RASA2 a new gatekeeper for TCR signaling
Abstract
RASA2 is a new checkpoint inhibitor suppressing T cell activation and promoting T cell exhaustion.
Summary and Analysis
, , , et al. RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature 2022; 609(7925): 174–182.
Alloimmunity and tumor immunity are in many ways similar because both involve understanding the innate and adaptive immune responses to tissues that resemble and differ from the host. However, the scientific objectives are diametrically opposed. Alloimmunity seeks to promote tolerance of the recipient immune system towards donor tissue, whereas antitumor immunity seeks to provoke activation, proliferation, and effector activities of the host immune cells towards cancer cells. In transplant patients who also develop cancers, addressing these dual responses is a challenging task. Nonetheless, the cellular and molecular pathways involved are often the same, and studies of one model often inform the other.
In the September issue of Nature, Carnovale et al. describe a novel gene called RASA2 as a critical regulator of antitumor immunity. RASA2 is a RAS GTPase-activating protein (RasGAP), a family of proteins that are known to suppress RAS signaling pathways downstream of T cell receptor (TCR) activation by conversion of RAS-GTP to RAS-GDP. Although RASA2 has been previously shown to be the only gene in its family that is selectively expressed in human CD8+ and CD4+ T cells, its role in T cell functions has not been examined, especially in tumor models, in which chronic exposure of effector T cells to tumors often induces T cell exhaustion, a state with impaired T cell functions.
The authors initially performed unbiased, genome-wide CRISPR/Cas9 screening in vitro in human T cells, attempting to identify novel checkpoint inhibitors that prevent T cell activation and promote T cell exhaustion in an immunosuppressive milieu. Six different T cell immunosuppressive conditions were modeled in the CRISPR/Cas9 screening, including intrinsic calcineurin inhibition with tacrolimus and cyclosporine and extrinsic inhibition with adenosine agonists, TGF-β cytokine and T-regulatory cells. The human T cells were transduced with single guide RNA and Cas9 designed to target genes of interests; then they were stimulated, and their proliferation was determined by flow cytometry and CFSE dilution assays. They found that targeted deletion of two candidate genes (RASA2 and TMEM222) resulted in markedly enhanced T cell proliferation across all immunosuppressive conditions.
The authors then characterized the effects of targeted RASA2 deletion on T cell activities following TCR stimulation, and demonstrated increased levels of RAS-GTP, MEK and ERK phosphorylation, key components of MAPK signaling pathway, as well as higher levels of effector cytokines, compared with control-edited T cells, suggesting that the T cell response is enhanced following RASA2 inactivation. RNA sequencing and gene set enrichment analysis of RASA2-inactivated T cells demonstrated multiple upregulated pathways, including pathways in the cell cycle, cell metabolism, oxidative phosphorylation and glycolysis. They further showed that RASA2-deleted T cells had higher sensitivity to antigens across a wide range of concentrations, even at low antigen levels. However, in the absence of TCR stimulation, RASA2 ablation neither resulted in unregulated MAPK signaling nor altered T cell activation, proliferation and viability, suggesting that the effect of RASA2 deletion on T cell activities is activation dependent.
In a repetitive stimulation assay in which T cells were cocultured with tumor cells, RASA2 was acutely downregulated and then rapidly upregulated upon repeated exposures to tumor antigens, which led to markedly decreased cytotoxic activity over time, consistent with a putative checkpoint role. RASA2-ablated T cells, however, were resistant to anergy and/or exhaustion, thus maintaining their tumor killing capacity over time. This was further validated with available murine and human datasets. The authors then tested the translational relevance of these findings to clinical care, where the performance of RASA2 knockout T cells was assessed in multiple preclinical models. In all models, RASA2-ablated T cells demonstrated significantly increased cytotoxic functions resulting in reduced tumor growth and improved host survival compared with control-edited T cells. Notably, there were no apparent safety risks in these preclinical models involving RASA2-ablated T cells compared with control-edited T cells.
Clearly, this work identifies RASA2 as an important repressor of RAS signaling in T cells and its ablation boosting antitumor immunity, suggesting that therapeutically targeting this pathway may have important clinical implications. Further studies are now warranted to determine whether means to enhance RASA2 activation would suppress the alloimmune response and extend graft survival. Reciprocal roles for molecules involved in antitumor versus alloimmunity are not without precedent. This is perhaps best demonstrated by the checkpoint inhibitor PD-1, whose blockade has been efficacious against cancers and whose agonists favor allograft survival.
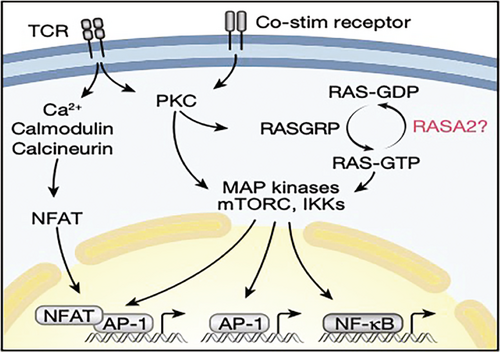
Figure 1. TCR stimulation activates multiple signaling pathways in T cells, which converge on the activation of NFAT, AP-1 and NF-kB transcription factors. RASA2 is a RAS GTPase-activating protein, a family of proteins that convert RAS-GTP to RAS-GDP, to inhibit RAS signaling and T cell activation following TCR stimulation.
Ashton Connor, MD, is assistant professor of surgery, and Xian C. Li, MD, PhD, is professor and director of the Immunobiology and Transplant Science Center and Department of Surgery at Houston Methodist Hospital in Houston, Texas.




