. Chemokines are subdivided into the following four groups according to the arrangement of the amino terminal cysteine residues: CC, CXC, C, and CXC3C (Fig. 1). Approximately forty chemokines can be linked to nineteen chemokine receptors, which are coupled to G proteins expressed by a large variety of immune cells. Each of these receptors can be linked to several molecules of the same group. Chemokines are mostly expressed by endothelial and epithelial cells. Thus, when immune cells expressing a chemokine receptor travel through the blood and lymphatic vessels, they are attracted by their matching ligands. Adhesion molecules such as integrins are also involved in the process to stabilize circulating cells against the vessel wall 1. Four major steps govern this process called chemotaxis: rolling, activation, arrest, and transendothelial migration. When chemokines are secreted into the outer-cell medium, targeted cells will position themselves toward the gradient. The first step of this migration is the rolling of the cells on the endothelial cell layer. This step depends on the interaction between integrins and selectins, such as E-selectin, P-selectin, or L-selectin (CD62L), expressed only by naïve cells. Once the leukocyte is fixed on the endothelium, the chemokine receptor binds to its ligand to immobilize the cell. This interaction induces conformational changes that lead to increased affinity to integrins, reinforcing adhesion to the endothelium. Finally, the cell crosses the endothelium through cell junctions 2.
Once a chemokine has bound to its receptor, a signaling cascade triggers cytosolic calcium elevation: The activation of the G protein induces the activation of phospholipase C, which cleaves the phosphatidyl-inositol diphosphate into two parts, the diacylglycerol and the inositol triphosphate, which activate the protein kinase C for the first and trigger calcium elevation for the second. These chain reactions lead to signaling cascades, such as those mediated by MAPK, ERK, and JAK, which trigger the expression of various molecules responsible for cell migration 3.
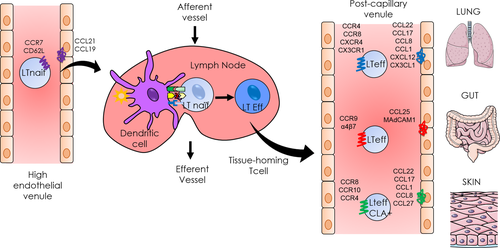
Most of the chemokines and chemokine receptors are not essential for life, as shown by targeted gene deletions in mice. CXCL12 is the only exception because, in the fetal state, CXCL12-knockout mice do not survive because of multiple organ failure. Indeed, CXCL12 is responsible for the migration of haematopoietic progenitor cells in the bone marrow 4. Immune cells can express various chemokine receptors at the same time, and the pattern of expression can be classified by homeostatic and inflammatory receptors 1. Homeostatic receptors, such as CCR7, CCR9, CCR10, and CXCR4, are chemokine receptors expressed under basal conditions, but some of them can also be overexpressed in inflammatory states. The homeostatic receptors are mainly expressed during organogenesis or for development of tissues, such as secondary lymphoid organs, including the lymph nodes (LN). CCR7, mainly expressed by naïve T cells, allows these cells to enter into secondary lymphoid organs owing to its interaction with CCL21 and CCL19. The pair CCL25/CCR9 is involved in thymus development: Immature T cells are attracted by the expression of CCL25 by thymic dendritic cells 5. Inflammatory chemokine receptors are expressed in inflamed tissues by resident immune cells and cells stimulated by the cytokine environment 6. Indeed, the presence of pro-inflammatory cytokines or pathogens induces the expression of chemokine receptors on cells. However, the expression of tissue-specific chemokine receptors is imprinted by dendritic cells in secondary lymphoid organs, which activate lymphocytes. Thus, the specific homing is due to the activation site 7. For example, lymphocytes in the mesenteric LN are imprinted by CD103+ dendritic cells during antigenic sensitization to express CCR9 and α4β7, which lead to their migration to the intestine. In the skin, Langerhans cells activate T cells to express CCR10, CCR4, CCR8, and the CLA 8. While these mechanisms are well known for the skin and the intestine, the migration of immune cells within the lungs remains unclear.
Chemokines and chemokine receptors are involved in several mechanisms of the immune system. Their first and main role is chemotaxis. The cell migration occurs when chemokines are expressed in the extracellular medium. Chemokine expression is generally due to epithelial and endothelial cells and correlated with the receptor expression by immune cells. For example, in asthma, the increased expression of CCR4 by T cells from broncho-alveolar lavage has been correlated with the increased expression of CCL22 measured by enzyme-linked immunosorbent assay 9. Moreover, the increase in CCL25 within the intestine in chronic inflammatory diseases was correlated with the increase in CCR9-expressing T cells in mesenteric LN 10. Finally, in atopic dermatitis, the increased expression of CCL18 by skin epithelial cells has been correlated with increases in CLA+ T cells and CCR8+ T cells 11. As a consequence, it has been shown that it is the increased expression of chemokines that precedes the homing of the cells. The secretion of CCL17 by bronchial epithelial cells and dendritic cells in asthmatic subjects preceded the homing of Th2 cells 12. In another study using a model of acute asthma, the authors showed that a higher concentration of CCL22 found in the lungs, expressed by monocytes and macrophages, preceded the migration of Th2 cells 13. In the same manner, during the first stage of airway inflammation, the secretion of CCL11 led to eosinophil infiltration 14. Besides this role, the chemokine/chemokine receptor system is also involved in cell retention, survival, or cell proliferation. Indeed, chemokines also regulate the time spent by lymphocytes inside the LN. Within the LN, T cells follow the CCL19 and CCL21 gradient to encounter DCs. This migration is due to a CCR7-mediated retention signal which ends with the expression of the sphingosine-1-phosphate (S1P) gradient and the exit of the T cell out of the LN 15. In terms of retention, the CXCR4/CXCL12 couple is very important for the differentiation of hematopoietic stem cells into various immune cells. Indeed, the blockade of the CXCR4/CXCL12 axis induces the release of neutrophils from the bone marrow, indicating a strong role of the chemokine receptor in their retention 16. CXCR4 is also required for the B-cell retention within the bone marrow 17. In terms of survival, CX3CR1 was described to provide a prosurvival signal to monocytes 18 and is also involved in the survival of Th2 cells in allergic asthma 19.
In allergic diseases, it has been shown that multiple chemokine receptors are overexpressed depending on the affected tissue (Table 1). In this review, we explain the types of chemokine receptors that are involved in three major allergic diseases: asthma, food allergies, and atopic dermatitis. Chemokine receptors are expressed by all the immune cells, but considering that T cells are critical upstream mediators of the allergic inflammation and are essential for the adaptive response, we choose to focus our interest mainly on T-cell homing (Fig. 2). Moreover, we detail the respective antagonists that have been developed.
| Chemokine receptor | Chemokine | Cell type | Allergic diseases |
|---|---|---|---|
| CCR1 | CCL5, CCL3 | Monocytes, eosinophils, basophils T lymphocytes | Asthma |
| CCR3 | CCL11 | Eosinophils, Th2, basophiles, mast cells | Asthma |
| CCR4 | CCL17, CCL25 | Th2, Tregs, CD8, monocytes | Asthma, AD |
| CCR8 | CCL1, CCL8 | T lymphocytes, thymocytes, monocytes, splenocytes | AD, Asthma |
| CCR6 | CCL20 | T and B lymphocytes, dendritic cells | Asthma |
| CCR9 | CCL25 | T lymphocytes, thymocytes | Food allergy |
| CCR10 | CCL27 | T lymphocytes, melanocytes, Langerhans cells | AD |
| CX3CR1 | CX3CL1 | T, B, and gamma delta lymphocytes, NK, monocytes | Asthma, AD, food allergy |
| CXCR4 | CXCL12 | T and B lymphocytes, eosinophils, monocytes | Asthma |
Chemokine receptors in asthma
Asthma is characterized by airway hyper-responsiveness and immune cell infiltration of the bronchi, composed of neutrophils, eosinophils, mast cells, and lymphocytes, particularly the Th2 subtype 20, leading to airway narrowing and respiratory symptoms. It has been shown that the accumulation of Th2 cells results from the recruitment of T cells from the mediastinal LN to the lungs and not from any proliferation of cells at the site 21. This recruitment depends on the chemokine expression by the inflamed tissue. Nevertheless, the exact mechanism directing this accumulation remains unclear. Thus, the overexpression of various chemokine receptors is a good parameter to explain the major infiltration of T cells, particularly Th2 cells, observed during asthma exacerbation 22. As its name suggests it, CCR1 was the first chemokine receptor discovered. Mostly expressed by macrophages, eosinophils, and basophils, its ligands CCL3 and CCL5 could be found in the broncho-alveolar lavage of patients with asthma 23. However, CCR3, a receptor typically expressed by eosinophils, plays a greater role in the pathology of asthma. CCR3 is one of the most relevant chemokine receptors in asthma because it triggers the migration of eosinophils owing to expression of the eotaxin family, mostly CCL11, CCL24, and CCL26 24. In a murine model of acute asthma, it has been shown that the suppression of CCL11 decreased eosinophil recruitment and thus airway hyper-responsiveness 25. As well as in mice, CCR3 is equally important among human beings. Indeed, patients with asthma displayed higher levels of CCL11 in plasma than healthy volunteers 26. Finally, CCR3 deletion in mice significantly decreased the homing of eosinophils to the lungs 27 and airway hyper-responsiveness in a model of airway inflammation induced by ovalbumin 28. Nevertheless, in another model of murine asthma to ovalbumin, the deletion of CCL11 did not completely inhibit the eosinophil recruitment 29. Another type of receptor involved in asthma is CCR4. Expressed mostly by Th2 cells, CCR4 plays a major role in the recruitment of T cells both in mice and in human beings 30. This receptor can bind two chemokines, CCL22 and CCL17, which have been found in higher concentrations in the broncho-alveolar lavage of patients with asthma 12, 31. The use of antibodies against CCR4 inhibited the normal migration of T cells to the lungs 32. Some studies have shown a decrease in allergic markers, such as Th2 cytokines, in a model of asthma, but some others have not reported any differences between knockout and wild-type mice 33-35. Indeed, CCR4-knockout mice seemed to be partially protected from symptoms in a model of allergy to Aspergillus fumigatus 36. CCR8 is mostly expressed by T cells and particularly Th2 cells, and its expression, as well as that of its ligand CCL1, was increased in the broncho-alveolar lavage of patients with asthma 37. In a model of asthma in CCR8-knockout mice, Th2 recruitment and mucus production were impaired 38, as well as the eosinophil infiltrate 39. If some studies made on mouse models correlate with the observations on human beings, there are contradictory results. Indeed, some studies observed no correlations between the absence of CCR8 and decreased respiratory symptoms 40, 41. CCR6 is expressed by different cell subsets, including T and B cells and dendritic cells 42. Among T cells, CCR6 is mostly expressed by Th17 cells, which were described as involved in asthma through the secretion of IL-17 in a mouse model 43. Ligation of CCR6 to CCL20 resulted in the migration of Th17 cells to various tissues, including the lungs and skin 42. Similarly, the expression of CCR6 on Th cells in PBMCs was enhanced in patients with asthma, compared to control subjects 44. CXCR4, which binds to CXCL12, induced the migration of T cells and eosinophils 45. Accordingly, high levels of CXCL12 were observed in the broncho-alveolar lavage of patients with asthma 46. The signaling pathway of CXCL12 ends in the activation of MMP9 (matrix metalloproteinase), a protein known to be involved in asthma remodeling 47. Finally, some recent studies have examined the role of CX3CR1 and its ligand CX3CL1 in asthma. The receptor CX3CR1 is expressed by various types of cells, including lymphocyte CD4, CD8, and Tγδ, and its expression was increased in the broncho-alveolar lavage of patients with asthma 48. Moreover, tobacco consumption is associated with an increase in CX3CL1 expression by the endothelium which attracts CX3CR1+ cells and increases their transendothelial migration 49. Finally, the expression of CX3CR1 by Th2 cells stimulated their survival and their retention within the airways 19.
Given the complexity and the redundancy of this system, human studies are needed to confirm or inform mouse models and understand the role of chemokine receptors. The access to biological fluid such as blood and BALF from patients suffering from asthma has significantly increased our knowledge on chemokine and their receptor in human beings. But although these samples are reasonably accessible, tissue studies require a more invasive procedure. Using mice facilitates the access to entire organs whose responses can be very different than fluid cells such as blood or BAL cells. Nevertheless, mouse models do not reflect the real disease, and particularly for asthma where the bronchi are not the same, the immune response can be different. But surprisingly, knockout mouse models have yielded encouraging results. All these contradictory results suggest that the increase in a given chemokine and/or its receptor may not reflect the pathology but be a more protective or a compensatory effect 50.
Chemokine receptors in atopic dermatitis
Atopic dermatitis is the most common skin disease in the world among children. The prevalence is 60% among children younger than 1 year, and it increases to 85% among children younger than 5 years 51. Although this prevalence decreases with age, some studies have shown a significant increase in the disease in the past few decades. This increase has been correlated with the increase in all atopic diseases, such as asthma, especially among people living in urban areas. The main characteristics of the disease are a dysfunction of the skin barrier and alteration of the immune system. The most widespread symptoms are a thickening of the skin and cell infiltration composed of mast cells, eosinophils, and lymphocytes, generally the Th2 subtype 52. Thus, chemokines and chemokine receptors play crucial roles in this pathology by recruiting immune cells to the skin. Many receptors have been studied, such as CCR4, CCR10, CCR8, and CX3CR1. In human beings, 90% of skin-resident lymphocytes express CLA (cutaneous lymphocyte-associated antigen), which links to adhesion molecules such as E-selectin that are expressed during flares of skin inflammation 53. Moreover, CCR4 and CCR10 seem to be associated with these CLA+ lymphocytes 54. The first chemokine receptor described in atopic dermatitis was CCR10, which binds to CCL27. Both molecules were highly expressed in skin biopsies of patients suffering from atopic dermatitis 55. CCL27 is exclusively expressed by keratinocytes under homeostatic conditions, and its expression can be stimulated by pro-inflammatory cytokines. Its neutralization induces a decrease in the skin inflammation in a mouse model of atopic dermatitis 56. Moreover, its expression was controlled by the secretion of inflammatory cytokines, such as IL-1β and TNF-α, within inflamed tissues 57. Finally, the inhibition of CCL27 decreased inflammation and T-cell recruitment toward the skin 58. Another receptor, CCR4, also expressed by CLA+ lymphocytes, binds to CCL22 and CCL17, which are mainly expressed by keratinocytes, endothelial cells, and dendritic cells. Their expression is particularly greater in the skin and in the serum of atopic patients, as well as in NC/nga mice 55, 59. CCL17 and CCL22 initiate the recruitment of Th2 lymphocytes, making CCR4 a potential target for therapeutic strategies. Indeed, proliferation of Th2 cells could be found in atopic dermatitis, in correlation with the increase in CCR4 expression 60. More recently, the receptor CCR8 and its main ligand CCL1 were found to be involved in a mouse model of contact hypersensitivity. CCR8 is expressed by helper T cells, thymocytes, macrophages, and dendritic cells 61. CCL18, a recently discovered ligand of CCR8, induced the migration of IL-5-secreting lymphocytes, leading to eosinophil infiltration 61. Finally, it was shown that CCR8 is able to retain dendritic cells within the skin, which prevents them to migrate toward secondary lymphoid organs. In a model of contact hypersensitivity in CCR8−/− mice, antigen-presenting cells could migrate toward LN from the skin after sensitization 62. Finally, CX3CR1 was also studied in cutaneous inflammation. CX3CR1-expressing T lymphocytes (Th1 and Th2) seemed to be involved in the induction of atopic dermatitis in mice 52. CX3CR1−/− mice displayed a decrease in cutaneous inflammation and a decrease in inflammatory cells, such as mast cells, eosinophils, and lymphocytes. Similarly, among patients, CX3CL1 was overexpressed in endothelial cells and in serum, compared to healthy volunteers 63. If some receptors have been found to be involved in skin inflammation and atopic dermatitis, to date, none of them is actually used in therapeutics or tested in any clinical trials. The redundancy of the system can explain this issue: for example, the fact that the anti-CCR4 targets both Th2 cells and Treg cells may explain the difficulty to develop suitable antagonists 64. But the complexity of the diseases, which are often very different from the animal models, is also an issue. Indeed, the models of contact hypersensitivity do not reflect totally the atopic dermatitis in human beings. All these parameters must be taken into account to transform chemokine receptor inhibition into new therapeutic treatment.
Chemokine receptors in food allergy
IgE-mediated food allergies induce various symptoms and can affect the skin, the intestine, and the lungs. They are triggered by food allergens, which are usually harmless for healthy people. The prevalence is very difficult to estimate because of the different tests used (skin prick tests, self-awareness, IgE concentration, etc.), but there are approximately 4–8% of food-allergic people among children and 1–3% among adults 65. Within the lamina propria, the antigen-presenting cells are dendritic cells, particularly cells expressing CD103 66. After antigen capture, they migrate to the mesenteric LN owing to the expression of CCR7 to activate naïve T cells. In the same manner, naïve T cells enter the LN through high endothelial venules and express CD62L and CCR7, which bind to their respective ligands on endothelial cells (GlyCAM-1, CCL21, and CCL19) 67. In the LN, CD103+ cells induce the homing molecules CCR9 and α4β7 on T cells, which allow them to migrate to the intestine. Regulatory T cells are also imprinted by dendritic cells in the mesenteric LN to maintain oral tolerance in the intestine. Thus, oral tolerance to food allergens can be impaired in mice lacking the gene for α4β7, MadCam1, or CCR9 68, 69. However, these results are still subject to debate 67. Despite contradictory results, everyone agrees that food allergy is a breach in oral tolerance. The mechanisms are not clear, but the expression of the homing molecules is a crucial step in cell recruitment to the intestine. Moreover, the allergic response is characterized by Th2 proliferation, and Th2-associated cytokines and chemokines can be found in the serum of allergic patients. In a model of food allergy in mice, the receptor CCR8 and the ligands of CCR4, CCL17, and CCL22 were overexpressed in the intestine 70. Once again, the receptor CX3CR1 is also involved in food allergies. In CX3CR1−/− mice, oral tolerance is impaired because of inhibition of the migration of regulatory T cells. The secretion of IL-10 was also decreased in this model 68. It was shown that Tregs leave the mesenteric LN, multiply among themselves, and migrate toward the lamina propria owing to CX3CR1-expressing myeloid cells, which also secrete IL-10 67, 71. The receptor CCR6 is also involved in oral tolerance because IL-10-secreting Treg cells express CCR6 on their membrane. Mice lacking this gene displayed impaired migration of Treg cells toward the intestine 72. Finally, it was shown that CX3CR1+ regulatory B cells played a role in the establishment of oral tolerance. Indeed, a decreased level of CX3CR1+ regulatory B cells could be found in the blood of allergic patients 73. Moreover, adoptive transfer of CD5+CD19+CX3CR1+ regulatory B cells induced a decrease in the allergic reaction in a mouse model 74. Relatively little is known about chemokine receptors in food allergy. If some molecules are undoubtedly involved in the process, none of them seem to predominate among the others. Within the gut disease-like inflammatory bowel disease, CCR9 was found to be particularly involved, and the clinical trial launched with its antagonist is well advanced 75, 76.
Despite the large number of chemokine receptors found to be involved in various diseases, none of these receptors seem more important than the others. Indeed, if some studies have clearly shown that the deletion of one gene in particular decreased symptoms of the pathology, many of them have found contradictory results. The type of model used, the type of allergen, or the genetic background of the mice could explain these conclusions. These issues are recurrent in the field of chemokine receptors, and they demonstrate their primary role in homeostasis. In general, studies made on knockout mice reflect the studies made with antagonists and show sometimes more convincing results. Indeed, the deletion of CCL2 induces a great protection from experimental autoimmune encephalomyelitis (EAE), while CCR2 can link also with CCL7 and CCL8, and none of their deletion showed any effect on EAE 77. Regarding CCR4, it has been shown that Tregs also expressed this receptor because 80% of them are CD4+CD25+CCR4+ 78. Thus, CCR4-knockout mice spontaneously develop skin inflammation due to the impaired migration of Tregs toward the epithelium 79. In asthma, the expression of CCR4 by Tregs is essential to regulate airway inflammation. Indeed, the adoptive transfer of CD4+CD25+CCR4+ T cells in asthmatic mice decreased lung inflammation, while the transfer of CD4+CD25+CCR4KO cells did not 80. The solution could be the extensive use of conditional knockout mice to analyze a particular cell subtype. Thus, results from knockout mouse studies must be taken with caution. In some models, knockout mice show no differences with the wild-type phenotype until they are submitted to an inflammatory stimulus 50. Moreover, chemokine receptor may have a different role according to their different ligand. For example, the chemokine CCL5 induces the recycling to the cell membrane only after the binding to CCR5 and not with its other receptors (CCR1 and CCR3) 81. The degree of internalization and/or recycling to the cell membrane of the receptor may change the response depending on the associated chemokine. Finally, chemokines can also bind to glycosaminoglycanes which are carbohydrate structures that are expressed on the cell surface or in the extracellular matrix 82. They have an important role in chemokine retention and could regulate their concentration. Indeed, CCL5 has a greater affinity for GAG than CCL2 or CCL3 which can change receptor binding 83. Finally, the differences encountered between the mouse and the human studies can be explained by the genetic differences. Indeed, the two species do not possess the same number of chemokines and chemokine receptors. If all mammals have roughly 50 chemokines and 20 receptors, human beings have 27 CCL chemokines, 17 CXCL, two XCL, and one CX3CL, whereas mice have respectively 22, 15, 1, and 1 82. Moreover, in human beings, chemokines genes are mapped on the chromosomes 4q12-21 and 17q11.2, while mouse chemokines are mapped on the chromosomes 5 and 11 84. Chemokines are known to be one of the most rapidly changing protein families 82. And some chemokines that exist in human beings cannot be found in mice, such as CCL13 and CCL14. The opposite is also true because the chemokine CXCL15 does not exist in human beings 85. There is a great difference in gene cluster between mice and human beings as is shown with the MCP: Monocyte Chimoattractant Protein group, a good example of structural difference. Indeed, there is no human counterpart of the MCP family 84, 85. Mice represent great models and great opportunities to understand immune mechanisms, but we must be always careful while extrapolating data from mouse studies to human diseases.
Chemokine receptor antagonists in therapeutic strategies against allergies
Chemokines and their receptors orchestrate the spatial distribution of immune cells toward inflammation sites under basal conditions as well as in various pathologies. The understanding of these receptors allowed for the emergence of new therapeutic strategies, such as the development of specific antagonists. Today, some antagonists are still being tested in clinical trials for different diseases, including allergies (Table 2). Nevertheless, despite a great number of clinical trials of chemokine receptor antagonists, very few of them have yielded real treatments. Several pharmaceutical industries developed antagonists which target the most important chemokine receptors involved in allergies, such as CCR3 and CCR4. For example, the molecules GW824575 and GSK2239633 targeting CCR3 and CCR4, respectively, developed by GSK, failed to prove their efficacy in asthma, and the clinical trials were stopped in phase I 86. Until today, only two drugs targeting chemokine receptors have been marketed: maraviroc, an anti-CCR5 developed by Pfizer and used in HIV therapy, and plerixafor, an anti-CXCR4 developed by Anormed and used in oncology 87, 88. However, some drugs have been encouraging, and clinical trials are still ongoing. The anti-CCR4 called mogamulizumab, developed by Amgen and Kyowa-Hakko, is currently being tested to treat lymphoma and allergic diseases 89, 90. The anti-CCL11 (bertilimumab), developed by Immune Pharmaceuticals, is currently in a phase II clinical trial to treat various diseases, such as asthma, Crohn's disease, and macular degeneration 91. The anti-CCR3 agent ASM8, developed by Pharmaxis, was tested to treat asthma, and an anti-CCR9 called vercirnon, developed by Chemocentryx, is currently in phase III trials to treat Crohn's disease and celiac disease 75, 76, 92. Chemokine receptors belong to the super family of G-protein-coupled receptors (GPCRs), and they represent good candidates for drug development. Indeed, the GPCR family is the most successful target for drug discovery because 30% of all medicines exert their effects via these receptors 93. Many receptors have been targeted, such as the β2-adrenoceptor for the treatment of asthma, the angiotensin receptor for the treatment of arterial hypertension, and the opioid receptor for the treatment of pain. The reasons for failure are many and are not always known. The principal explanations are the high redundancy of the chemokine system, disease heterogeneity and insufficient blockade of the receptor, poor predictive data from animal models, and our limited understanding of the system 86. Indeed, because of the very complex system and its redundancy, it is very complicated to target one cell type at a time. Chemokine receptors are not specific to one cell type, and several share the same function; therefore, the inhibition of one receptor only is not sufficient to prevent cell migration. For example, an antagonist against CCR4 targets effector T cells, as well as regulatory T cells, which can worsen the condition rather than improving it 94. The anti-CCR4 antagonist mogamulizumab has been implicated in severe cases of skin inflammation during clinical trials because of the depletion of Tregs 64. Moreover, it was discovered that the ligands of CCR4, CCL17, and CCL22 did not respond in the same manner and induced different signaling pathways 95. To overcome the problem of redundancy, drugs have been developed to target two chemokine receptors at the same time. The use of an antagonist blocking CCR5 and CXCR3 for the treatment of organ transplant rejection was successful in a mouse model of heart transplantation 96. Moreover, dual inhibition of CCR3 and the H1 histamine receptor has been used for the treatment of asthma and atopic dermatitis in vitro and in a mouse model of ovalbumin-induced asthma 97. Finally, data from animal models have misled the development of new drugs, and many antagonists proved their efficacy in rodents but failed to induce the same effects in human beings. The limited development of real treatments from chemokine receptor antagonist has mainly been due to the complexity of the chemokine/chemokine receptor system. However, the targeted diseases are also very complex. Allergic diseases are multifactorial, and many cells contribute to inflammation according to the various endotypes and phenotypes involved. This last parameter is very important and must be considered for the elaboration of new treatments.
| Chemokine receptor targeted | Drug name | Company | Disease | Clinical phase | Publication |
|---|---|---|---|---|---|
| CCR4 | KW-0761 (mogamulizumab) | Amgen/Kyowa | Lymphoma and allergic disease | Phase I | 92, 93 |
| CCR9 | CCX282 (vercirnon) | Chemocentryx | Crohn's disease, celiac disease | Phase III | 78, 79 |
| CCR3 | ASM8 | Pharmaxis | Asthma | Phase II | 95 |
| CCL11 | Bertilimumab | Immune Pharmaceuticals | Asthma, macular degeneration, Crohn's disease | Phase II | 94 |
| CXCR4 | Plerixafor | Anormed | Cancer | Marketed | 91 |
| CCR5 | Maraviroc | Pfizer | HIV | Marketed | 90 |
Conclusion
Our knowledge of chemokines and chemokine receptor family has developed significantly over the past decades. It has allowed us to enhance our understanding of the mechanisms of allergic reactions. Now the greatest challenge is in converting this knowledge into new therapies. Small molecules can now be used as antagonists of chemokine receptors to block immune cell migration. As part of the GPCR family, the chemokine receptors represent quite interesting targets for the development of new drugs. Nevertheless, the high complexity of these diseases and the high redundancy of the chemokine/chemokine receptor system can explain the multiple failures. Antagonists administered orally do not target a specific tissue but exert their effects systemically. Moreover, because of redundancy, the blockade of one chemokine receptor is not sufficient. To overcome this problem, it could be interesting to target specific cells with cocktails of antagonists administered as ointments on the skin or by aerosols for the lungs.
Acknowledgments
This work was supported by the ‘Société Française d'Allergologie’, ‘Fondation pour la recherché Médicale’, ‘La fondation du souffle’, ‘Institut de Recherche en Santé Respiratoire’, and LC was supported by the ‘Ministère de la Recherche’ and the University of Nantes. GB was supported by The People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007–2013) under REA grant agreement no. 624910.
Conflicts of interest
The authors declare that they have no conflicts of interest.