Patterns of curative treatment for non-small cell lung cancer in New South Wales, Australia
Abstract
Introduction
There is a lack of large population-based studies examining patterns of curative treatment for non-small cell lung cancer (NSCLC) in Australia. This study aimed to evaluate the utilization of curative treatment for NCSLC at a population level and identify factors associated with its use in New South Wales (NSW), Australia.
Methods
Patients diagnosed with localized or locoregional NSCLC between 2009 and 2014 were identified from the NSW Central Cancer Registry. Curative treatment was defined as surgery or radiotherapy with a 45 Gy minimum dose. Univariate and multivariable analyses were performed to investigate factors associated with the receipt of curative treatment. A Cox proportional-hazards regression model was used to analyze the factors associated with 2-year overall survival (OS).
Results
Of the 5722 patients diagnosed with NSCLC in the study period, 3355 (59%) patients received curative treatment and 2367 (41%) patients did not receive curative treatment. The receipt of curative treatment was significantly associated with younger patients, female gender, localized disease, and Charlson Comorbidity Index (CCI) = 0. The use of curative treatment increased significantly over time from 2009 (55%) to 2014 (63%) and varied significantly from 24% to 70% between local health districts (LHDs) of residence. Younger age, female gender, localized disease, CCI = 0, and overseas country of birth were significantly associated with 2-year OS. The 2-year OS significantly improved from 70% in 2009 to 77% in 2014 for patients who received curative treatment.
Conclusion
The use of curative treatment for patients with potentially curable NSCLC was low at 59%. However, the use of curative treatment and survival have increased over time. Significant variation was noted in the use of curative treatment between LHDs.
1 INTRODUCTION
Lung cancer is the fifth most commonly diagnosed cancer in Australia and accounts for an estimated 18% of all cancer deaths, making it the most common cause of cancer deaths in Australia.1 The 5-year relative survival rate from lung cancer in Australia (2012–2016) was only 19%.1 Survival from lung cancer may be improved if more patients potentially curable by stage are treated with curative therapy.
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for around 85% of cases.1 For stages I and II NSCLC, the standard of care is surgery; however, many patients are also referred for radiotherapy (RT) if they are medically inoperable or do not want surgery. Surgery has a more limited role in stage III NSCLC, with the majority of patients being treated with RT with or without chemotherapy. The Australian lung cancer guidelines2 and other international guidelines3-5 recommend surgery or curative RT with or without chemotherapy and immunotherapy for suitable patients.
Poor survival in NSCLC may be partially due to the low use of curative treatment. The use of curative treatment in stages I–II NSCLC varies from 56% to 71%6-8 and is even lower in stage III NSCLC, ranging from 10% to 62%.6, 8-11 Previous Australian studies with smaller study cohorts have reported underutilization of curative treatment, with a large proportion of patients receiving either palliative or no treatment.12-17 This study aimed to evaluate the use of curative treatment for localized and locoregional NCSLC at a population level and identify factors associated with the use of curative treatment in New South Wales (NSW), Australia.
2 MATERIALS AND METHODS
2.1 Study population
The cohort comprised all patients diagnosed with localized and locoregional NSCLC in NSW between 2009 and 2014. NSW has the largest population (32%) of any state in Australia. Cases were identified from a linked dataset comprising diagnosis data recorded in NSW Central Cancer Registry, the NSW Cancer Institute Electronic RT Oncology Data (extract of RT data from each NSW public and private radiation oncology facility), Admitted Patient Data Collection (APDC), and Registry of Births, Deaths, and Marriages (RBDM). Probabilistic data linkage was performed by the Centre for Health Record Linkage (CHeReL). Due to the lack of data completeness in the staging data, degree of spread (DOS) was used as a surrogate for stage (DOS 1: localized to tissue of origin; DOS 2: regional spread, adjacent organs and/or regional lymph nodes). DOS 1 and 2 correspond to stages I–III NSCLC.18 The study was approved by the NSW population and health services research ethics committee.
2.2 Primary outcomes and covariables
The primary outcome was to identify the use of curative treatment in NSCLC. For this study, curative treatment was defined as surgery and/or RT (minimum dose 45 Gy). A minimum dose of 45 Gy was chosen because this is the recommended minimum RT dose for neoadjuvant chemoradiotherapy for superior sulcus tumors.19 This also allows inclusion of the minimum stereotactic ablative dose of 48 Gy in four fractions in use during the latter part of the study period. Curative surgery was defined as partial resection of the lung (endoscopic wedge resection, wedge resection, radical wedge resection, or segmental resection), lobectomy of the lung and pneumonectomy. Factors potentially associated with curative treatment were evaluated, including age, sex, socioeconomic status (SES), geographic remoteness of area of residence, distance to the closest RT center, Charlson Comorbidity Index (CCI), country of birth, year of treatment, and residence local health district (LHD). The CCI was calculated by including non-cancer chronic conditions identified from International Classification of Diseases 10th edition diagnoses codes20 recorded in the APDC up to 12 months before the cancer diagnosis and 6 months after diagnosis. Survival outcome was defined as 2-year overall survival (OS) from the date of diagnosis. Univariate analyses were performed using chi-square tests to examine factors associated with curative treatment. For multivariable analyses, multivariable generalized estimating equations with a binomial distribution were used. A multivariable Cox proportional-hazards regression model was used to analyze the factors associated with survival.
3 RESULTS
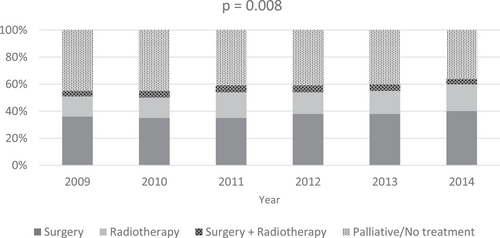
A total of 5722 patients were available for analysis; 3355 (59%) patients received curative treatment and 2367 (41%) patients received palliative or no treatment (Table 1). Of the patients who received curative treatment, 46% were DOS 1 and 54% were DOS 2. Table 2 shows factors associated with curative treatment in NSCLC. In univariate analyses, age 70 years and older, DOS 2, lower SES, regional/remote area of residence, distance to center ≥50 km, and CCI score ≥1 were associated with reduced use of curative treatment, while female gender, overseas country of birth, and diagnosis 2013 onward were significantly associated with increased use of curative treatment. In multivariable analyses, age 70+ years, DOS 2, multiple comorbidities (CCI ≥1), male gender, and diagnosis before 2011 onward were associated with lower rates of curative treatment. SES, distance to center, and country of birth were no longer significantly associated with the use of curative treatment on multivariable analysis. Variation in the use of curative treatment was also seen by LHD, with seven of 14 LHDs showing significantly lower use of curative treatment in both univariate and multivariable analyses. The use of curative treatment between LHDs of residence ranged from 24% to 70% (Figure 1). The use of surgery alone ranged from 8% to 55% by LHDs of residence, while the use of RT alone ranged from 9% to 23% (p < .0001). The use of curative treatment increased over time from 2009 (55%) to 2014 (64%) (p < .0001). The time trend shows an increase in the use of surgery (from 36% in 2009 to 40% in 2014) and RT (from 15% in 2009 to 20% in 2014) over time.
| Curative (n = 3355, 59%) | Palliative/no treatment (n = 2367, 41%) | |||||
|---|---|---|---|---|---|---|
| DOS 1 (n = 1562, 46%) | DOS 2 (n = 1793, 54%) | Total | DOS 1 (n = 875, 37%) | DOS 2 (n = 1492, 63%) | Total | |
| Age (year) | ||||||
| <50 | 47 (25%) | 83 (43%) | 130 (68%) | 11 (6%) | 50 (26%) | 61 (32%) |
| 50–59 | 188 (27%) | 293 (42%) | 481 (69%) | 53 (8%) | 156 (23%) | 209 (31%) |
| 60–69 | 538 (30%) | 676 (37%) | 1214 (67%) | 194 (11%) | 404 (22%) | 598 (33%) |
| 70–79 | 580 (29%) | 598 (29%) | 1178 (58%) | 329 (16%) | 516 (26%) | 845 (42%) |
| >80 | 209 (21%) | 143 (14%) | 352 (35%) | 288 (29%) | 366 (36%) | 654 (65%) |
| Sexa | ||||||
| Male | 813 (25%) | 1014 (31%) | 1827 (56%) | 532 (16%) | 904 (28%) | 1436 (44%) |
| Female | 741 (31%) | 755 (31%) | 1496 (62%) | 343 (14%) | 588 (24%) | 931 (38%) |
| Socioeconomic status | ||||||
| Most disadvantaged | 392 (27%) | 449 (31%) | 841 (58%) | 198 (14%) | 406 (28%) | 604 (42%) |
| Second quintile | 289 (24%) | 348 (28%) | 637 (52%) | 248 (20%) | 351 (28%) | 599 (48%) |
| Third quintile | 361 (29%) | 397 (32%) | 758 (61%) | 180 (15%) | 299 (24%) | 479 (39%) |
| Fourth quintile | 257 (27%) | 325 (34%) | 582 (61%) | 137 (14%) | 246 (25%) | 383 (39%) |
| Least disadvantaged | 263 (31%) | 274 (33%) | 537 (64%) | 112 (13%) | 190 (23%) | 302 (36%) |
| Remoteness of residency | ||||||
| Major city | 1003 (29%) | 1154 (33%) | 2157 (62%) | 449 (13%) | 851 (25%) | 1300 (38%) |
| Inner regional | 361 (27%) | 396 (30%) | 757 (57%) | 217 (16%) | 358 (27%) | 575 (43%) |
| Outer regional/remote/very remote | 198 (21%) | 243 (26%) | 441 (47%) | 209 (22%) | 283 (31%) | 492 (53%) |
| Distance to centera (km) | ||||||
| <50 | 1287 (28%) | 1478 (33%) | 2765 (61%) | 623 (14%) | 1155 (25%) | 1778 (39%) |
| 50–99 | 114 (26%) | 128 (29%) | 242 (55%) | 75 (17%) | 121 (28%) | 196 (45%) |
| 100–149 | 65 (22%) | 84 (28%) | 149 (50%) | 64 (21%) | 86 (29%) | 150 (50%) |
| >150 | 70 (20%) | 74 (21%) | 144 (41%) | 97 (27%) | 112 (32%) | 209 (59%) |
| Charlson Comorbidity Index | ||||||
| 0 | 952 (28%) | 1152 (34%) | 2104 (62%) | 411 (12%) | 869 (26%) | 1280 (38%) |
| 1 | 431 (29%) | 417 (28%) | 848 (57%) | 269 (18%) | 383 (25%) | 652 (43%) |
| ≥2 | 179 (21%) | 224 (27%) | 403 (48%) | 195 (23%) | 240 (29%) | 435 (52%) |
| County of birth | ||||||
| Australia | 926 (26%) | 1079 (31%) | 2005 (57%) | 577 (16%) | 852 (27%) | 1529 (43%) |
| Overseas | 658 (29%) | 746 (33%) | 1404 (62%) | 306 (13%) | 556 (25%) | 862 (38%) |
| Year | ||||||
| 2009 | 224 (25%) | 270 (30%) | 494 (55%) | 168 (19%) | 243 (26%) | 411 (45%) |
| 2010 | 253 (27%) | 265 (28%) | 518 (55%) | 142 (15%) | 274 (30%) | 416 (45%) |
| 2011 | 239 (26%) | 296 (33%) | 535 (59%) | 113 (13%) | 254 (28%) | 367 (41%) |
| 2012 | 266 (27%) | 310 (32%) | 576 (59%) | 137 (14%) | 278 (27%) | 415 (41%) |
| 2013 | 288 (28%) | 330 (32%) | 618 (60%) | 165 (16%) | 245 (24%) | 410 (40%) |
| 2014 | 292 (30%) | 322 (33%) | 614 (63%) | 150 (16%) | 198 (21%) | 348 (37%) |
- Abbreviations: DOS, degree of spread; DOS 1, localized to tissue of origin; DOS 2, regional spread, adjacent organs, and/or regional lymph nodes.
- a Missing data for sex and distance to center for some patients.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI for OR | p-Value | OR | 95% CI for OR | p-Value | |
| Age (year) | <.0001 | <.0001 | ||||
| <50 | Reference | Reference | Reference | Reference | ||
| 50–59 | 1.10 | .77–1.55 | .6 | 1.13 | .78–1.63 | .5 |
| 60–69 | .96 | .70–1.32 | .8 | .98 | .69–1.38 | .9 |
| 70–79 | .64 | .47–.88 | .007 | .63 | .45–.89 | .008 |
| >80 | .26 | .18–.36 | <.0001 | .23 | .16–.32 | <.0001 |
| Sex | <.0001 | .01 | ||||
| Male | Reference | Reference | Reference | Reference | ||
| Female | 1.30 | 1.17–1.45 | <.0001 | 1.18 | 1.05–1.33 | .01 |
| Degree of spread | <.0001 | <.0001 | ||||
| DOS 1 | Reference | Reference | Reference | Reference | ||
| DOS 2 | .66 | .60–.74 | <.0001 | .51 | .45–.58 | <.0001 |
| Socioeconomic status | <.0001 | .1 | ||||
| Most disadvantaged | Reference | Reference | Reference | Reference | ||
| Second quintile | .73 | .62–.85 | <.0001 | .91 | .76–1.09 | .3 |
| Third quintile | 1.10 | .94–1.29 | .2 | 1.06 | .88–1.28 | .5 |
| Fourth quintile | 1.04 | .88–1.23 | .6 | .92 | .75–1.13 | .4 |
| Least disadvantaged | 1.23 | 1.04–1.47 | .02 | 1.21 | .93–1.58 | .2 |
| Remoteness of residency | <.0001 | .1 | ||||
| Major city | Reference | Reference | Reference | Reference | ||
| Inner regional | .78 | .68–.89 | <.0001 | 1.12 | .93–1.35 | .2 |
| Outer regional/remote/very remote | .52 | .45–.60 | <.0001 | .84 | .58–1.23 | .4 |
| Distance to center (km) | <.0001 | .06 | ||||
| <50 | Reference | Reference | Reference | Reference | ||
| 50–99 | .77 | .63–.94 | .01 | 1.17 | .86–1.58 | .3 |
| 100–149 | .62 | .49–.78 | <.0001 | 1.01 | .68–1.50 | .9 |
| >150 | .4 | .32–.51 | <.0001 | .70 | .46–1.06 | .1 |
| Charlson Comorbidity Index | <.0001 | <.0001 | ||||
| 0 | Reference | Reference | Reference | Reference | ||
| 1 | .81 | .72–.92 | .001 | .81 | .71–.93 | .003 |
| ≥2 | .57 | .49–.67 | <.0001 | .63 | .53–.75 | <.0001 |
| Country of birth | <.0001 | .1 | ||||
| Australia | Reference | Reference | Reference | Reference | ||
| Overseas | 1.25 | 1.12–1.39 | <.0001 | 1.11 | .98–1.25 | .1 |
| Year of diagnosis | .0001 | <.0001 | ||||
| 2009 | Reference | Reference | Reference | Reference | ||
| 2010 | 1.03 | .85–1.23 | .79 | 1.07 | .88–1.31 | .5 |
| 2011 | 1.20 | .99–1.44 | .06 | 1.29 | 1.05–1.57 | .02 |
| 2012 | 1.18 | .98–1.41 | .08 | 1.27 | 1.04–1.55 | .02 |
| 2013 | 1.26 | 1.05–1.51 | .01 | 1.27 | 1.04–1.54 | .02 |
| 2014 | 1.52 | 1.26–1.83 | <.0001 | 1.65 | 1.34–2.02 | <.0001 |
| Residence local health district | <.0001 | <.0001 | ||||
| A | Reference | Reference | Reference | Reference | ||
| B | .99 | .72–1.36 | .96 | 1.06 | .76–1.49 | .7 |
| C | .94 | .67–1.32 | .7 | 1.05 | .73–1.51 | .8 |
| D | .87 | .63–1.21 | .4 | .90 | .64–1.28 | .6 |
| E | .84 | .61–1.15 | .3 | .91 | .65–1.29 | .6 |
| F | .70 | .48–1.02 | .06 | .83 | .53–1.30 | .4 |
| G | .69 | .47–1.00 | .05 | .84 | .53–1.33 | .5 |
| H | .63 | .45–.89 | .008 | .68 | .47–.98 | .04 |
| I | .55 | .40–.75 | .0002 | .54 | .37–.78 | .001 |
| J | .47 | .34–.66 | <.0001 | .49 | .34–.70 | .001 |
| K | .50 | .36–.68 | <.0001 | .58 | .42–.82 | .002 |
| L | .44 | .30–.64 | <.0001 | .50 | .32–.79 | .003 |
| M | .07 | .04–.13 | <.0001 | .07 | .04–.13 | <.0001 |
| N | .14 | .10–.21 | <.0001 | .11 | .07–.18 | <.0001 |
- Abbreviations: CI, confidence interval; DOS, degree of spread; OR, odds ratio.
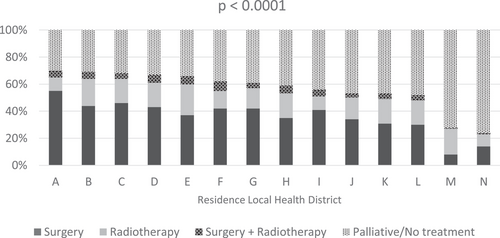
Curative treatment was used in only 27% of DOS 1 NSCLC (21% surgery, 5% RT alone, 1% surgery and RT) and 31% of DOS 2 NSCLC (16% surgery, 11% RT, 4% surgery and RT). Of the patients who received RT, 93% received conventional fractionation and 7% received stereotactic ablative body RT (SABR). Figure 2 shows the breakdown of treatment type by LHDs of residence and year of treatment.
For patients who received curative treatment, significant variation in 2-year OS was apparent in relation to age (higher 2-year OS in patients aged <50 years, p < .0001), sex (higher 2-year OS in female, p < .0001), DOS (higher 2-year OS in DOS 1, p < .0001), residence LHD (ranging from 64% to 82%, p < .0001), CCI (higher 2-year OS in patients with CCI = 0, p < .0001), country of birth (higher 2-year OS in patients born overseas, p < .0001), and year of treatment (higher 2-year OS from 2012 onward, p = .02) (Table 3). For patients who did not receive curative treatment, significant variation in 2-year OS was apparent in relation to age (higher 2-year OS in patients aged <50 years, p < .0001), sex (higher 2-year OS in female, p = .0002), DOS (higher 2-year OS in DOS 1, p < .0001), residence LHD (ranging from 18% to 61%, p = .001), remoteness of residency (higher 2-year OS in regional/remote area, p = .0005), CCI (higher 2-year OS in patients with CCI = 0, p < .0001), country of birth (higher 2-year OS in patients born overseas, p < .0001), and year of treatment (higher 2-year OS from 2013 onward, p = .009). Similar findings were noted in multivariable analyses (Table 4).
| Curative | Palliative/no treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Alive at 2 years | Alive at 2 years | |||||||
| Characteristics | n | n | % | p-Value | n | n | % | p-Value |
| Age (year) | <.0001 | <.0001 | ||||||
| <50 | 130 | 106 | 82 | 61 | 27 | 44 | ||
| 50–59 | 481 | 374 | 78 | 209 | 75 | 36 | ||
| 60–69 | 1214 | 940 | 77 | 598 | 204 | 34 | ||
| 70–79 | 1178 | 829 | 70 | 845 | 243 | 29 | ||
| ≥80 | 352 | 216 | 61 | 654 | 133 | 20 | ||
| Sexa | <.0001 | .0002 | ||||||
| Male | 1827 | 1232 | 67 | 1436 | 373 | 26 | ||
| Female | 1496 | 1210 | 81 | 931 | 309 | 33 | ||
| Degree of spread | <.0001 | <.0001 | ||||||
| DOS 1 | 1562 | 1315 | 84 | 875 | 300 | 34 | ||
| DOS 2 | 1793 | 1150 | 64 | 1492 | 382 | 26 | ||
| Residence local health district | <.0001 | .001 | ||||||
| A | 176 | 118 | 67 | 73 | 13 | 18 | ||
| B | 468 | 347 | 74 | 212 | 53 | 25 | ||
| C | 287 | 221 | 77 | 134 | 32 | 24 | ||
| D | 362 | 289 | 80 | 179 | 44 | 25 | ||
| E | 393 | 279 | 71 | 205 | 60 | 30 | ||
| F | 148 | 105 | 71 | 90 | 20 | 22 | ||
| G | 149 | 107 | 72 | 94 | 22 | 23 | ||
| H | 223 | 149 | 67 | 159 | 41 | 26 | ||
| I | 314 | 249 | 79 | 248 | 68 | 27 | ||
| J | 230 | 164 | 71 | 209 | 38 | 18 | ||
| K | 383 | 277 | 72 | 340 | 71 | 21 | ||
| L | 114 | 73 | 64 | 105 | 42 | 40 | ||
| M | 48 | 38 | 79 | 127 | 78 | 61 | ||
| N | 60 | 49 | 82 | 189 | 100 | 53 | ||
| Socioeconomic status | .2 | .05 | ||||||
| Most disadvantaged | 841 | 623 | 74 | 604 | 158 | 26 | ||
| Second quintile | 637 | 467 | 73 | 599 | 207 | 35 | ||
| Third quintile | 758 | 543 | 72 | 479 | 122 | 25 | ||
| Fourth quintile | 582 | 418 | 72 | 383 | 112 | 29 | ||
| Least disadvantaged | 537 | 414 | 77 | 302 | 83 | 27 | ||
| Remoteness of residency | .07 | .0005 | ||||||
| Major city | 2157 | 1613 | 75 | 1300 | 333 | 26 | ||
| Inner regional | 757 | 538 | 71 | 575 | 194 | 34 | ||
| Outer regional/remote/very remote | 441 | 314 | 71 | 492 | 155 | 32 | ||
| Distance to centera (km) | .7 | .05 | ||||||
| <50 | 2765 | 2041 | 74 | 1778 | 485 | 27 | ||
| 50–99 | 242 | 180 | 74 | 196 | 62 | 32 | ||
| 100–149 | 149 | 104 | 70 | 150 | 53 | 35 | ||
| ≥150 | 144 | 104 | 72 | 209 | 71 | 34 | ||
| Charlson Comorbidity Index | <.0001 | <.0001 | ||||||
| 0 | 2104 | 1642 | 78 | 1280 | 493 | 39 | ||
| 1 | 848 | 596 | 70 | 652 | 129 | 20 | ||
| ≥2 | 403 | 227 | 56 | 435 | 60 | 14 | ||
| Country of birth | <.0001 | <.0001 | ||||||
| Australia | 1976 | 1397 | 71 | 1511 | 391 | 26 | ||
| Overseas | 1379 | 1068 | 78 | 856 | 291 | 34 | ||
| Year of treatment | .02 | .009 | ||||||
| 2009 | 494 | 347 | 70 | 411 | 104 | 25 | ||
| 2010 | 518 | 373 | 72 | 416 | 96 | 23 | ||
| 2011 | 535 | 373 | 70 | 367 | 108 | 29 | ||
| 2012 | 576 | 433 | 75 | 415 | 127 | 31 | ||
| 2013 | 618 | 467 | 76 | 410 | 130 | 32 | ||
| 2014 | 614 | 472 | 77 | 348 | 117 | 34 | ||
- Abbreviation: DOS, degree of spread.
- a Missing data for sex and distance to center for some patients.
| Curative | Palliative/no treatment | |||||
|---|---|---|---|---|---|---|
| Characteristics | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Age (year) | ||||||
| <50 | Reference | Reference | ||||
| 50–59 | .86 | .51–1.45 | .6 | .72 | .39–1.34 | .3 |
| 60–69 | .84 | .51–1.37 | .5 | .73 | .41–1.30 | .3 |
| 70–79 | .56 | .34–.92 | .02 | .61 | .35–1.08 | .09 |
| ≥80 | .34 | .20–.57 | <.0001 | .40 | .23–.72 | .002 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 1.87 | 1.57–2.23 | <.0001 | 1.48 | 1.21–1.81 | .0001 |
| Degree of spread | ||||||
| DOS 1 | Reference | Reference | ||||
| DOS 2 | .29 | .24–.34 | <.0001 | .58 | .47–.72 | <.0001 |
| Residence local health district | ||||||
| A | Reference | Reference | ||||
| B | 1.21 | .79–1.85 | .4 | 1.91 | .93–3.92 | .08 |
| C | 1.52 | .96–2.42 | .08 | 1.73 | .81–3.70 | .2 |
| D | 1.83 | 1.16–2.89 | .0009 | 1.69 | .82–3.50 | .2 |
| E | 1.16 | .76–1.79 | .5 | 2.43 | 1.19–4.95 | .01 |
| F | 1.06 | .57–1.94 | .9 | 1.89 | .79–4.55 | .2 |
| G | 1.30 | .70–2.41 | .4 | 3.00 | 1.22–7.37 | .02 |
| H | .91 | .57–1.46 | .7 | 2.24 | 1.07–4.70 | .03 |
| I | 1.65 | .99–2.73 | .05 | 1.89 | .90–3.97 | .09 |
| J | 1.12 | .70–1.80 | .6 | 1.09 | .52–2.27 | .8 |
| K | 1.03 | .66–1.61 | .9 | 1.76 | .87–3.59 | .1 |
| L | .80 | .43–1.49 | .5 | 4.20 | 1.84–9.60 | .0006 |
| M | 1.98 | .48–8.08 | .95 | 9.17 | 4.11–20.49 | <.0001 |
| N | 1.96 | .87–4.42 | .1 | 5.64 | 2.68–11.89 | <.0001 |
| Socioeconomic status | ||||||
| Most disadvantaged | Reference | Reference | ||||
| Second quintile | .98 | .75–1.29 | .9 | .99 | .73–1.34 | .9 |
| Third quintile | .95 | .72–1.25 | .7 | .92 | .66–1.28 | .6 |
| Fourth quintile | .81 | .61–1.09 | .2 | .94 | .65–1.35 | .7 |
| Least disadvantaged | .90 | .63–1.31 | .6 | .96 | .59–1.54 | .9 |
| Remoteness of residency | ||||||
| Major city | Reference | Reference | ||||
| Inner regional | .96 | .74–1.25 | .8 | .88 | .63–1.23 | .5 |
| Outer regional/remote/very remote | .82 | .46–1.48 | .50 | .50 | .28–.91 | .02 |
| Distance to center (km) | ||||||
| <50 | Reference | Reference | ||||
| 50–99 | 1.24 | .79–1.95 | .4 | 1.38 | .85–2.23 | .2 |
| 100–149 | 1.29 | .71–2.36 | .4 | 1.75 | .95–3.23 | .07 |
| ≥150 | 1.51 | .77–2.94 | .2 | 1.57 | .83–2.97 | .2 |
| Charlson Comorbidity Index | ||||||
| 0 | Reference | Reference | ||||
| 1 | .67 | .55–.81 | <.0001 | .37 | .29–.48 | <.0001 |
| ≥2 | .42 | .33–.54 | <.0001 | .29 | .19–.36 | <.0001 |
| Country of birth | ||||||
| Australia | Reference | Reference | ||||
| Overseas | 1.51 | 1.25–1.82 | <.0001 | 1.62 | 1.30–200 | <.0001 |
| Year of treatment | ||||||
| 2009 | Reference | Reference | ||||
| 2010 | 1.01 | .75–1.36 | .9 | .77 | .54–1.09 | .1 |
| 2011 | .97 | .72–1.30 | .8 | 1.12 | .79–1.59 | .5 |
| 2012 | 1.31 | .98–1.76 | .07 | 1.27 | .91–1.78 | .2 |
| 2013 | 1.40 | 1.04–1.88 | .02 | 1.49 | 1.06–2.08 | .02 |
| 2014 | 1.45 | 1.08–1.95 | .01 | 1.41 | 1.00–2.00 | .04 |
- Abbreviations: CI, confidence interval; DOS, degree of spread; OR, odds ratio.
4 DISCUSSION
In this large Australian population-based study of stage I–III NSCLC patients, we found that 59% of patients received curative treatment. Other population-based studies have reported lower rates of 20% in New Zealand (2004)8 and 44% in Netherlands (2017–2019).21 In our cohort, the use of curative treatment among DOS 1 patients, which is roughly equivalent to stage I NSCLC, was only 27%. However, other studies show higher rates of curative treatment in stage I NSCLC, being 72% in England, 85% in Norway, and 91% in Netherlands (2015–2016).22 A previous Australian population-based study for lung cancer diagnoses between 2006 and 2011 evaluated the use of guideline treatment within one local health district, where guideline treatment was defined as surgery or curative RT in stages I–III NSCLC patients with good performance status and palliative treatment in those with poor performance status.6 Sixty-six percent of patients received guideline treatment, varying from 81% of stage I to 39% of stage IIIB NSCLC patients. In inoperable stages I–II NSCLC during a similar time period to this study, 57% of patients seen at three Australian radiation oncology clinics were treated with curative RT,12 while at the population level in an earlier time period, 32% of patients seen at the British Columbia Cancer Agency received curative RT.23
In our study, factors that correlated with increased use of curative treatment included younger age, female gender, no comorbidity, and overseas country of birth. Previous studies have also identified age and comorbidity as factors that correlated significantly with the use of curative treatment in patients with NSCLC.13, 14, 22, 24 The negative association between age and curative treatment may be due to patient preference,25 frailty, clinician nihilism or the perceived toxicities of treatment. The introduction of SABR in particular has improved survival at the population level as patients who previously received no treatment for early-stage NSCLC received stereotactic RT.26 The negative association of curative treatment with comorbidity is understandable. NSCLC patients frequently have comorbidities, especially cardiovascular or respiratory diseases, which may impact the ability to deliver curative treatment, as well as impact their life expectancy.27-29 These patients are best discussed at a multidisciplinary team meeting where all factors can be taken into account in deriving a treatment plan.
Interestingly, we found that female gender was significantly associated with the use of curative treatment. Proportionally, females have a lower incidence of smoking than males in the Australian population and are less likely to have smoking-related comorbidities. They may have been perceived to be fitter for curative treatment, although our analysis did account for DOS and comorbidities. Of note, SES and the remoteness of residence were not significantly associated with the use of curative treatment in multivariable analyses, suggesting equitable access to curative treatment.
We found that the use of curative treatment increased over time from 2009 (55%) to 2014 (63%). This was due to an increase in both surgery from 36% (2009) to 40% (2014) and RT from 15% (2009) to 20% (2014). The increase in RT utilization may also have been driven by the introduction of SABR, which has added a new treatment option for medically inoperable patients with early-stage NSCLC who may not have been thought fit enough to receive a long course of fractionated RT previously. Minimally invasive surgery and improvements in RT technology such as four-dimensional computed tomography and intensity-modulated RT may also have changed the risk:benefit ratio of curative treatment, thereby increasing the population clinically eligible for this. Access to RT in Australia also improved over time from 2010 to 2015, where the number of sites delivering RT increased from 61 in 2010/2011 to 75 in 2014/2015.30
Our study identified a wide variation in the proportion of curative treatment used between LHDs of residence ranging from 24% to 70%. Similar findings have been reported in the literature across different jurisdictions. Variation in the use of curative treatment has been described between different countries (72% in England, 85% in Norway, 91% in Netherlands).22 Differences in the use of surgery versus RT for stages I and II NSCLC and in the use of concurrent chemoradiotherapy versus sequential chemoradiotherapy in stage III NSCLC have been reported in the Netherlands.25 In the USA, for patients with medically inoperable stage I NSCLC, the use of RT varies according to institution type and patient volume.31 Patients seen at academic centers and high volume centers were more likely to receive RT over no treatment and SABR over conventional RT.31 The variation in the use of curative treatment seen in this study may be due to a number of reasons, including equity of access to cardiothoracic surgery, RT, or specific RT technology, or even access to expert advice and up-to-date knowledge of developments in surgery and RT, such as the more recent availability of SABR. The health funding available may also vary from center to center and among the LHDs. For example, a review of the radiation oncology health program grant (ROHPG) scheme in 2016 found that significant disparities in funding levels continued to exist among centers, especially between public and private facilities.32 These funding disparities will further influence issues such as workforce shortages, lack of expertise and resources to introduce new technology, and lack of investment in clinician education and training to adhere to evidence-based practice.
The existence of and clinician engagement in multidisciplinary team meetings may also vary between LHDs. Multidisciplinary team discussion is important in overcoming clinician bias and nihilism. These meetings result in increased utilization of non-surgical treatment and guideline treatment.33, 34 However, it is important to recognize that patient casemix can also result in different treatment patterns. A Scottish study found that the differences in the use of surgery and curative RT for NSCLC throughout regions in Scotland were largely explained by differences in patient comorbidities and pulmonary function between the regions.28
Despite the large variation in the use of curative treatment between the LHDs, this did not correlate with the differences in 2-year survival. This may be due to an imbalance of other prognostic factors, such as performance status, smoking, pathological subtype, and mutation status, which were not available in the registry dataset. Other factors associated with improved 2-year OS were younger age, female gender, DOS 1, CCI = 0, overseas country of birth, and recent years of treatment 2013 and 2014. Previous studies have also identified female gender as a factor associated with improved OS.35, 36
There are several limitations to this study. We were unable to ascertain patients’ treatment facilities; therefore, LHD of patient residence was used as a surrogate and assumed to be the treatment facility. In reality, a small proportion of patients may have received treatment in a facility outside of their residence LHD. We also acknowledge that these large datasets lacked clinical-level information; therefore, some factors such as performance status, respiratory function, and smoking status were not available for analysis. In addition, survival was measured without taking other factors into consideration (subsequent treatment including chemotherapy or immunotherapy, treatment received on relapse, and cause of death). While the study results pertain to a cohort of patients treated from 2009 to 2014, these findings are still relevant, as transparent public reporting is a necessary first step to spark change and reduce variation in care. However, we acknowledge that more recently, SABR has been increasingly utilized in curative treatment for NSCLC since the publications of the stereotactic precision and conventional radiotherapy evaluation (SPACE) trial in 201637 and TROG 09.02 CHISEL trial in 2019.38 NSCLC management is likely to continue to evolve as more mature data become available, and the proportion of patients who are eligible for curative treatment is likely to increase over time. Despite these limitations, these data captured the overall practice pattern of curative treatment use in stages I–III NSCLC.
In conclusion, the use of curative treatment between 2009 and 2014 for patients with localized or locoregional NSCLC was low at 59% in NSW, when compared with other countries in similar study periods. It is encouraging to note that the use of curative treatment and survival improved over time. Significant variation was noted in the use of curative treatment between LHDs, although this did not directly correlate with survival. This study provides a benchmark from which future improvements in care can be measured, ideally with the implementation of a centralized lung cancer clinical quality registry.39
ACKNOWLEDGMENTS
Dr. Batumalai was supported by a Sydney Partnership for Health, Education, Research, and Enterprise (SPHERE) Translational Research Fellowship. We acknowledge support from Cancer Institute NSW.
Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
CONFLICTS OF INTEREST
No conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.




