Anaplastic thyroid cancer: A review of recent evidence and summary of an Australian institutional protocol
Abstract
Anaplastic thyroid cancer (ATC), a rare and highly aggressive malignancy, is characterized by an exceptionally poor prognosis, where the majority of patients present with extensive local invasion and/or distant metastases. 20–30% of ATCs harbor the BRAF-V600E mutation. Neoadjuvant BRAF-targeted therapy may have the potential to downstage and facilitate surgical resection for patients with locally advanced and unresectable primary tumors with BRAF mutation and may convey a survival advantage in those with metastatic disease. There is emerging evidence to support the use of other targeted agents, including multikinase inhibitors, as well as the incorporation of immunotherapy into the treatment regimen. Rapid molecular and pathological diagnosis and expert multidisciplinary discussion at specialized treatment centers are critical to expedite investigations and initiate treatment for this complex and rapidly progressive disease.
1 BACKGROUND
Anaplastic thyroid cancer (ATC) is an undifferentiated form of thyroid cancer that arises from follicular cells. It is an aggressive and almost universally fatal malignancy, with a median reported overall survival (OS) of less than 6 months from diagnosis and disease-specific mortality close to 100%.1 Surgical resection is considered first-line in resectable disease and is associated with improved survival, particularly if clear resection margins can be achieved. However, all cases of ATC are classified as stage IV disease, with up to 90% of patients presenting with extensive locoregional invasion (stage IVB) and/or distant metastases (stage IVC) at diagnosis.2-5 Complete surgical resection is often difficult to achieve and, thus, goals of care and an initial appropriate management plan must be established quickly, with promptly coordinated involvement of surgeons, anatomical pathologists, radiation and medical oncologists, endocrinologists, and palliative care teams.
Although survival rates for ATC appear to be slowly increasing,5 these improvements are modest, and the prognosis remains very poor. Further, traditional systemic treatment with radioactive iodine therapy is less effective in ATC as most tumors do not concentrate iodine. There remains a clear need for more efficacious systemic treatment, as well as refinement of local treatments (surgery and radiation therapy). Here, we discuss recent advances in the management of ATC and provide specific treatment recommendations for use in an Australian setting, using an institutional protocol developed at the authors’ high-volume thyroid cancer referral center (Royal North Shore Hospital, Sydney) as an illustrative example (Figure 1).
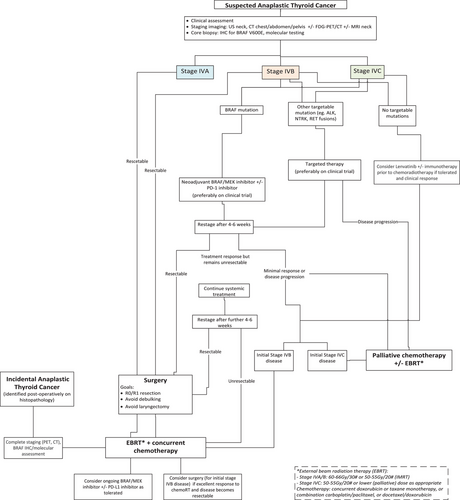
2 ROYAL NORTH SHORE HOSPITAL (RNSH) INSTITUTIONAL PROTOCOL
Patients with a new diagnosis of ATC should be alerted to the Endocrine Surgical Fellow, who will then convene a rapid full board review within a week, with involvement of endocrine surgeon, ENT surgeon, pathologist, endocrinologist, radiation and medical oncologists, and palliative care teams. All patients should be discussed as soon as possible at a dedicated Thyroid Cancer Multidisciplinary Meeting prior to commencing treatment. Initial management is guided by disease stage and results of molecular testing (if available), in line with current guidelines by the American Thyroid Association and National Comprehensive Cancer Network (NCCN).6, 7 All patients should be considered for clinical trials where available.
For all patients with ATC, proactive and early involvement of the palliative care team is advised (i.e., Routine referral where feasible at the time of diagnosis) as some patients will be recommended or elect for best supportive care from the outset. In particular, those with advanced disease, significant medical comorbidities, or poor performance status should be referred as early as possible. Even for those undergoing more aggressive treatment, early involvement of the palliative care team is recommended to assist with local symptoms related to disease burden and/or side effects of treatment.8
2.1 Initial investigations in suspected ATC
Baseline blood tests include full blood count, electrolytes (including creatinine as well as calcium, magnesium, and phosphate), liver function tests, thyroid function tests (thyroid stimulating hormone and free thyroxine), thyroglobulin and anti-thyroglobulin antibodies. Initial clinical examination includes assessment of the vocal cords with laryngoscopy.
Pathologic confirmation of ATC and exclusion of other differential diagnoses is time-critical due to the rapid growth of ATC and the potential for airway compromise, often necessitating early intervention. However, this can be challenging due to the highly dedifferentiated nature of ATC and its variable spectrum of morphological patterns (e.g., Epithelioid, spindle, giant cell, pleomorphic, squamoid, rhabdoid, angiomatoid, and pancicellular patterns).9 Fine needle aspiration biopsy provides a rapid result but often yields insufficient material for immunohistochemistry and molecular testing; therefore core biopsy is preferred (often with concurrent fine needle aspiration for rapid on-site assessment). The pathological diagnosis of ATC requires judicious use of immunohistochemistry (IHC) to exclude morphologic mimics including medullary thyroid carcinoma, metastatic carcinomas, sarcomas, melanoma, and lymphoma, and is best undertaken by an experienced endocrine pathologist. Assessment of tumor mutation profile guides subsequent treatment.10, 11 Mutation-specific immunohistochemistry for the BRAF V600E mutation is highly specific12 and can serve as a surrogate for this mutation (with results available within 24 h of the first H&E-stained slide being prepared). All ATCs are RNSH are submitted for further molecular testing to confirm BRAF mutation and/or detect other potential molecular alterations to guide targeted therapies (i.e., non-V600E BRAF mutations, RAS, ALK, RET, and NTRK mutations and rearrangements). Targeted molecular sequencing using a 50 gene panel which assesses the most common mutations and gene rearrangements typically takes a further 7 days after the initial pathology report is finalized. PD-L1 expression within the tumor (assessed by IHC) and tumor mutational burden (determined by next-generation sequencing) may help guide the likely benefit of checkpoint inhibitor immunotherapy. Mismatch repair deficiency has been reported in more than 10% of anaplastic thyroid carcinomas in some series, and identification of DNA mismatch repair defects may predict sensitivity to immunotherapy treatment.13 IHC for MLH1, PMS2, MSH2, and MSH6 is routinely performed by endocrine pathologists at RNSH.
Initial investigations for ATC should include neck ultrasound and CT scan of the head, neck, mediastinum, chest, abdomen, and pelvis with multiphase contrast to assess for locoregional disease extent (and whether this is resectable) and for distant metastases. A whole body FDG-PET/CT scan is recommended for the staging of metastatic disease and is Medicare-funded for rare or uncommon cancer types including thyroid cancer.14 MRI scan of the neck may assist with surgical planning through delineation of locoregional disease extent. Brain MRI (or CT with contrast) should be considered as clinically indicated.
2.2 Stage IVA/IVB resectable disease
2.2.1 Recommendations
Prompt assessment of the resectability of ATC is required. Patients with upfront resectable disease and adequate performance status should be offered surgical resection (resection of gross disease, total thyroidectomy, and central lymph node resection) followed by adjuvant radiation therapy with concurrent chemotherapy (Figure 1).
2.2.2 Background and evidence
Surgery
Surgical resection is only recommended if the local disease is resectable, that is, complete macroscopic resection can be foreseeably achieved with acceptable morbidity (including avoidance of laryngectomy). This approach is associated with improved survival in ATC.15 Surgery should be performed by an experienced thyroid cancer surgeon to balance the potential morbidity of surgery with expected benefits in the context of patients’ overall prognosis. Patients should be assessed for resectability, and surgery should be considered unless there is gross involvement of adjacent anatomical structures (trachea, esophagus, cervical spine, mediastinum) that will require major reconstructive surgery, or if there is a significant risk of airway compromise.
Radiation therapy
Radiation therapy (RT) is recommended postoperatively for resected stage IVA and IVB disease and is associated with improved OS following surgical resection.16, 17 Typically, conventional fractionation (1.8–2 Gy per fraction delivered once daily, 5 days per week) is utilized. A prescribed dose of 66–70 Gy is recommended for the gross tumor(s), 60–66 Gy for the surgical bed, and 45–54 Gy for the elective areas at risk of harboring micrometastatic disease. Intensity-modulated radiation therapy or volumetric modulated arc therapy techniques are preferred due to the ability to deliver a more conformal dose and maximally spare nearby organs at risk.18 RT should start as soon as possible once the patient has recovered from surgery, ideally within 2–3 weeks and no later than 6 weeks.
Accelerated hyperfractionated treatment (1.2 Gy per fraction delivered twice daily) is an alternative regime, with some studies suggesting superior local control to conventional fractionation.19, 20 However, these time-intensive regimes can be significantly burdensome for patients and carers. Hypofractionated and accelerated RT (e.g., 54 Gy in 18 fractions, or 55 Gy in 20 fractions) is a potential alternative approach, particularly if concurrent chemotherapy is not administered. The shortened overall treatment time may be expected to minimize the repopulation of this rapidly proliferating tumor, as well as reduce the time until adjuvant systemic treatment can be commenced. Observational data suggest this approach is safe and feasible, and a systematic review with pooled analysis suggests such regimes are noninferior to standard fractionation in the definitive setting.21, 22
Chemotherapy
Chemotherapy (where feasible) is recommended concurrently with RT, with improved OS compared with postoperative RT alone for resected ATC.16 The aim is to improve the therapeutic ratio by potentiating the effects of RT, increase the chance of local control, and possibly control the micrometastatic disease. However, this comes at the cost of increased acute toxicities, and thus may not be appropriate for all patients.
There is limited literature regarding the optimal chemotherapy agent to combine with radiation therapy for ATC. Commonly used regimes include doxorubicin or taxane monotherapy (paclitaxel or docetaxel) or combination therapy with carboplatin/paclitaxel or docetaxel/doxorubicin.6, 7, 19, 23
2.3 Stage IVB/C unresectable disease (BRAF V600E mutation-positive or presence of another molecular target)
2.3.1 Recommendations
The overall goal of management of Stage IVB/C disease is to alleviate symptoms by avoiding local progression whilst limiting treatment-related morbidity and adverse effects. Clear expectations and goals, including early palliative care involvement for all patients, should be discussed within the clinical team and shared with the patient and family.
Neoadjuvant dabrafenib (150 mg twice daily) and trametinib (2 mg once daily) are recommended for patients with unresectable ATC and BRAF V600E mutation. Alternative BRAF/MEK inhibitors (e.g., BRAF inhibitors vemurafenib or encorafenib; MEK inhibitors cobimetinib or binimetinib) may also be used. Treatment can be commenced after a positive BRAF V600E IHC result is confirmed, which is highly specific, but has the disadvantage of not identifying non-V600E BRAF mutations. ECG and baseline blood tests (hematology, electrolytes, liver, and kidney function) should be considered prior to commencing BRAF/MEK inhibitors. Anti-PD1 immunotherapy (pembrolizumab or nivolumab) can be added to neoadjuvant BRAF/MEK inhibitors if available. Assessment of response to neoadjuvant treatment and re-assessment of surgical resectability should be made 4–6 weeks after commencing neoadjuvant treatment (Figure 2). At a minimum, a CT neck should be performed.
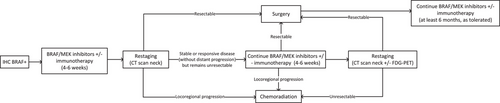
If considered suitable for surgery after 4–6 weeks of neoadjuvant targeted therapy (i.e., local disease has been downgraded from unresectable to resectable), patients should proceed to surgical resection. Dabrafenib should be ceased one day prior, and trametinib 3–5 days prior to surgery. Both drugs can be recommenced 5–7 days postoperatively. If R0/R1 resection is achieved, adjuvant RT with or without concurrent chemotherapy is recommended. BRAF/MEK inhibitors ± immunotherapy can be continued postoperatively depending on tolerability and ongoing tumor response. For patients who continued on targeted therapy, neoadjuvant responses leading to successful surgical resection have been reported up to 13.5 months after the start of treatment.24
After 4–6 weeks of neoadjuvant treatment, if there is a stable or responsive disease (in the absence of distant progression) but the disease remains unresectable, systemic treatment can be continued for another 4–6 weeks before repeat re-assessment, ideally with CT neck and FDG-PET/CT scan.
If there is a lack of adequate response to treatment after 8–12 weeks such that surgery is not feasible, or evidence of locoregional progression (clinically or radiologically) at any time, then BRAF/MEK inhibitors should be ceased and chemoradiation can be initiated. If repeat restaging after completion of chemoradiation suggests the tumor has become potentially resectable (and in the absence of distant progression), then surgical resection can be considered. BRAF/MEK inhibitors ± immunotherapy can be continued after chemoradiation depending on tolerability and ongoing tumor response.
For patients without a BRAF V600E mutation, but with the presence of other targetable alterations identified on next-generation sequencing (e.g., ALK, RET, or NTRK rearrangements), selective kinase inhibitors can be considered as neoadjuvant treatment.
2.3.2 Background and evidence
BRAF V600E mutation
Improved understanding of the molecular classification of ATC and emerging evidence for the efficacy of neoadjuvant combined BRAF/MEK inhibitors in BRAF-mutated ATC has led to its incorporation into the treatment pathway in the neoadjuvant, locally advanced, and metastatic settings.
Cohort studies support the potential for neoadjuvant BRAF-targeted therapy to downstage ATC and facilitate surgical resection in select patients. An early case series of six patients with unresectable ATC demonstrated that neoadjuvant BRAF/MEK inhibitors facilitated complete surgical resection in all patients, with high pathologic response rates.25 A subsequent series of 57 patients from the same unit (all with stage IVB or IVC disease) demonstrated that 70% of patients treated with neoadjuvant BRAF-directed therapy ultimately had surgical resection of residual disease.26 For those who underwent surgical resection, 12-month OS was 94% and progression-free survival (PFS) 84%, compared with 39% and 15% in those who did not undergo surgery. Patients with pathological complete response had 12-month OS and PFS of 100%. Hence, in patients with BRAF-mutated ATC, neoadjuvant BRAF-targeted therapy followed by surgery provided favorable outcomes in a traditionally lethal disease with respect to reduction in the size of primary neck lesions, surgical complexity, and morbidity, PFS, and OS. Patient tumor-derived spheroids have shown similar responses to combination BRAF/MEK inhibition as patient treatment response, with the potential to be used as preclinical models for further evaluation of targeted therapies.27
The 2022 European Society of Medical Oncology Guidelines recommend dabrafenib/trametinib as first-line treatment for unresectable/M1 BRAF V600E mutated ATC.28 The 2021 Anaplastic Thyroid Association (ATA) guidelines recommend dabrafenib/trametinib as first-line treatment in unresectable stage IVC disease and second-line treatment (chemoradiation is generally preferred) in unresectable stage IVB disease.6
Currently, in Australia, dabrafenib and trametinib are approved by the Therapeutic Goods Administration (TGA) but are not PBS-subsidized for the treatment of ATC.29 If given at the recommended dosage of dabrafenib 150 mg twice daily and trametinib 2 mg once daily, this combination treatment costs approximately $14,000 AUD per month.30
Other targetable molecular alterations
Although less robust evidence exists for non-BRAF systemic agents in ATC, there is increasing interest in specific pathogenic gene rearrangements, including ALK, NTRK, and RET fusion fusions. There is less established data on the effectiveness of these treatments, and clinical trial enrolment is generally recommended. There is evidence for disease activity of larotrectinib and entrectinib in ATC with NTRK gene fusions,31, 32 as well as both selpercatinib and pralsetinib in the presence of RET fusions.33 These drugs are all US Food and Drug Administration (FDA)-approved for solid tumors with these specific gene mutations. Selpercatinib, entrectinib, and pralsetinib are all TGA-approved for NSCLC with NTRK and RET fusions (but not ATC), while larotrectinib is approved for any NTRK gene fusion.34-37 Based on low quality of evidence, the current ATA guidelines conditionally recommend initiation of NTRK inhibitor (larotrectinib or entrectinib) or RET inhibitor (selpercatinib or pralsetinib) for NTRK or RET fusion ATC respectively, preferably in a clinical trial.6 None of these drugs are PBS-funded for ATC.
Immunotherapy
Evidence remains limited for the use of neoadjuvant/adjuvant immunotherapy for ATC. Preclinical studies suggest the distinct immune microenvironment of ATC may render it more sensitive to immunotherapy than papillary thyroid cancer.38 Immunotherapy appears to potentiate the effectiveness of BRAF-targeted therapy, with preclinical studies demonstrating increased CD8+ T-cell infiltration into the tumor microenvironment and upregulation of PD-L1 expression on tumor cells.39 This is supported by two retrospective studies from MD Anderson demonstrating that the addition of immunotherapy to targeted therapy for BRAF V600E-mutated ATC was associated with improved overall and progression-free survival.5, 40 This specialized center's current treatment approach incorporates pembrolizumab in addition to BRAF/MEK inhibitors in neoadjuvant systemic therapy for potentially resectable ATC, with an ongoing phase II trial investigating the role of adjuvant immunotherapy after radiotherapy for stage IVB disease.5, 41 The use of anti-PD-1 immunotherapy as salvage treatment at progression on BRAF-targeted therapy may also provide survival benefits, although further data are required to support this.40
Radiation therapy
For unresectable disease or disease nonresponsive to neoadjuvant systemic treatment after 8–12 weeks, RT with concurrent chemotherapy is recommended. The approach to dose and fractionation is similar to the postoperative setting, acknowledging a larger volume will receive 66–70 Gy due to the presence of macroscopic disease. Higher doses (60–75 Gy compared with 45–59.9 Gy) have been associated with improved OS for unresectable disease.42
Practical notes
Combination BRAF/MEK inhibition is well tolerated. The ROAR basket study reported the commonest adverse events (across several tumor subtypes) were fatigue (38%), pyrexia (37%), and nausea (35%).43 Safety data, extrapolated from its use in melanoma, recognizes early side effects include fever, anorexia, fatigue, arthralgias/myalgias, diarrhea, skin toxicities (including secondary cutaneous malignancies, hyperkeratosis, and maculopapular rash), mild alopecia, and ocular changes (retinal vein occlusion or central serous retinopathy). Late side effects include pulmonary and cardiac toxicities. It is recommended that BRAF/MEK inhibitor therapy be administered by clinicians experienced in the use of tyrosine kinase inhibitor drugs. Guidelines for the safe prescribing of systemic cancer therapy have been published by the Clinical Oncology Society of Australia,44 and patient information on dabrafenib/trametinib (for metastatic melanoma) is available via the NSW Cancer Council.45
2.4 Stage IVB/C unresectable disease (in the absence of BRAF V600E mutation or other molecular targets)
2.4.1 Recommendations
Definitive chemoradiation can be considered for select patients with stage IVB or low-volume metastatic stage IVC disease without BRAF V600E mutation. Surgical resection can be considered for select patients with IVB disease with an excellent response to chemoradiation. Lenvatinib ± immunotherapy can be considered for up to 4 weeks prior to chemoradiation if tolerated and evidence of clinical response.
2.4.2 Background and evidence
Multikinase inhibition ±/− immunotherapy
Lenvatinib, a multikinase inhibitor of VEGFR1-3, FGFR 1–4, PDGFR, RET, and KIT, has demonstrated activity in ATC. Phase II trials in metastatic ATC demonstrated an overall response rate of 24% and a median PFS of 7.4 months with lenvatinib monotherapy in a small Japanese study,46 and stable disease in half of patients in a subsequent multicenter international phase II study.47
Lenvatinib appears to have increased efficacy with pembrolizumab, with a reported median PFS of 9.5 months.5, 48-50 Patients should be counseled about the risk of bleeding and fistula with such antiangiogenic drugs, specifically given the extensive locoregional disease commonly observed in ATC. In Australia, lenvatinib is PBS-subsidized for the treatment of radioiodine refractory differentiated thyroid cancer but not ATC.
2.5 Stage IVB/C disease, not suitable for locoregional treatment
2.5.1 Recommendations
Palliative systemic treatment should be initiated for patients not suitable for locoregional treatment, where the patient is of adequate performance status and active treatment is desired. In the absence of a BRAF V600E mutation or other targetable mutations, treatment options include combination immunotherapy (pembrolizumab or nivolumab) with Lenvatinib, single agent Lenvatinib, or chemotherapy alone 48. Selective kinase inhibitors can be considered in the presence of targetable mutations identified on next-generation sequencing. Palliative RT should be considered for symptomatic locoregional and/or metastatic disease in conjunction with referral to specialist palliative care service.
2.5.2 Background and evidence
BRAF V600E mutation
The efficacy and safety of combined dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) for ATC was first demonstrated in advanced disease in the phase II Rare Oncology Agnostic Research (ROAR) basket study.43, 51 The overall response rate was 56% (including three complete responses) and OS 51.7% and 31.5% at 12- and 24 months, respectively. Dabrafenib/trametinib was approved by the US FDA for BRAF-mutated ATC in 2018 after the initial results of this study were published.52
Absence of BRAF V600E mutation
As described previously, selective kinase inhibitors may be considered in the presence of a targetable mutation (other than BRAF V600E) or Lenvatinib ± pembrolizumab in the absence of a targetable mutation.
Immunotherapy
Retrospective data as well as early prospective phase II data supports improved OS with addition of immunotherapy to targeted therapy in metastatic ATC,5, 48, 49 and is recommended in select patients with ATC in both the ATA and NCCN guidelines.6, 7
A retrospective series of 12 patients who received combination kinase inhibitors plus pembrolizumab (at time of progression on kinase inhibitor monotherapy) demonstrated partial response in 42% and stable disease in 33%, with median OS 6.9 months.50 As discussed earlier, retrospective data suggests improved OS with the addition of immunotherapy to targeted therapy for ATC.5
NCCN guidelines include pembrolizumab monotherapy as a treatment option for metastatic ATC, but only in the setting of high tumor mutational burden (≥10 mut/MB) based on the results of KEYNOTE-158, although this study only included differentiated thyroid cancers.7, 53 Several case reports support the use of pembrolizumab in patients with both high and low tissue tumor mutational burden.54, 55
In Australia, pembrolizumab is approved by the TGA for the treatment of unresectable or metastatic tumors with high tumor mutational burden that has progressed following prior treatment, but not specifically for ATC outside these indications.56 For patients self-funding pembrolizumab, the cost is about $6000 per dose (administered every 3 weeks), capped at around $70,000. High PD-L1 expression may select patients who are more likely to benefit from this approach.
Radiation therapy
Palliative RT to the thyroid and/or neck is recommended for stage IVC patients with local symptoms, or those with stage IVA/B disease not suitable for curative intent treatment. Commonly utilized dose schedules include 30–40 Gy in 10 fractions (daily over two weeks) or 20–25 Gy in five fractions (daily over 1 week), depending on the patient's performance status, feasibility of overall treatment time, and expected prognosis.
RT for distant metastases is individualized based on patient and disease factors. Stereotactic body radiation therapy or stereotactic radiosurgery may be appropriate for oligometastatic lesions, with conventional palliative RT more suitable for symptomatic sites in patients with widely metastatic disease and/or poor prognosis.
2.6 Role of specialized centers in ATC management
Given the rapid progression of ATC and the potential for delay in treatment, a strong argument exists for the centralization of care at specialized centers. Data from MD Anderson demonstrate that after instituting the Facilitating Anaplastic Thyroid Cancer Specialized Treatment improvement project, the mean time from referral to the scheduling of the first appointment decreased from 8.7 to 0.5 days, with clinical trial participation increasing to 34% although survival data has not yet been published.57 In an Australian context, early referral to specialized centers may streamline and expedite crucial investigations, minimizing the time from presentation to initiation of treatment and potentially improving survival and quality of life. However, there are often significant challenges for patients residing in rural and remote areas, with significant distances between patients and their nearest healthcare provider.
Core biopsy of suspected ATC should be performed at a site that can facilitate rapid diagnosis, BRAF V600E IHC assessment, and prompt initiation of systemic therapy where appropriate. Outside such sites, a centralized pathology review is strongly recommended. Initial and re-staging imaging can be performed locally, but review at a dedicated thyroid MDT is recommended, in particular, regarding surgical resectability. BRAF/MEK inhibitor therapy should be commenced by specialists familiar with prescribing these treatments. For patients undergoing chemoradiotherapy, treatment should be delivered locally where feasible.
2.7 Generalizability of recommendations
The differing resource availability across other major jurisdictions in the Asia/Pacific region will likely necessitate tailored approaches specific to each region, such as Koda et al.’s proposed treatment guidelines.58 However, a detailed discussion of the intricacies of region-specific access to and funding models for imaging, pathology, and systemic therapies is beyond the scope of this paper and an acknowledged limitation.
3 CONCLUSION
Management of ATC varies across Australia, specifically access to and availability of functional imaging, molecular testing, targeted therapies, and expert multidisciplinary care. Impeded access to many of these services is particularly pronounced in rural and regional areas. Close and early collaboration with surgical, endocrinology, oncology, radiology, and anatomical pathology colleagues in a multidisciplinary team setting is crucial in managing this rare disease, particularly if neoadjuvant targeted therapies are being considered. This may require a hub-and-spoke approach to treatment and/or the establishment of a dedicated statewide service as exists for other cancer subtypes.
The purpose of this literature review is to provide updated recommendations for ATC managementfor in an Australian context. Our institutional protocol seeks to provide an example of unified and rapid access to multidisciplinary care, tumor molecular testing, and initiation of targeted therapy with BRAF/MEK inhibitors ± immunotherapy in line with international guidelines.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
No funding was received for this study.
ETHICS STATEMENT
Ethics approval was not required as this protocol did not involve patients or their data.




