Real-world outcomes for patients with pleural mesothelioma: A multisite retrospective cohort study
Abstract
Aim
To evaluate the real-world treatment patterns and outcomes for patients with pleural mesothelioma (PM) in the era of immunotherapy.
Methods
This retrospective audit included patients with PM diagnosed within three tertiary referral centers in Queensland, Australia from January 2017 to July 2023. Patient and treatment characteristics and outcomes were recorded. Data was analyzed using descriptive statistics and the Kaplan-Meier survival method.
Results
A total of 90 patients were included: 84% were male, the median age was 75 years (range 70–79) and 85% had baseline Eastern Group Cooperative Group of 0–1. Subtypes included 54% epithelioid, 17% biphasic, 12% sarcomatoid, and 17% unspecified/unknown. First-line treatment was received by 57/90 patients (63%) and 33/90 patients (37%) received the best supportive care (BSC). Chemotherapy was most used (63%) overall, but first-line immunotherapy was more commonly used since ipilimumab/nivolumab was reimbursed by the Australian Pharmaceutical Benefits Scheme in July 2021. After first-line treatment, only 40% received second-line treatment and 60% received BSC.
12-month overall survival (OS) and progression-free survival for all patients were 53% (95% confidence interval [CI]: 43–65) and 25% (95% CI 15–40) respectively. 12-month OS was 72%, 64%, and 29% for immunotherapy, chemotherapy, and BSC, respectively. There was no significant difference in survival between chemotherapy and immunotherapy (hazard ratio 1.28, 95% CI: 0.65–2.5, p = 0.5).
Conclusion
In our unselected real-world cohort, both chemotherapy and immunotherapy are active against PM, but the prognosis remains guarded. There remains a need for better treatment options, especially in the first-line setting. Enrolment in clinical trials is crucial to improving outcomes in this debilitating disease.
1 INTRODUCTION
Pleural mesothelioma (PM) is a rare but aggressive malignancy accounting for just 0.2% of all cancer diagnoses.1 The causal link between asbestos exposure and PM is well established. Australia is known to have one of the highest rates of asbestos exposure and PM incidence in the world.2 Despite introducing a ban on asbestos-containing materials from 2003 onwards, the incidence continues to rise due to the long latency period between exposure and development of PM, typically 30–50 years.3-5
Historically, PM is classified into three broad histologic subtypes namely: epithelioid (60%–80%); sarcomatoid (10%), and biphasic (10%–15%).6 The epithelioid subtype is associated with a better prognosis compared to non-epithelioid histology. In Australia, age-adjusted PM survival rates have shown gradual improvements, particularly 1-year survival outcomes.5 Nevertheless, global overall survival (OS) outcomes remain poor with 6-, 12-, and 60-month survival rates of 55%, 33%, and 5%, respectively.7
The role of treatment modalities including surgery8 and radiotherapy remains controversial and should only be offered to a small subgroup of selected patients following multidisciplinary team consensus.9 In surgically resectable PM patients, the MARS2 trial showed that surgery (pleurectomy decortication) plus chemotherapy had inferior OS (hazard ratio [HR] 1.28, 95% confidence interval [CI] 1.02–1.60, p = 0.032), higher rates of adverse events, and poorer quality of life outcomes compared to chemotherapy alone.10 Thus, palliative systemic therapy is the treatment of choice in most patients. Historically, this was treatment with platinum chemotherapy (cisplatin or carboplatin) and pemetrexed, with or without bevacizumab.11, 12 However efficacy is limited, particularly for non-epithelioid subtypes. The landmark CheckMate-743 trial established the role of immunotherapy in treating PM.13 This trial compared dual immunotherapy with ipilimumab and nivolumab to platinum chemotherapy in the first-line setting. The study showed superior OS for patients treated with immunotherapy, with a median OS of 18.1 versus 14.1 months (HR 0.73, 95% CI: 0.61–0.80) for ipilimumab/nivolumab and chemotherapy respectively.13 The largest benefit was seen for those with non-epithelioid histology, who historically did poorly with chemotherapy. Following the publication of the trial in February 2021, the drug was approved for first-line use on the Australian pharmaceutical benefits scheme (PBS) in July 2021.
More recently, three ongoing phase III clinical trials are investigating first-line chemoimmunotherapy against chemotherapy alone in PM. These include the DREAM3R (NCT04334759) investigating the benefit of adding durvalumab to platinum/pemetrexed; BEAT-MESO (NCT03762018) adding atezolizumab to platinum/pemetrexed/bevacizumab and IND.227 (NCT02784171) with the addition of pembrolizumab to chemotherapy. Preliminary results from the IND.227 trial were presented at the American Society of Clinical Oncology 2023 annual meeting which demonstrated improvements in OS, progression-free survival (PFS), and overall response rate with the addition of pembrolizumab. Similar to immunotherapy doublet, the benefit of adding immunotherapy was greatest in the non-epithelioid subtype. Patient selection and biomarkers for benefit from chemo-immunotherapy combination versus chemotherapy or immunotherapy alone remains unclear.
With the recent evolution in the treatment landscape of PM, we sought to evaluate real-world outcomes in patients with PM in an Australian context and understand the impact of immunotherapy treatment regimens on outcomes.
2 MATERIALS AND METHODS
We conducted a multicentre retrospective cohort study of patients with pleural mesothelioma (PM) diagnosed within the Sunshine Coast and Metro North Hospital and Health Services in Queensland, Australia (including three tertiary hospitals). Patients with a histologically confirmed diagnosis between July 2017 and June 2023 were eligible. Those with mesothelioma of non-pleural origin, without adequate follow-up, or enrolled in clinical trials were excluded.
Cases were identified from the statewide cancer repository and systemic therapy prescribing platforms. Data were extracted through a retrospective review of medical records by investigators. Baseline characteristics were recorded including demographics (age, sex, and Eastern Group Cooperative Group [ECOG] performance status) and clinical characteristics (histology and staging as per the American Joint Committee on Cancer 8th Edition). Treatment details were collected including surgery, radiotherapy, and systemic treatment (type and number of treatment cycles). Clinical outcomes were recorded including dates of progression and date of death.
The outcomes of interest in this study were OS, PFS, and time to progression (TTP) as defined below. Treatment toxicity data was not recorded as it was unable to be reliably extracted from a retrospective review of medical records.
This study was exempted by the Human Research Ethics Committee (Approval number: LNR/2020/QPCH/52968) and was conducted in accordance with the National Health and Medical Research Council guidelines.
2.1 Statistical analysis
OS was defined as the time from diagnosis to death from any cause. PFS was calculated for patients who received systemic therapy and was defined as the time from diagnosis to radiologically documented disease progression or death, whichever occurred first. Given the observational nature of the study and the possibility for PFS to be inflated by a lack of scheduled imaging, TTP was also calculated, with the exclusion of participants who did not have radiological surveillance of their disease. TTP was defined as the date of diagnosis to the date of radiological progression.
Descriptive statistics were used for baseline characteristics including median and interquartile range for continuous variables and count and proportions for categorical variables. Time-to-event outcomes were analyzed using the Kaplan-Meier method. Outcomes were compared for participants who received any immunotherapy versus chemotherapy alone using the Cox Proportional Hazards model. All data analysis was carried out using RStudio Statistical Software v2022.02.3.
3 RESULTS
3.1 Demographics
A total of 111 patients had a confirmed diagnosis of PM between July 2017 to June 2023, of which 21 patients were excluded due to enrolment in clinical trials. Ninety patients met the inclusion criteria and were included in our study analysis.
Patient demographics and clinical characteristics are summarised in Table 1. The majority of patients were male (84%), with a median age of 75 years (interquartile range 70–79). Most patients had an ECOG of 0–1 (85%) and 66% had a documented history of asbestos exposure. The most common histological subtype was epithelioid (54%), followed by biphasic (17%) and sarcomatoid (12%). The majority of patients had unresectable stage IIIB disease (87%).
| Values | ||||
|---|---|---|---|---|
| Characteristics | All patients (N = 90) | Chemotherapy (N = 36) | Immunotherapy (N = 21) | BSC (N = 33) |
| Median age (IQR)—Years | 75 (70–79) | 73 (69–77) | 73 (70–78) | 78 (71–83) |
| Sex—n (%) | ||||
| Male | 76 (84) | 32 (89) | 19 (91) | 25 (76) |
| Female | 14 (16) | 4 (11) | 2 (9) | 8 (24) |
| ECOG at diagnosis—n (%) | ||||
| 0–1 | 76 (85) | 32 (89) | 21 (100) | 23 (70) |
| ≥2 | 12 (13) | 3 (8) | 0 | 9 (27) |
| Unknown | 2 (2) | 1 (3) | 0 | 1 (3) |
| Asbestos exposure—n (%) | ||||
| Yes | 59 (66) | 22 (61) | 2 (10) | 22 (67) |
| No | 12 (13) | 4 (11) | 15 (71) | 6 (18) |
| Unknown | 19 (21) | 10 (28) | 4 (19) | 5 (15) |
| Smoking history—n (%) | ||||
| Never | 33 (37) | 10 (28) | 7 (33) | 16 (49) |
| Former | 41 (46) | 22 (61) | 9 (43) | 10 (30) |
| Current | 6 (7) | 2 (6) | 2 (10) | 5 (6) |
| Unknown | 10 (10) | 2 (5) | 3 (14) | 5 (15) |
| Histological subtype—n (%) | ||||
| Epithelioid | 49 (54) | 25 (69) | 9 (43) | 15 (46) |
| Biphasic | 15 (17) | 4 (11) | 7 (33) | 4 (12) |
| Sarcomatoid | 11 (12) | 2 (6) | 4 (19) | 5 (15) |
| Unspecified | 15 (17) | 5 (14) | 1 (5) | 9 (27) |
| Clinical staging at diagnosis (AJCC 8th Edition)—n (%) | ||||
| I | 2 (2) | 0 | 1 (5) | 1 (3) |
| II | 0 | 0 | 0 | 0 |
| IIIA | 0 | 0 | 0 | 0 |
| IIIB | 78 (87) | 32 (89) | 20 (95) | 26 (79) |
| IV | 8 (9) | 2 (6) | 0 | 6 (18) |
| Unable to be determined | 2 (2) | 2 (5) | 0 | 0 |
| Surgical management—n (%) | ||||
| VATS pleurodesis | 30 (33) | 9 (25) | 12 (57) | 9 (27) |
| Pleurectomy/Decortication | 1 (1) | 0 | 0 | 1 (3) |
| None | 59 (66) | 27 (75) | 9 (43) | 23 (70) |
| Upfront radiotherapy—n (%) | ||||
| Yes | 0 90 (100) | 0 | 0 | 0 |
| No | 36 (100) | 21 (100) | 33 (100) | |
- Abbreviations: AJCC, American Joint Committee on Cancer; ECOG, Eastern Oncology Cooperative Group; IQR, interquartile range.
Patients receiving BSC had a higher median age (78 years), greater percentage of ECOG ≥2 (27%), and stage IV disease (18%) compared to those who received chemotherapy and immunotherapy. Note that, 89% of patients who received chemotherapy had an ECOG of 0–1, compared to all of the patients who received immunotherapy. The immunotherapy group had a larger proportion of non-epithelioid subtypes (33% biphasic, 19% sarcomatoid, and 5% unspecified) as compared to those receiving chemotherapy (11% biphasic, 6% sarcomatoid, and 14% unspecified). Note that, 57% of patients receiving immunotherapy had VATS pleurodesis compared to 25% of those receiving chemotherapy.
3.2 Treatment patterns
Of the 90 patients, 57 (63%) patients received active treatment while 33 (37%) received best supportive care (BSC) alone. Chemotherapy with platinum/pemetrexed was given as first-line treatment in 36/57 (63%) patients. Platinum backbone was carboplatin in 61% or cisplatin in 39% of chemotherapy-treated patients respectively. First-line immunotherapy was received by 21/57 (37%) treated patients, with all receiving ipilimumab/nivolumab except for one who received pembrolizumab.
Table 2 summarises the first-line treatment choice by histological subtype and the first-line treatment choice before and after July 2021, when ipilimumab/nivolumab was reimbursed for first-line use on the PBS scheme. Chemotherapy was prescribed more commonly than immunotherapy for epithelioid and unspecified histology. In contrast, immunotherapy was more commonly given for non-epithelioid subtypes of sarcomatoid and biphasic histology. Prior to July 2021, chemotherapy was more commonly prescribed compared to immunotherapy, whereas immunotherapy was more commonly used after. This was shown across all histological subtypes, shown in Figure 1.
| Histology/Treatment received—N = 90 | Epithelioid (n = 49) | Biphasic (n = 15) | Sarcomatoid (n = 11) | Unspecified (n = 15) |
|---|---|---|---|---|
| Platinum/pemetrexed—n (%) | 25 (51) | 4 (27) | 2 (18) | 5 (33) |
| Immunotherapyb—n (%) | 9 (18) | 7 (47) | 4 (36) | 1 (7) |
| BSC—n (%) | 15 (31) | 4 (26) | 5 (46) | 9 (60) |
| Treatment received—N = 57 | Before July 2021 | After July 2021 |
|---|---|---|
| Platinum/pemetrexed—n | 33 | 3 |
| Immunotherapyb—n | 5 | 16 |
- Note: BSC = best supportive care.
- a First-line treatment between 2017 and 2023.
- b Ipilimumab/Nivolumab immunotherapy reimbursed for first-line use on the Australian Pharmaceutical Benefits (PBS) scheme from July 2021 onward.
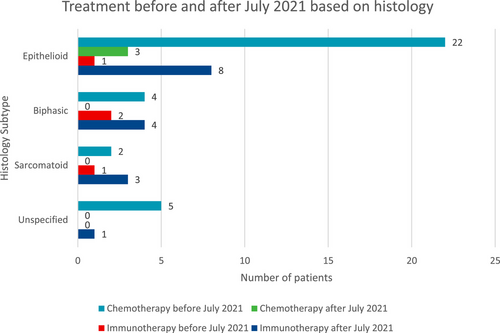
Figure 2 summarizes the lines of treatment received by all included patients. Of the 57 treated patients with a documented progression event, 23 (40%) received second-line treatment while 34 (60%) received no further systemic therapy. Second-line treatments included chemotherapy in 14 patients (61%) (platinum/pemetrexed in 12 and vinorelbine in two patients) and immunotherapy in nine patients (predominantly single agent PD1-inhibitor).
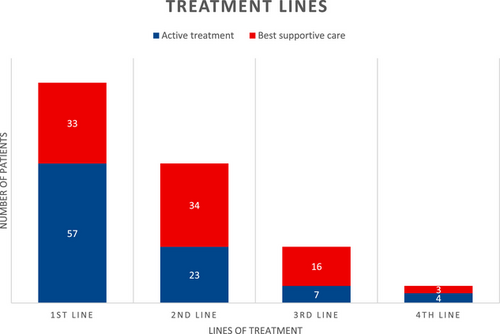
Of those who received second-line treatment, 7/23 (30%) received third-line treatment. Only four patients received fourth-line treatment.
3.3 PFS and TTP
Median follow-up was 55.8 months in the chemotherapy arm and 25.7 months in the immunotherapy arm. PFS was analyzed in 56 patients who received any first-line treatment, with one patient excluded due to incomplete data as the patient was treated outside of the health service. The median PFS for all patients was 8.3 months (95% CI: 7.1–10.2). Landmark PFS at 12 and 24 months were 25% (95% CI: 15–40) and 4% (95% CI: 1–16) respectively.
There was no statistically significant difference in PFS between the chemotherapy and immunotherapy groups, with a median of 8.4 months (95% CI: 6.7–10.6) with chemotherapy compared to 7.8 months (95% CI 6.2–NR) with immunotherapy (HR 1.31, 95% CI: 0.69–2.40, p = 0.58) (Figure 3).
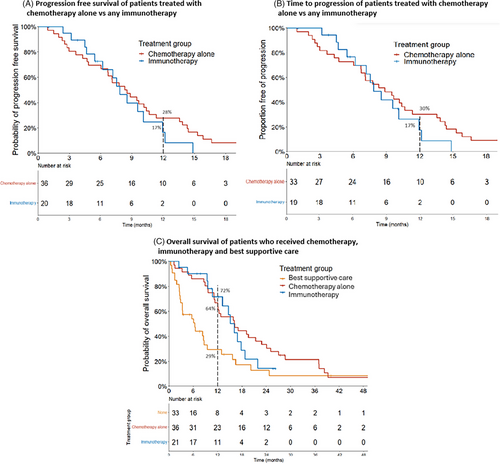
The estimated 12-month PFS for chemotherapy and immunotherapy groups were 28% (95% CI: 16–47) and 17% (95% CI: 5–55) respectively. (Figure 3A)
An additional four patients were excluded from the final TTP analysis due to not having subsequent imaging (N = 52). Median TTP was 8.8 months (95% CI: 6.7–13.6) with first-line chemotherapy compared to 7.8 months (95% CI: 7.2–NR) for immunotherapy. Estimated 12-month TTP for chemotherapy and immunotherapy were 30% (95% CI: 18–51) and 17% (95% CI: 5–59) respectively. (Figure 3B) There was no statistical difference between the two groups (HR 1.32, 95% CI: 0.68–2.55, p = 0.66).
3.4 Overall survival
Median OS for all patients was 12.8 months (95% CI: 9.6–16.2). Landmark OS at 12 and 24 months were 53% (95% CI: 43–65) and 23% (95% CI: 15%–34%) respectively. (Figure 3C)
Survival by treatment type is shown in Figure 3C. Without treatment, the median OS was 6.3 months (95% CI: 3.3–13), with an estimated 12-month OS of 29% (95% CI: 18–52). Compared to no treatment, patients who received chemotherapy had a longer OS, with a median of 16.2 months (95% CI: 12–24.1 months) (HR 0.48, 95% CI: 0.29–0.82, p < 0.01) and estimated 12-month OS of 64% (95% CI: 50%–82%). Those treated with immunotherapy had a median OS of 16.1 months (95% CI: 13.2–NR) and an estimated 12-month OS of 72%. Immunotherapy compared to no treatment did not show a statistically significant improvement in OS (HR 0.52, 95% CI: 0.26–1.01, p = 0.05). There was no difference in survival between the chemotherapy alone and immunotherapy groups in this cohort (HR 1.28, 95% CI: 0.65–2.5, p = 0.5).
Patients with non-epithelioid histology had a shorter median OS of 9.5 months (95% CI: 4.6–16.4) compared to epithelioid subtype, median 14.7 months (95% CI: 11.3–21.3) (HR 1.6, 95% CI: 1.0–2.6, p = 0.04). Twelve-month OS for non-epithelioid and epithelioid PM were 44% (95% CI: 30–63) and 61% (95% CI: 48–77), respectively.
4 DISCUSSION
In this retrospective study, we highlight the real-world clinical outcomes and treatment patterns of PM patients across 3 large tertiary referral centers in Queensland, Australia. This is the first study to examine patterns of care for PM in Queensland, Australia. Whilst previous studies within Australia predominantly focused on the outcomes and biomarkers for immunotherapy,14, 15 our study is the first to report outcomes of all treatments including chemotherapy, immunotherapy, and BSC within an unselected real-world cohort.
There was a lower representation of patients with epithelioid (54%) and higher non-epithelioid subtypes (29%) in our cohort compared to published historical epidemiological data and other recent real-world studies (epithelioid ranging from 66% to 75%).2, 16-18 A significant proportion of our patients (37%) did not receive any active treatment or BSC alone, which was higher compared to several recent real-world PM studies ranging between 21% and 31%. This may be attributed to the other studies having a younger median age between 68 and 70.16-18 In our study, among those who received BSC alone, 27/33 patients were over 70 years old.
At our institutions, we enroll patients into any available clinical trials as the first-line option. Outside of clinical trials, the standard of care was rapidly evolving as new clinical evidence emerged. During the study period, we used either first-line chemotherapy or ipilimumab/nivolumab for epithelioid PM, considering patient factors such as comorbidities, anticipated tolerance to chemotherapy, social circumstances, and autoimmune conditions. For non-epithelioid PM, chemotherapy was used initially due to the lack of other options but once immunotherapy was funded, most investigators offered that regimen if patients were suitable.
Overall, first-line treatment was largely consistent with international guideline recommendations.19 Carboplatin was used more commonly (61%) in combination with pemetrexed in our patient cohort compared to other countries,16-18 likely also reflecting the older population in our study. Prescribing patterns in Australia reflect the availability of treatment options at that time as immunotherapy was funded in Australia from July 2021. Since then, first-line ipilimumab/nivolumab has been more commonly used in all subtypes compared to chemotherapy, reflecting the published data from Checkmate 743.20
We demonstrated that both chemotherapy and immunotherapy are active treatment options with similar outcomes to published data in our study. Nevertheless, the median OS for the immunotherapy arm in our study (16.1 months) was shorter compared to Checkmate 743 (18.1 months).20 The relatively small sample size in our study did not allow for comparisons between treatment groups for epithelioid and non-epithelioid histology separately. Our study had an older population (median age of 75 years compared to 69 years for Checkmate 743) and a larger proportion of non-epithelioid subtypes, which is known to carry a more guarded prognosis.21 With chemotherapy being the most used first-line treatment option, immunotherapy has only been available since July 2021 and a relatively short median follow-up time of 25.7 months, the benefit of immunotherapy may not have been seen in our study. A similar finding was seen in the RIOMeso Australian retrospective study, which demonstrated no significant difference in median OS between epithelioid and non-epithelioid histologies in patients who received ipilimumab and nivolumab.14
Only one patient within the BSC arm received pleurectomy/decortication, indicating that most PM present with unresectable disease. With the results of the MARS2 trial showing no significant benefits from the addition of surgery to chemotherapy,10 the future role and utility of surgery will likely diminish in Queensland. On the other hand, there is limited good-quality evidence for the OS benefit of pleurodesis. One retrospective analysis of 49 clinical trials compared survival rates between those receiving talc pleurodesis versus surgery (extrapleural pneumonectomy and radical pleurectomy decortication) reported a median OS of 14 months for talc pleurodesis and 17–24 months for surgery. However, the studies had significant heterogeneity and the number of studies with talc pleurodesis was low.22 In our study, the higher percentage of patients who received talc pleurodesis in the immunotherapy compared to the chemotherapy arm may not have impacted outcomes.
There is no clear standard of care for second or subsequent-line treatments in PM. In our population, chemotherapy was the most used treatment in the second-line setting. One meta-analysis of 49 trials consisting of various second-line treatments including immunotherapy, chemotherapy (gemcitabine, vinorelbine, platinum/pemetrexed) and targeted therapies showed a median pooled PFS of 3.4 months (95% CI 2.87–3.93) and OS of 7.9 months (95% CI 7.01–8.72). There was a trend towards improved OS for immunotherapy compared to targeted and chemotherapy.23 The CONFIRM trial randomized patients who progressed on first- or second-line platinum-based chemotherapy to nivolumab or placebo, whereby nivolumab demonstrated a statistically significant improvement in PFS (median 3.0 months vs. 1.8 months, HR 0.67, 95% CI 0.53–0.85, p = 0.0012) and OS (median 10.2 vs. 6.9 months, HR 0.69, 95% CI 0.52–0.91, p = 0.009) compared to placebo.24 However, the PROMISE-meso trial failed to show a benefit of pembrolizumab compared to institutional choice single-agent chemotherapy (gemcitabine and vinorelbine) with similar PFS (HR 1.06, 95% CI 0.73–1.53, p = 0.76) and OS (HR 1.12, 95% CI 0.74–1.69, p = 0.59) despite adjusting for 63% cross-over from the chemotherapy arm.25 With the low number of patients receiving subsequent treatments in our study, we did not perform a time-to-event analysis of these later-line treatments. There is a substantial drop off from first to second-line treatments, highlighting the need to optimize first-line treatment as many patients in a real-world setting receive no subsequent therapy.
There were several limitations in our study. This was a retrospective analysis with a relatively small sample size. There were patients who died without subsequent imaging which may have led to an overestimation of PFS. However, with the exclusion of these patients, the calculation of TTP yielded similar survival estimates, suggesting the lack of radiographic surveillance in some patients did not have a large influence on outcome estimates. Given the retrospective nature of the study, toxicity data was unable to be reliably extracted as there were missing or conflicting data surrounding the documentation of these outcomes. This was also limited by inter-clinician variability of toxicity grading, and it was difficult to determine causation between the toxicities and treatment received. Programmed-death ligand 1 (PD-L1) expression was not routinely tested within biopsy samples, therefore the association between PD-L1 expression and the efficacy of immunotherapy is unknown in our study.
Both chemotherapy and immunotherapy have a role to play in the treatment paradigm of PM. Based on the findings of this study, we recommend treatment be individualized based on patient factors. Both chemotherapy and immunotherapy have comparable median OS in this cohort. Chemotherapy or immunotherapy should be considered for patients with epithelioid histology but this space is rapidly evolving and we await the result of the recently closed DREAM3R study which should answer this question definitively. The choice of first-line treatment should be the one that will likely be best tolerated, given that many patients will not be suitable for later-line treatments. As we were unable to compare the outcomes between epithelioid and non-epithelioid subtypes, we would recommend immunotherapy as a first-line treatment based on published literature.
5 CONCLUSION
This study showed that the overall prognosis of patients with PM remains poor in the real world. Our study demonstrated that both chemotherapy and immunotherapy are active treatments for PM, but the considerable drop-off between first- and second-line treatments showed that better treatments are needed especially in the first-line setting. With the rapidly changing landscape of PM treatment with ongoing phase 3 trials of combination chemoimmunotherapy, further real-world studies along with identifying prognostic and predictive biomarkers will be crucial for patient selection. We encourage participation and enrolment in clinical trials to improve the treatment paradigm for this debilitating disease.
CONFLICT OF INTEREST STATEMENT
Associate Professor Brett Hughes holds a consulting or Advisory Role for Merck and Co., Bristol-Myers Squibb, Roche, Pfizer, Astra Zeneca, Eisai, and Sanofi/Regeneron, and received grant funding (institutional) from Amgen. Associate Professor Zarnie Lwin holds an honoraria or advisory role for Bristol-Myers Squib, Astra Zeneca, Roche, Pfizer, Takeda, Merck and Co., and Johnson and Johnson. Associate Professor Bryan Chan holds an honoraria or advisory role for Astra Zeneca, Roche, and Merck and Co. The other authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




