PRIORITI: Phase 4 study of triptorelin or active surveillance in high-risk prostate cancer
Abstract
Aim
To evaluate the efficacy and safety of triptorelin after radical prostatectomy (RP) in patients with negative lymph nodes.
Methods
PRIORITI (NCT01753297) was a prospective, open-label, randomized, controlled, phase 4 study conducted in China and Russia. Patients with high-risk (Gleason score ≥ 8 and/or pre-RP prostate-specific antigen [PSA] ≥ 20 ng/mL and/or primary tumor stage 3a) prostate adenocarcinoma without evidence of lymph node or distant metastases were randomized to receive triptorelin 11.25 mg at baseline (≤ 8 weeks after RP) and at 3 and 6 months, or active surveillance. The primary endpoint was biochemical relapse-free survival (BRFS), defined as the time from randomization to biochemical relapse (BR; increased PSA > 0.2 ng/mL). Patients were monitored every 3 months for at least 36 months; the study ended when 61 BRs were observed.
Results
The intention-to-treat population comprised 226 patients (mean [standard deviation] age, 65.3 [6.4] years), of whom 109 and 117 were randomized to triptorelin or surveillance, respectively. The median BRFS was not reached. The 25th percentile time to BRFS (95% confidence interval) was 39.1 (29.9–not estimated) months with triptorelin and 30.0 (18.6–42.1) months with surveillance (p = 0.16). There was evidence of a lower risk of BR with triptorelin versus surveillance but this was not statistically significant at the 5% level (p = 0.10). Chemical castration was maintained at month 9 in 93.9% of patients who had received triptorelin. Overall, triptorelin was well tolerated and had an acceptable safety profile.
Conclusion
BRFS was observed to be longer with triptorelin than surveillance, but the difference was not statistically significant.
Plain language summary
After a diagnosis of prostate cancer, one of the current treatments is surgical removal of the prostate and its cancer from the body. This is called radical prostatectomy. This may cure the person. Unfortunately, sometimes the tumor may have already spread into neighboring cells (lymph nodes). If this has happened, we know that giving people a chemical castration therapy to lower their levels of male sex hormones may reduce the risk of the cancer spreading further. This is achieved by giving people an androgen deprivation therapy (ADT), such as triptorelin (which is used in this study). What we do not yet know, and what we investigated in this study, is whether ADT can also benefit people who do not have signs that their cancer has already spread to the lymph nodes. Our study involved 226 men from China and Russia and investigated whether giving triptorelin for 9 months would lead to better outcomes compared with giving no additional treatment (active surveillance). All people in the study had radical prostatectomy but only half of them were given triptorelin in the following 8 weeks. The time frame of 8 weeks was used because this is the time needed to make sure that the surgery had gone well, and that no biological markers of cancer were still present in the person's blood (the marker is called prostate-specific antigen [PSA]). High levels of PSA are a sign that the prostate cancer has returned. People were monitored for 3 years with regular checks of their castration status and levels of the cancer marker (PSA). At the end of the study, we saw that it took longer for PSA levels to increase for people who had taken triptorelin than it did for those who had not had additional treatment, but the difference was not statistically significant. There were no unexpected safety concerns seen among the people taking triptorelin. Our findings are promising but more studies are needed to confirm if starting triptorelin shortly after surgery can benefit this group of people who have had their prostate cancer treated by radical prostatectomy and have no signs that their cancer has spread to the lymph nodes.
[Correction added on 31th July 2024, after first online publication: Plain language summary is added in this version.]
1 INTRODUCTION
In men worldwide, prostate cancer is the second most common malignancy and the fifth most common cause of cancer-related deaths.1, 2 In 2018, in China and Russia, respectively, prostate cancer accounted for 2.3% and 7.4% of all new cancer cases, and 3.9% and 4.6% of cancer-related deaths.3, 4 While the incidence and mortality of prostate cancer in China are currently relatively low compared with the global average, the age-standardized incidence rate of 17.3/100,000 reported for China in 2019 was a rise of 95.2% compared with 1990, which is much higher than the global increase in incidence reported for the same period (13.2%).5 In a recent retrospective single-center study in China that included 1,287 patients with prostate cancer, 46% had prostate cancer that was classified as ‘high-risk’ according to the European Association of Urology criteria.6 High-risk prostate cancer is associated with an increased risk of biochemical relapse (BR), metastases, and death.7, 8
Treatment options for prostate cancer include radical prostatectomy (RP), radiotherapy, and/or androgen deprivation therapy (ADT). However, consensus on the optimal treatment for patients with high-risk localized prostate cancer has yet to be established.7-10 The use of early ADT following radiotherapy has demonstrated clinical benefit in patients with high-risk localized disease.11-14 At the time of this study, early ADT following RP was reported to benefit patients with node-positive disease,15 but the benefit after RP in patients with high-risk node-negative disease was unclear and ADT was not recommended.12, 16, 17 The potential benefit of adjuvant ADT in prostate cancer is still under investigation in several randomized controlled trials.18
Triptorelin is a synthetic decapeptide analog of gonadotrophin-releasing hormone (GnRH) with a 100-fold higher affinity for the GnRH receptor than the natural hormone and is six times more active than leuprorelin in vitro.19 The 3-month sustained-release formulation of triptorelin is marketed in China and Russia and is indicated for the treatment of patients with prostate cancer.20, 21
The PRIORITI study (ClinicalTrials.gov identifier NCT01753297) aimed to evaluate the efficacy and safety of early adjuvant triptorelin administered in the 8 weeks after RP for 9 months in patients with high-risk (node-negative) prostate cancer in China and Russia. When this study was designed, data indicated that after RP, immediate ADT improved survival and reduced the risk of recurrence in patients with node-positive prostate cancer.22 Therefore, this study was designed to confirm the anticipated benefit of early ADT in patients with high-risk node-negative disease. At the time of study initiation, the definition of successful RP was prostate-specific antigen (PSA) ≤ 0.2 ng/mL; however, more recently, PSA levels of < 0.1 ng/mL have been used to define successful RP.23
2 PATIENTS AND METHODS
2.1 Study design
The PRIORITI study was a prospective, open-label, randomized, controlled, phase 4 study conducted in patients with locally advanced prostate cancer who were undergoing RP at nine centers each in Russia and China. Eligible patients were randomly assigned (1:1), using a computer-generated list with a block size of four, to receive triptorelin or to be under surveillance (Figure 1). Patients in the triptorelin group received an intramuscular injection of triptorelin 11.25 mg at baseline and at 3 and 6 months. Patients in the surveillance group were monitored according to the standard of care of each participating center. Study visits took place every 3 months for at least 36 months. The study ended when all patients completed 36 months of monitoring or when 61 BR events had been observed. BR was defined as increased PSA > 0.2 ng/mL confirmed in a second measurement carried out 4–6 weeks later.
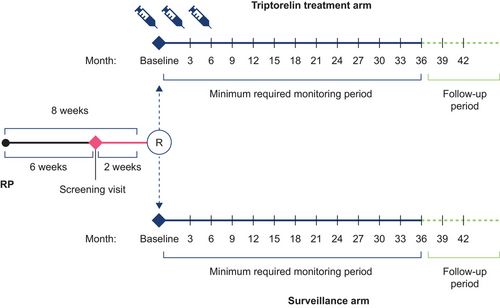
No adjuvant treatment with any method (hormonal treatment, surgical castration, or radiation therapy) was permitted in the surveillance group prior to evidence of disease recurrence (clinical or biochemical). New or additional therapy was permitted for patients in either treatment arm who developed BR or clinical disease progression (whichever occurred first), in accordance with the local standard of care.
The study was conducted according to the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice and in compliance with applicable local regulations. The protocol and informed consent forms were approved by the independent ethics committees of all participating centers. Written informed consent was obtained from all patients before study entry.
2.2 Patients
Eligible patients were males ≥ 18 years of age with histopathologically confirmed adenocarcinoma of the prostate, who had undergone RP < 8 weeks before randomization. Other key inclusion criteria were an Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO) performance status of 0 or 1, post-RP PSA level of ≤ 0.2 ng/mL at 6 weeks, and a high risk of disease progression, defined as prostatectomy specimen with Gleason score ≥ 8 on and/or pre-RP PSA level of ≥ 20 ng/mL and/or primary tumor stage 3a. Key exclusion criteria were evidence of lymph node or distant metastasis, positive surgical margins, evidence of other malignancy not treated with curative intent, previous or current treatment for prostate cancer other than RP, prior surgical castration, and a life expectancy of < 5 years.
2.3 Endpoints
The primary efficacy endpoint was BR-free survival (BRFS), defined as the time from randomization to the first BR (i.e., the first elevated PSA measurement > 0.2 ng/mL).
Secondary efficacy endpoints included: median time to event-free survival (EFS; time from randomization to time of first clinical disease progression or death from any cause); overall survival (OS); disease-specific mortality; PSA doubling time (PSADT); percentage change in PSA level between baseline and months 3, 6, and 9; and, in the triptorelin group, change in serum testosterone level between baseline and months 3, 6, and 9. Health-related quality of life (HRQoL) in both groups at baseline and 9, 24, and 36 months was also assessed using the Functional Assessment of Cancer Therapy—Prostate (FACT-P) questionnaire, version 4, and the 36-item Short-Form Health Survey (SF-36), version 2.
Safety endpoints included the incidence and severity of adverse events (AEs) and treatment discontinuations due to toxicity. AEs in this study were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.0, and were monitored until month 9 (i.e., 3 months following the last triptorelin injection for those patients in the triptorelin treatment group). After month 9 and until the end of the study, only treatment-related AEs and serious AEs were reported.
2.4 Statistical analyses
Efficacy was evaluated in the intention-to-treat (ITT) population, which included all randomized patients analyzed according to the treatment assigned. Safety was evaluated in the safety population comprising all randomized patients who received at least one injection (triptorelin group) or who underwent at least 1 day of surveillance (surveillance group), and analyzed according to treatment received.
For the primary efficacy analysis, a sample size of 226 patients reporting 61 BR events was estimated to provide 90% power to detect a decrease from 40% to 20% in the probability of BR at 36 months.
A two-sided log-rank test was used to compare BRFS between groups with alpha set at 5%. Kaplan–Meier methodology was used to estimate the median or 25th percentile (if the median was not reached) BRFS in each group. A Cox proportional hazard model was fitted to compute hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) with treatment group, country, center, and Gleason score of prostatectomy specimen as fixed factors. Similarly, a post hoc analysis of BRFS was performed using the Cox proportional hazard model. The following covariates were included: treatment group, country, age, and the number of high-risk factors (1, 2, or 3). Interactions between treatment groups and covariates were tested in separate models and were considered suggestive if p < 0.20. Changes in serum testosterone levels from baseline and HRQoL measurements were analyzed with a repeated measures analysis of covariance (ANCOVA) using mixed linear models. For serum testosterone levels, the patient was included as a random effect and baseline and visit as fixed factors. For HRQoL measurements, the patient was included as a random effect, with baseline, group, visit (months 9, 24, and 36), and the interaction between the treatment group and visit as fixed effects. Missing data were not imputed and dropouts were not replaced. Statistical evaluations were performed using the Statistical Analysis System (SAS) (version 9.4).
3 RESULTS
3.1 Patient characteristics
Between December 11, 2012, and September 9, 2019, 226 patients (163 in China and 63 in Russia) of a mean (standard deviation) age of 65.3 (6.4) years were randomized (ITT population), of whom 109 and 117 were assigned to receive triptorelin or to be under surveillance, respectively (Figure S1). RP characteristics were similar between treatment groups (Table 1). The distribution of high-risk disease characteristics in the treatment groups is shown in Table 2. The median (quartile [Q]1–Q3) duration of triptorelin exposure was 9.0 (8.8–9.1) months, and of study exposure (defined as the time between informed consent and the last visit attended) was 43.3 (36.2–54.5) months.
| Triptorelin (n = 109) | Surveillance (n = 117) | |
|---|---|---|
| Patient demography | ||
| Age, years | ||
| Median (range) | 65.0 (49−80) | 66.0 (48−79) |
| Mean (SD) | 65.4 (6.1) | 65.3 (6.8) |
| Race, n (%) | ||
| Asian | 79 (72.5) | 84 (71.8) |
| Caucasian/White | 30 (27.5) | 33 (28.2) |
| ECOG/WHO PS score, n (%) | ||
| 0 | 71 (65.1) | 84 (71.8) |
| 1 | 38 (34.9) | 33 (28.2) |
| Prostate cancer characteristics | ||
| Time between surgery and screening, median (range), weeks | 6.14 (5.1−24.0) | 6.29 (4.9−8.7) |
| Pelvic lymphadenectomy, n (%) | 109 (100) | 117 (100) |
| Extended | 30 (31.6) | 44 (43.6) |
| Not extended | 65 (68.4) | 57 (56.4) |
| Missing | 14 | 16 |
| Total Gleason score, n (%) | ||
| ≤ 6 | 11 (10.1) | 17 (14.5) |
| 7 (3 + 4) | 35 (32.1) | 29 (24.8) |
| 7 (4 + 3) | 16 (14.7) | 27 (23.1) |
| ≥ 8 | 47 (43.1) | 44 (37.6) |
| pTNM staging, n (%) | ||
| T1 | 0 | 1 (0.9)a |
| T2 | 49 (45.0) | 62 (53.0) |
| T3 | 59 (54.1) | 54 (46.2) |
| T4 | 1 (0.9) | 0 |
| NX | 0 | 1 (0.9) |
| N0 | 109 (100) | 116 (99.1) |
| N1 | 0 | 0 |
| MX | 0 | 1 (0.9) |
| M0 | 109 (100) | 116 (99.1) |
| M1 | 0 | 0 |
- a One patient in the surveillance group had a T1 tumor stage: he had a pre-RP PSA level of ≥ 20 ng/mL and a Gleason score < 8.
- Abbreviations: ECOG/WHO PS, Eastern Cooperative Oncology Group/World Health Organization Performance Status; ITT, intention-to-treat; pTNM, pathological tumor-node-metastasis; SD, standard deviation.
| Triptorelin (n = 109) | Surveillance (n = 117) | |
|---|---|---|
| Gleason score ≥ 8 on prostatectomy specimen, n (%) | 47 (43.1) | 44 (37.6) |
| Mean (95% CI) pre-RP PSA level, ng/ml | 22.6 (19.4−25.8) | 18.9 (16.6−21.2) |
| Pre-RP PSA level ≥ 20 ng/mL, n (%) | 57 (53.3) | 51 (44.7) |
| Missing, n | 2 | 3 |
| Primary tumor stage ≥ T3a, n (%) | 56 (51.4) | 51 (43.6) |
| Gleason score ≥ 8 on prostatectomy specimen and pre-RP PSA level ≥ 20 ng/mL, n (%) | 17 (15.7) | 8 (6.9) |
| Missing, n | 1 | 1 |
| Gleason score ≥ 8 on prostatectomy specimen and primary tumor stage ≥ T3a, n (%) | 18 (16.5) | 14 (12.0) |
| Pre-RP PSA level ≥ 20 ng/mL and primary tumor stage ≥ T3a, n (%) | 24 (22.4) | 10 (8.8) |
| Missing, n | 2 | 3 |
| Gleason score ≥ 8 on prostatectomy specimen, pre-RP PSA level ≥ 20 ng/mL and primary tumor stage ≥ T3a, n (%) | 7 (6.5) | 2 (1.7) |
| Missing, n | 1 | 1 |
- Abbreviations: CI, confidence interval; ITT, intention-to-treat; PSA, prostate-specific antigen; RP, radical prostatectomy.
3.2 Primary efficacy endpoint
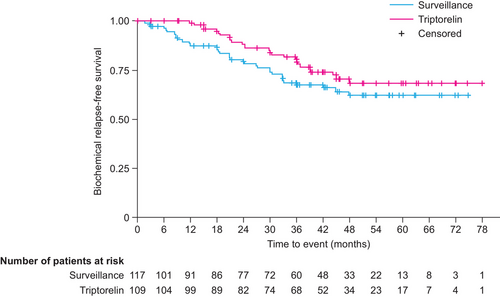
Sixty-one BR events were observed (triptorelin, n = 26; surveillance, n = 35) and the median time to BRFS was not reached. The Q1 time to BRFS (95% CI) was 39.1 (29.9–not estimated [NE]) months and 30.0 (18.6−42.1) months in the triptorelin and surveillance groups, respectively (Figure 2). There was no statistically significant difference between treatment groups in the Q1 time to BRFS (p = 0.16). We observed some evidence of a lower risk of BR in patients in the triptorelin group than in the surveillance group (HR 0.65, 95% CI 0.38−1.09; p = 0.10), although the difference was not statistically significant at the 5% level. Regardless of treatment assignment, we observed some evidence of a higher risk of BR in patients with a Gleason score of 7 (HR 2.35, 95% CI 0.87−6.39; p = 0.093) and ≥ 8 (HR 2.03, 95% CI 0.74−5.55; p = 0.17) than in those with a score of ≤ 6, although the differences were not statistically significant at the 5% level. No significant interaction between the treatment group and country (p = 0.97), center (p = 0.24), or Gleason score (p = 0.27) was observed. In a separate (post hoc) Cox model (Table S1), investigating whether the number of high-risk factors was a prognostic factor of BRFS adjusting for the treatment group, country, and age, there were no statistically significant differences in BR rate between patients with three (vs one) high-risk factors (HR 2.21, 95% CI 0.66−7.42; p = 0.20), or in those with two (vs one) high-risk factors (HR 0.86, 95% CI 0.47−1.56; p = 0.61). Similarly, patient age (by 1 unit) was not associated with increased risk of BR (HR, 0.99, 95% CJ 0.95−1.03; p = 0.51).
3.3 Secondary efficacy endpoints
No significant differences in EFS (triptorelin, three events; surveillance, two events; p = 0.64) or OS (triptorelin, two deaths; surveillance, one death; p = 0.56) were observed between the two groups. Owing to a low number of events during the study, median and Q1 time-to-event analyses could not be performed. No deaths due to prostate cancer were observed; therefore, disease-specific mortality was not estimated.
After six PSADT events in the triptorelin group and nine in the surveillance group, the Q1 times to PSADT (95% CI) were 9.1 (1.6–NE) months and 13.4 (6.0–NE) months, respectively (p = 0.53). At baseline, the median (Q1, Q3) PSA level was 0.074 (0.074−0.074) ng/mL in the triptorelin and surveillance groups, and levels did not vary during the course of the study. In the triptorelin group, 99.0%, 95.9%, and 93.9% of patients were castrated at months 3, 6, and 9, respectively. Among patients receiving triptorelin, the median (Q1–Q3) testosterone level was 462.0 (333.0−612.0) ng/dL at baseline (n = 106), 12.7 (12.7, 18.4) ng/dL at month 3 (n = 102), 12.7 (12.7−15.4) ng/dL at month 6 (n = 97), and 12.7 (12.7−19.0) ng/dL at month 9 (n = 99).
Changes in the FACT-P subscale and SF-36 scores from baseline to month 9 are shown in Table S2. No deterioration in HRQoL was observed in either cohort, hence triptorelin had no detrimental effect on patients’ wellbeing. Based on the repeated measures of ANCOVA, neither treatment nor time had a significant effect on any of the included HRQoL measurements, with the exception of the mental health domain scale of the SF-36, which favored triptorelin. Visit and the visit*treatment interaction had no significant effect on any of the HRQoL scores evaluated.
3.4 Safety
The safety population prior to month 12 included 217 patients (triptorelin, n = 105; surveillance, n = 112) (Table 3). Of the nine patients excluded from the safety population, three in the triptorelin group did not receive any injections and six in the surveillance group did not undergo at least 1 day of surveillance (Figure S1). One patient who was randomized to receive triptorelin was included in the surveillance group for safety analyses because he participated in the study but refused all injections. Up until month 12, 80 treatment-emergent AEs (TEAEs) were reported in 33.3% of patients who had received triptorelin, and 58 AEs were reported in 30.4% of those who had been under surveillance (Table 3). There were three deaths during the study (not due to prostate cancer), one in the surveillance group and two in the triptorelin group, which were unrelated to treatment and occurred > 2 years after the last injection.
| Before Month 12b | Month 12 onwards | ||
|---|---|---|---|
| Triptorelin (n = 105) | Surveillance (n = 112) | Total (N = 217) | |
| Any AE | 35 (33.3) | 34 (30.4) | 8 (3.7) |
| Any TEAE | 33 (31.4) | – | – |
| NCI-CTCAE grade of TEAEs or AEsa | |||
| Grade 1 | 27 (25.7) | 23 (20.5) | 5 (2.3) |
| Grade 2 | 7 (6.7) | 7 (6.3) | 4 (1.8) |
| Grade 3 | 1 (1.0) | 7 (6.3) | 1 (0.5) |
| Grade 4 | – | 2 (1.8) | – |
| Grade 5 | – | 1 (0.9) | – |
| Serious TEAEs or AEsa | 3 (2.9) | 11 (9.8) | 2 (0.9) |
| TEAEs or AEs leading to study withdrawala | 0 (0) | 1 (0.9) | 1 (0.5) |
| TEAEs or AEs leading to deatha | 0 (0) | 1 (0.9) | 0 (0) |
| TEAEs leading to study drug discontinuation | 2 (1.9) | – | – |
| Causality of TEAEs | |||
| Related | 14 (13.3) | – | – |
| Not related | 23 (21.9) | – | – |
| Causality and NCT-CTCAE grade of TEAEs | – | – | |
| Related and grade 1 | 13 (12.4) | – | – |
| Related and grade 2 | 2 (1.9) | – | – |
| Not related and grade 1 | 18 (17.1) | – | – |
| Not related and grade 2 | 5 (4.8) | – | – |
| Not related and grade 3 | 1 (1.0) | – | – |
| Causality of serious TEAEs | |||
| Related | 0 (0) | – | – |
| Not related | 3 (2.9) | – | – |
- Abbreviations: AE, adverse event; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; TEAE, treatment-emergent adverse event.
- a TEAEs reported for the triptorelin arm and AEs reported for the active surveillance arm.
- b AEs were collected until month 9, which corresponded to 3 months after the final triptorelin injection for those patients in the triptorelin treatment arm. After this visit, only AEs and serious AEs related to triptorelin according to the investigator's judgment were reported.
Twenty-six TEAEs related to triptorelin were reported in 14 patients, with the most frequent being hot flush (9.5% of patients) and hyperhidrosis (1.9%) (Table S3). Three grade 1 TEAEs (asthenia, peripheral edema, and hot flush) were reported in two patients and led to the discontinuation of triptorelin. Two patients experienced grade 2 triptorelin-related TEAEs: fatigue (n = 1) and decreased platelet count (n = 1). In patients receiving triptorelin, serious TEAEs were reported in three patients (alanine aminotransferase increased, papillary thyroid cancer, and urethral stenosis). No treatment-related serious TEAEs were reported.
In the surveillance group, grade 3 AEs were experienced by seven patients. Two patients experienced grade 4 AEs (incisional hernia, urothelial stenosis), and one experienced a grade 5 AE (sudden death). At least one serious AE was reported for 11 patients under surveillance (Table 3).
From month 12 onwards, 15 AEs were reported in eight patients (3.7%) (Table 3). Among patients who had received triptorelin, one experienced grade 2 breast hyperplasia, which was classified as a treatment-related non-serious AE, and the other experienced grade 2 gynecomastia, which was a treatment-related serious AE that led to the patient's withdrawal from the study.
4 DISCUSSION
The role of adjuvant ADT following RP in patients with high-risk prostate cancer is unclear owing to the paucity of prospective clinical trials in this setting.7 In men with high-risk node-positive disease, ADT after RP is a recommended strategy and has been shown to significantly improve OS, PFS, and prostate cancer-specific survival compared with surveillance after 11.9 years of follow-up.15, 24 Other studies, which included men with node-negative disease, have suggested that ADT after RP is associated with favorable OS and disease-free survival. However, limitations of these studies include the lack of a control arm and retrospective data collection.25, 26
The PRIORITI study evaluated the efficacy and safety of immediate ADT with triptorelin, administered every 3 months, in patients with high-risk prostate cancer but without evidence of residual disease after RP. Although the median time to BRFS was not reached, the Q1 time to BRFS was numerically longer in patients receiving triptorelin, and we observed evidence of a lower risk of BR in the triptorelin group than in the surveillance group (although the difference was not statistically significant). However, in line with findings from prior studies,27 triptorelin was active, with testosterone below castrate levels during the monitoring period and maintenance of chemical castration in 93.9% of patients at month 9. Regardless of the treatment group, we observed evidence of a higher risk of BR in patients with a Gleason score of 7 and ≥ 8 than in those with a score of ≤ 6 (although these differences were not statistically different). Survival could not be evaluated in this study owing to the low number of deaths, which might be related to improved surgical techniques in recent years. Safety findings in this study were in line with the previously reported safety profile of triptorelin in patients with high-risk prostate cancer, and similar to those reported for patients receiving ADT.19, 28-31 It is possible that the 3-month formulation of triptorelin may improve adherence with treatment while maintaining castration compared with treatments that are administered more frequently; however, further studies would be required to test this hypothesis.
In this study, BRFS was selected as the most appropriate primary endpoint to demonstrate a significant difference between triptorelin and surveillance during the 9-month treatment period after RP. However, the practical importance in the clinic of biochemical failure is unclear because PSA elevation might precede the onset of metastases by several years. Additionally, metastasis-free survival 3 years after PSA relapse has been reported to be 60%.32 Therefore, metastasis-free survival may have been a more clinically relevant endpoint than BRFS.
Strengths of this study include the randomized, prospective design, the large number of patients, and the inclusion of a comparator (surveillance) arm. Limitations include the open-label study design, the exploratory nature of the secondary efficacy analyses, and the choice of PSA cut-off value (> 0.2 ng/mL) for BR.33 In addition, most of the patients recruited had only one risk factor for disease progression, whereas in clinical practice patients may present with a number of risk factors.34 Furthermore, risk could be based on the tumor stage, which is a subjective parameter.35
5 CONCLUSIONS
In conclusion, in this evaluation of the efficacy and safety of triptorelin after RP in patients with negative lymph nodes, the PRIORITI study observed that BRFS was longer with triptorelin treatment than with surveillance alone, but the difference was not statistically significant. The question of the benefit of adjuvant therapy before or after radical therapy is of high importance in ensuring that patients with high-risk prostate cancer are not undertreated, and to decrease the risk of relapse after initial radical therapy. Further studies in patients with node-negative prostate cancer and more than one risk factor for disease progression may be warranted to identify those patients at the highest risk of disease progression, and, therefore, most likely to benefit from early ADT following RP.
AUTHOR CONTRIBUTIONS
Substantial contributions to study conception/design, or acquisition/analysis/interpretation of data: all authors; Drafting of the publication, or revising it critically for important intellectual content: all authors; Final approval of the publication: all authors.
ACKNOWLEDGMENTS
The authors thank Dr Emma Bolton and Dr Kirsty Walters of Oxford PharmaGenesis, Oxford, UK, for providing medical writing and editorial support, which was funded by Ipsen in accordance with Good Publication Practice (GPP22) guidelines. The authors also thank all patients involved in the study, as well as their caregivers, care team, investigators, and research staff in participating institutions. This study was sponsored by Ipsen. The sponsor was involved in the design of the study, analysis and interpretation of the data, and review of the manuscript.
CONFLICT OF INTEREST STATEMENT
Vsevolod Matveev has received honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, and Merck Sharp & Dohme, and reports advisory/consultancy roles for Bayer, Bristol Myers Squibb, Janssen, and Merck Sharp & Dohme. Patrick Cabri and Aude Houchard are employees of Ipsen. Adnan Mahmood was an employee of Ipsen at the time of writing. Xin Gao, Evgeny Kopyltsov, Jindan Luo, Qiang Wei, Dingwei Ye, Fangjian Zhou, and Li-Ping Xie declare no conflict of interest.
ETHICS STATEMENT
The study was conducted according to the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice and in compliance with applicable local regulations. The protocol and informed consent forms were approved by the independent ethics committees of all participating centers.
PATIENT CONSENT STATEMENT
All patients provided written informed consent before study entry.
Open Research
DATA AVAILABILITY STATEMENT
Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to [email protected] and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.




