Efficacy and safety of blinatumomab: Post hoc pooled analysis in Asian adults with relapsed/refractory B-cell precursor acute lymphoblastic leukemia
Abstract
Background
Global studies have demonstrated the efficacy and safety of blinatumomab—a BiTE® (bispecific T-cell engager) targeted immuno-oncology therapy that mediates the lysis of cells expressing CD19 in patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). Because limited data are available in Asian patients, we conducted a post hoc pooled analysis in 45 Asian adult patients with R/R ALL—19 from the blinatumomab arm of TOWER (NCT02013167) and 26 from Study 265, a phase 1b/2 study in Japanese adults (NCT02412306).
Methods
Patients received a maximum of two cycles of induction blinatumomab for 4 weeks by continuous intravenous infusion (cycle 1/week 1: 9 μg/day; cycle 1/weeks 2–4: 28 μg/day) followed by 2 weeks of no blinatumomab (each 6-week cycle); patients received 28 μg/day blinatumomab in subsequent cycles.
Results
Twenty of 45 patients enrolled (44%) achieved complete remission with full or partial hematologic recovery compared with 44% in TOWER and 80% and 38% in phase 1b and phase 2, respectively, of Study 265. The Kaplan–Meier (KM) median overall survival was 11.9 months (95% confidence interval [CI], 9.9–17.1) and the KM median duration of relapse-free survival was 8.9 months (95% CI, 3.8–10.7). Ninety-three percent of patients had grade ≥ 3 treatment-emergent adverse events (AEs) compared with 87% in TOWER and 80% and 100% in phase 1b and phase 2, respectively, of Study 265. Five patients (11.4%) had fatal AEs.
Conclusions
The safety and efficacy of blinatumomab in Asian patients were comparable with those reported in previous global studies with no new safety signals.
1 BACKGROUND
It is estimated that there are approximately 54,000 new cases of acute lymphoblastic leukemia (ALL) in Asia every year.1 By 2020, the incidence of ALL in Asia-Pacific is expected to be 0.4 per 100,000, with a prevalence of 0.37 per 100,000.2 Adult patients with relapsed/refractory (R/R) B-cell precursor ALL have a poor prognosis. Complete remission rates with the use of standard salvage chemotherapy are in the range of 30%–40% at first relapse and 20%–25% at second relapse.3, 4 The median overall survival (OS) in these patients ranges from 3 to 5 months, and 3- to 5-year survival rates are usually less than 10%.4-8
BLINCYTO® (blinatumomab; Amgen), a BiTE® (bispecific T cell engager) immuno-oncology therapy with dual specificity for cluster of differentiation (CD)–19 and CD3, enables endogenous T-cell recognition and elimination of CD19-positive ALL blasts.9-12 Blinatumomab has been approved by the US Food and Drug Administration for the treatment of R/R B-cell precursor ALL in adults and children.13 Additionally, blinatumomab has received regulatory approval in 57 countries globally, including Japan.14 The efficacy and safety of blinatumomab has been evaluated in global clinical trials in predominantly Caucasian patients with Philadelphia chromosome–negative (Ph−) R/R B-cell precursor ALL.15, 16 In the global phase 3 TOWER study, blinatumomab monotherapy versus standard-of-care chemotherapy resulted in a significantly higher rate of complete remission with full hematologic recovery (CR; 34% vs. 16%, P < 0.001) and complete remission with full, partial, or incomplete hematologic recovery (CR/CRh/CRi; 44% vs. 25%, P < 0.001) and a longer median OS (7.7 months vs. 4.0 months, P = 0.01).16
Currently, limited data are available on the efficacy and safety of blinatumomab in Asian patients, whose immunologic and genetic background may differ from Caucasian patients. A recent phase 1b/2 study (Study 265, NCT02412306) evaluated the safety and efficacy of blinatumomab in Japanese adults with Ph− R/R B-cell precursor ALL.17 No published study to date has evaluated the safety and efficacy of blinatumomab in the broader Asian population. Therefore, a patient-level, post hoc analysis was conducted to evaluate the efficacy and safety of blinatumomab in 45 Asian adult patients with Ph– R/R B-cell precursor ALL pooled from two studies—19 patients from the blinatumomab arm of the phase 3 TOWER study (NCT02013167) and 26 patients from the phase 1b/2 study (Study 265; NCT02412306).
2 METHODS
Patients in both studies were ≥18 years old with Ph– R/R B-cell precursor ALL, with >5% blasts, with an Eastern Cooperative Oncology Group performance status 0–2, and with no central nervous system pathology. Key exclusion criteria for both studies were the presence of other active cancers, a history of clinically relevant central nervous system pathology, autoimmune disease, any active acute graft-versus-host disease (GVHD) of grade 2 or higher or active chronic GVHD, allogeneic hematopoietic stem cell transplantation (allo-HSCT) within 12 weeks before the start of blinatumomab, autologous HSCT (auto-HSCT) within 6 weeks before the start of blinatumomab, chemotherapy or radiotherapy within 2 weeks before the start of blinatumomab, and immunotherapy within 4–6 weeks before the start of blinatumomab.
In TOWER and Study 265, patients were administered up to two cycles of induction therapy.16, 17 Responders were defined as patients who achieved CR (defined as ≤5% bone marrow blasts with no evidence of disease and full recovery of peripheral blood counts [platelets > 100,000/μl and absolute neutrophil count (ANC) > 1,000/μl]) or CR with partial hematologic recovery (CRh; defined as ≤5% bone marrow blasts with no evidence of disease and partial recovery of peripheral blood counts [platelets > 50,000/μl and ANC > 500/μl]). Responders received three cycles of consolidation therapy until they had disease progression, an intolerable adverse event, withdrew their consent or had received a maximum of five induction/consolidation cycles. In TOWER, patients with continued morphologic remission received up to 12 months of maintenance therapy.16 Induction and consolidation treatments with blinatumomab in both studies were administered in 6-week cycles. In each cycle, patients received treatment for 4 weeks: 9 μg/day during week 1 of induction cycle 1 and 28 μg/day during week 2–4 by continuous infusion, followed by no treatment for 2 weeks. In subsequent consolidation cycles, patients received blinatumomab at 28 μg/day during weeks 1–4, followed by no treatment for 2 weeks. Maintenance treatment was a 4-week continuous infusion of blinatumomab administered every 12 weeks. Patients could undergo HSCT at any time following the first treatment cycle.
Time-to-event outcomes OS and relapse-free survival (RFS) were summarized using Kaplan–Meier curves, survival estimates at select time points, and quartiles. Proportions of hematologic and minimal residual disease (MRD) responders were provided along with exact binomial 95% confidence intervals (CIs). Progressive disease was defined as an increase from baseline of at least 25% of bone marrow blasts or an absolute increase of at least 5,000 cells/μl in the number of circulating peripheral blasts. Treatment-emergent adverse events (TEAEs) were summarized using subject incidence rate. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities version 21.1, and severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
3 RESULTS
A total of 45 Asian patients were enrolled, of which 26 (57.8%) were female. Table S1 shows a comparison of the baseline demographics and clinical characteristics of patients from the current analysis with TOWER and Study 265. The median age of patients included in the current analysis was 43 years. A total of 30 (66.7%) patients received at least 1 prior salvage therapy and 20 (44.4%) patients received prior allo-HSCT. Table S2 shows prior treatment regimens received by each of the patients enrolled in this analysis. Forty-four patients received at least one cycle of blinatumomab 9–28 μg/day. Baseline demographic and clinical characteristics in the pooled patient group were generally comparable to the baseline characteristics in the blinatumomab arm of TOWER and Study 265. Of the 45 patients enrolled, 26 patients were enrolled at study sites in Japan, eight patients in the Republic of Korea, and five patients in Taiwan. In countries outside of Asia, two patients were enrolled in the USA and one each in Australia, Canada, Italy, and the United Kingdom.
The Kaplan–Meier median duration of OS was 11.9 months (95% CI, 9.9–17.1; Figure 1a and Table 1). The Kaplan–Meier median duration of RFS in patients who achieved CR/CRh in the first 12 weeks was 8.9 months (95% CI, 3.8–10.7; Figure 1b). Of the 45 patients enrolled, 20 patients (44.4%; 95% CI, 29.6%–60.0%) achieved CR/CRh, of which 14 (31.1%; 95% CI, 18.2%–46.6%) achieved CR and 6 (13.3%; 95% CI, 5.1%–26.8%) achieved CRh within 12 weeks of treatment initiation (Table 2). Six patients (13.3%; 95% CI, 5.1%–26.8%) achieved blast-free hypoplastic or aplastic bone marrow (without CRi), 3 (6.7%; 95% CI, 1.4%–18.3%) showed progressive disease, and 10 (22.2%; 95% CI, 11.2%–37.1%) were nonresponsive to treatment. Of the 20 patients who achieved CR/CRh, 15 (75%; 95% CI, 50.9%–91.3%) showed MRD remission, of which 12 (60%; 95% CI, 36.1%–80.9%) showed MRD complete remission. Of the 45 patients enrolled, 18 received on-study allo-HSCT prior to which nine patients showed CR, three patients showed blast-free hypoplastic or aplastic bone marrow, three patients showed no response, two patients showed progressive disease, and the status of one patient was unevaluable.
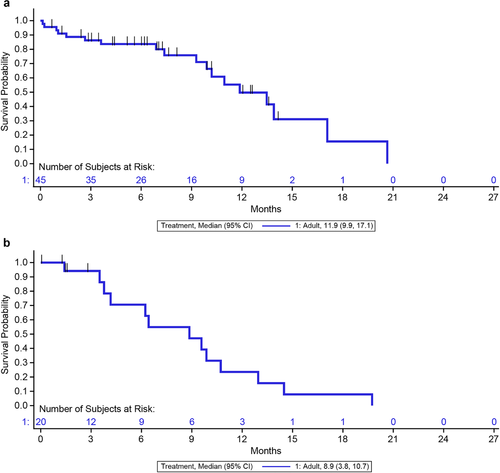
Kaplan–Meier estimates of (a) overall survival (b) relapse-free survival in Asian adult patients. aNote: CR is defined as ≤5% bone marrow blasts with no evidence of disease and full recovery of peripheral blood counts (platelets > 100,000/μl and ANC > 1,000/μl); CRh is defined as ≤5% bone marrow blasts with no evidence of disease and partial recovery of peripheral blood counts (platelets > 50,000/μl and ANC > 500/μl). Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; CR, complete remission with full hematologic recovery; CRh, complete remission with partial hematologic recovery of peripheral blood counts.
aPatients who achieved CR/CRh in the first 12 weeks.
Vertical bar | indicates a censored patient at the time of allogeneic hematopoietic stem cell transplantation
| Adult | |
|---|---|
| (N = 45) | |
| Overall survival | |
| Number of patients | 45 |
| Events, n (%) | 18 (40.0) |
| Deaths from any cause | 18 (40.0) |
| Censored, n (%) | 27 (60.0) |
| Alive at last follow-up | 18 (40.0) |
| Consent withdrawn | 5 (11.1) |
| Decision by sponsor | 4 (8.9) |
| Time to event (KM) (months)a | |
| Median | 11.9 |
| 95% CI | 9.9–17.1 |
| Q1, Q3 | 9.3, 17.1 |
| Min, Max | 0.1, 20.7 |
| Time to censoring (months)a,b | |
| Median | 7.6 |
| Q1, Q3 | 5.1, 13.6 |
| Min, Max | 0.7, 14.1 |
| Relapse-free survival | |
| Number of patientsc | 20 |
| Events, n (%) | 13 (65.0) |
| Relapse | 6 (30.0) |
| Progressive disease | 1 (5.0) |
| Death from any cause | 6 (30.0) |
| Censored, n (%) | 7 (35.0) |
| Alive w/o relapse | 7 (35.0) |
| Time to event (KM) (months)a | |
| Median | 8.9 |
| 95% CI | 3.8–10.7 |
| Q1, Q3 | 4.2, 10.7 |
| Min, Max | 1.4, 19.7 |
| Time to censoring (months)a,b | |
| Median | NE |
| Q1, Q3 | 1.5, NE |
| Min, Max | 0.0, 2.8 |
- Note: CR is defined as ≤5% bone marrow blasts with no evidence of disease and full recovery of peripheral blood counts (platelets > 100,000/μl and ANC > 1,000/μl); CRh is defined as ≤5% bone marrow blasts with no evidence of disease and partial recovery of peripheral blood counts (platelets > 50,000/μl and ANC > 500/μl).
- Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; CR, complete remission with full hematologic recovery; CRh, complete remission with partial hematologic recovery of peripheral blood counts; KM, Kaplan–Meier; N, total number of patients; n, patient subset; NE, not estimable; Q1, first quartile; Q3, third quartile.
- a Months are calculated as days from randomization or first dose date to event/censor date, divided by 30.5.
- b Time to censoring measures follow-up time by reversing the status indicator for censored and events.
- c In patients who achieved CR/CRh in the first 12 weeks.
| Adult | |
|---|---|
| (N = 45) | |
| Hematologic response | |
| CR/CRh, n (%) (95% CI) | 20 (44.4) (29.6–60.0) |
| CR | 14 (31.1) (18.2–46.6) |
| CRh | 6 (13.3) (5.1–26.8) |
| Blast-free hypoplastic or aplastic bone marrow (without CRi), n (%) (95% CI) | 6 (13.3) (5.1–26.8) |
| Partial remission, n (%) (95% CI) | 0 (0.0) (NE–NE) |
| Progressive disease, n (%) (95% CI) | 3 (6.7) (1.4–18.3) |
| Nonresponse, n (%) (95% CI) | 10 (22.2) (11.2–37.1) |
| Unevaluable/missing post-baseline assessment within 12 weeks, n (%) | 6 (13.3) |
| MRD remission in all patients analyzed | |
| Number of patients | 45 |
| MRD remission, n (%) (95% CI) | 19 (42.2) (27.7–57.8) |
| MRD complete remission, n (%) (95% CI) | 13 (28.9) (16.4–44.3) |
| No MRD remission, n (%) | 15 (33.3) |
| No post-baseline MRD assessment, n (%) | 11 (24.4) |
| MRD remission in patients with CR | |
| Number of patients with CR | 14 |
| MRD remission, n (%) (95% CI) | 11 (78.6) (49.2–95.3) |
| MRD complete remission, n (%) (95% CI) | 9 (64.3) (35.1–87.2) |
| No MRD remission, n (%) | 1 (7.1) |
| No post-baseline MRD assessment, n (%) | 2 (14.3) |
| MRD remission in patients with CR/CRh | |
| Number of patients with CR/CRh | 20 |
| MRD remission, n (%) (95% CI) | 15 (75.0) (50.9–91.3) |
| MRD complete remission, n (%) (95% CI) | 12 (60.0) (36.1–80.9) |
| No MRD remission, n (%) | 2 (10.0) |
| No post-baseline MRD assessment, n (%) | 3 (15.0) |
- Note: CR is defined as ≤5% bone marrow blasts with no evidence of disease and full recovery of peripheral blood counts (platelets > 100,000/μl and ANC > 1,000/μl); CRh is defined as ≤5% bone marrow blasts with no evidence of disease and partial recovery of peripheral blood counts (platelets > 50,000/μl and ANC > 500/μl); CRi is defined as ≤5% bone marrow blasts with no evidence of disease and incomplete recovery of peripheral blood counts (platelets > 100,000/μl or ANC > 1,000/μl); partial remission is defined as bone marrow blasts in the range of 6%–25% with at least a 50% reduction from baseline; progressive disease is defined as an increase from baseline of at least 25% of bone marrow blasts or an absolute increase of at least 5,000 cells/μl in the number of circulating peripheral blasts. MRD remission is defined as fewer than 10–4 detectable blasts, as determined by PCR or flow cytometry; MRD complete remission is defined as no detectable leukemic cells, as determined by PCR or flow cytometry.
- Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; CR, complete remission with full hematologic recovery; CRh, complete remission with partial hematologic recovery of peripheral blood counts; CRi, complete remission with incomplete hematologic recovery of peripheral blood counts; MRD; minimal residual disease; NE, not estimable; PCR, polymerase chain reaction.
- a Within the first 12 weeks of treatment.
Forty-four patients who received at least one dose of blinatumomab 9–28 μg/day were part of the safety analysis set. Forty-one patients (93.2%) reported grade ≥ 3 TEAEs and 21 (47.7%) reported serious AEs (Table 3). In addition, five deaths (11.4%) occurred due to acute respiratory failure, pneumonitis, suicide, pneumonia, and tumor lysis syndrome, all of which were considered as non-treatment related by site investigators. Grade ≥ 3 TEAEs of interest reported in greater than 20% of the patient population were cytopenia (63.6%), neutropenia (59.1%), and infections (43.2%).
| Adults (N = 44), n(%) | |
|---|---|
| All TEAEs | 44 (100.0) |
| Grade ≥ 2 | 44 (100.0) |
| Grade ≥ 3 | 41 (93.2) |
| Grade ≥ 4 | 26 (59.1) |
| Serious adverse events | 21 (47.7) |
| Leading to interruption of investigational product | 10 (22.7) |
| Serious | 3 (6.8) |
| Leading to discontinuation of investigational product | 3 (6.8) |
| Serious | 3 (6.8) |
| Life-threatening adverse events | 7 (15.9) |
| Fatal adverse events | 5 (11.4) |
| Grade ≥ 3 TEAEs of interest | 39 (88.6) |
| Cytopenia | 28 (63.6) |
| Neutropenia | 26 (59.1) |
| Infections | 19 (43.2) |
| Bacteraemia | 2 (4.5) |
| Bacterial sepsis | 2 (4.5) |
| Device-related infection | 3 (6.8) |
| Lower respiratory tract infection fungal | 1 (2.3) |
| Muscle abscess | 1 (2.3) |
| Acute otitis media | 1 (2.3) |
| Pneumonia | 1 (2.3) |
| Pseudomonal sepsis | 1 (2.3) |
| Pseudomonas infection | 1 (2.3) |
| Sepsis | 2 (4.5) |
| Septic shock | 1 (2.3) |
| Infusion reaction considering duration | 7 (15.9) |
| Lymphopenia | 7 (15.9) |
| Central neuropsychiatric events due to direct neurotoxicities | 4 (9.1) |
| Elevated liver enzyme | 3 (6.8) |
| Cytokine release syndrome | 1 (2.3) |
| Decreased immunoglobulins | 1 (2.3) |
| Embolic and thrombotic events | 1 (2.3) |
| Tumor lysis syndrome | 1 (2.3) |
- Abbreviations: N, total number of patients; n, patient subset; TEAE, treatment-emergent adverse event.
4 DISCUSSION
This pooled analysis demonstrated that blinatumomab was safe and efficacious in Asian adult patients with Ph− R/R B-cell precursor ALL. Overall, the efficacy results were comparable to the global TOWER study and Study 265 conducted in Japanese patients (Table 4).16, 17 There were no obvious differences in hematologic response between the current analysis and the blinatumomab arm of TOWER and Study 265. In addition, the median duration of OS in the current analysis (11.9 months) was slightly greater than that observed in the blinatumomab arm of TOWER (7.7 months). The median duration of RFS (8.9 months) was similar to that observed in the blinatumomab arm of TOWER (7.3 months) and greater than that observed in Study 265 (5 months). This difference in OS and RFS between the current analysis and TOWER could be attributed to factors such as difference in the ethnicity of the patient populations (Asian patients comprised 7% of the total patient population in TOWER), difference in the size of the patient population analyzed, and differences in the local standard treatment practices followed in some geographies wherein an aggressive frontline treatment approach could contribute to greater resistance at relapse thereby impacting OS in response to salvage therapies.
| Efficacy outcomes | Pooled Asian analysis (N = 45) | TOWER1 (blinatumomab) (N = 271) | Study 2652 | |
|---|---|---|---|---|
| Phase 1b (N = 5) | Phase 2 (N = 21) | |||
|
CR/CRh, n (%) [95% CI] |
20 (44) [30–60] |
119a (44) [38–50] |
4 (80) [28‒100] |
8 (38) [18–62] |
| Blast-free hypoplastic or aplastic bone marrow without CRh or CRi, n (%) [95% CI] |
6 (13) [5‒27] |
9 (3) [2‒6] |
0 (0) [0–52] |
6 (29) [11–52] |
| CR/CRh with evaluable MRD, n | 17 | 97a, b | 4 | 7 |
|
MRD remission,c n (%) [95% CI] |
15 (88) [64–99] |
74 (76) [67–84] |
4 (100) [40‒100] |
5 (71) [29‒96] |
|
MRD complete remission,c n (%) [95% CI] |
12 (71) [44–90] |
58 (60) [49–70] |
4 (100) [40‒100] |
3 (43) [10‒82] |
| KM median relapse-free survival (months) [95% CI] |
8.9 [3.8–10.7] |
7.3 [5.8–9.9] |
NA NA |
5 [3.5–6.4] |
| KM median overall survival (months)[95% CI] |
11.9 [9.9–17.1] |
7.7 [5.6–9.6] |
NE [7.4–NE] |
|
| Safety outcomes | Pooled Asian analysis (N = 44) | TOWER1 (blinatumomab) (N = 267) | Study 2652 | |
|---|---|---|---|---|
|
Phase 1b (N = 5) |
Phase 2 (N = 21) | |||
| Grade ≥ 3 TEAE, n (%) | 41 (93) | 231 (87) | 4 (80) | 21 (100) |
| Grade ≥ 3 TEAEs of interest (%) | ||||
| Neurologic events | 4 (9) | 27 (10) | 0 (0) | 1 (5) |
| Cytokine release syndrome | 1(2) | 13 (5) | 0 (0) | 1 (5) |
| Cytopenia | 28 (64) | 142 (53) | 3 (60) | 17 (81) |
| Infections | 19 (43) | 93 (35) | 2 (40) | 8 (38) |
| Tumor lysis syndrome | 1 (2) | 8 (3) | 0 (0) | 1 (5) |
- Note: CR is defined as ≤5% bone marrow blasts with no evidence of disease and full recovery of peripheral blood counts (platelets > 100,000/μl and ANC > 1,000/μl); CRh is defined as ≤5% bone marrow blasts with no evidence of disease and partial recovery of peripheral blood counts (platelets > 50,000/μl and ANC > 500/μl). MRD complete remission is defined as no detectable leukemic cells, as determined by PCR or flow cytometry.
- Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; CR, complete remission with full hematologic recovery; CRh, complete remission with partial hematologic recovery of peripheral blood counts; KM, Kaplan–Meier; MRD, minimal residual disease; NA, not available; NE, not estimable; TEAE, treatment-emergent adverse event.
- a Patients with CR/CRh/CRi.
- b Amgen data on file.
- c Percentage calculated with respect to patients with CR/CRh with evaluable MRD.
Overall, the percentage of patients who reported grade ≥ 3 TEAEs in the current analysis was comparable to that observed in the blinatumomab arm of TOWER and Study 265.16, 17 The percentage of patients with grade ≥ 3 TEAEs of interest such as neurologic events, cytokine release syndrome, cytopenia, infections, and tumor lysis syndrome in this study were generally comparable to that reported in TOWER and Study 265.
Although this subanalysis in Asian patients was valuable, the generalizability of the findings to routine clinical practice is limited by the relatively small sample size and the post hoc nature of the analysis. Key efficacy and safety parameters from the subpopulation of Asian patients in the blinatumomab arm of TOWER and Study 265 were provided along with results from the entire blinatumomab-treated patient population in these respective studies as context. The analysis was not powered to detect a difference between the Asian subgroup and rest of the population with respect to these key efficacy and safety parameters.
5 CONCLUSIONS
In conclusion, these results suggest that the efficacy and safety of blinatumomab in Asian patients were comparable to those reported in global TOWER study and Study 265 in Japanese patients, with similar disease response rates and a favorable safety profile with no new safety signals. A separate study in another Asian population of Chinese ethnicity is ongoing (NCT03476239).
ACKNOWLEDGMENTS
The authors thank all the site investigators and patients who participated in the TOWER study and Study 265. Medical writing support, in accordance with GPP guidelines, was provided by Swapnil Kher, PhD, of Cactus Life Sciences (part of Cactus Communications) and was funded by Amgen Inc.
AUTHORS’ CONTRIBUTION
Y. Kobayashi, I. Oh, T. Miyamoto, W.-S. Lee, H. Iida, H. Minami, Y. Maeda, J.H. Jang, S.-S. Yoon, S.-P. Yeh, and H. Kiyoi contributed in data collection, analysis, and interpretation. Q. Tran, J. Morris, and J. Franklin contributed toward study design, data analysis, and data interpretation. All authors contributed toward writing/review of the manuscript. All authors read and approved the final manuscript.
FUNDING STATEMENT
This analysis was funded by Amgen Inc. and Astellas Pharma Inc. The funder contributed to study design, data collection, data analysis, and data interpretation, and funded a professional medical writer to assist with writing the report.
CONFLICTS OF INTEREST
The authors had full access to all data in the study and had final responsibility for the decision to submit for publication. Yukio Kobayashi received research funding from Amgen Astellas BioPharma and Pfizer; received compensation from SymBio for an advisory role; and participated in a speaker's bureau for Amgen Astellas BioPharma and Pfizer. Hironobu Minami received grants from Asahi-Kasei Pharma, Astellas, Nippon Shinyaku, Taisho Toyama, Teijin Pharma, Yakult, CSL Behring, Nippon Kayaku, and Shionogi; received grants and personal fees from Bayer; received grants and personal fees from Boehringer Ingelheim, Eisai, Kyowa Kirin, Eli Lilly, Merck Serono, Nippon Chemiphar, Sanofi, Takeda, and Sumitomo Dainippon Pharma; received grants and personal fees, personal fees from Bristol Myers Squibb; received personal fees from Celgene, Janssen, Otsuka, Shire Japan, Genomic Health, AbbVie, and Kyowa; received grants and personal fees from Chugai; received grants from Daiichi Sankyo; received grants and personal fees from MSD; received grants and personal fees from Ono Pharmaceutical; received grants, personal fees from Pfizer; received grants and personal fees from Taiho; received personal fees from Novartis; and received personal fees from Nihon Medi-Physics, outside the submitted work. Yoshinobu Maeda received research funding from Bristol Myers Squibb and Astellas; and received honoraria from Bristol Myers Squibb, Mundipharma, and Kyowa Kirin. Sung-Soo Yoon received compensation for a consulting or advisory role from Janssen, Takeda, Amgen, Celgene, Novartis, Astellas; received honoraria from Novartis; received research funding from Yuhan Pharmaceutical, Kyowa Kirin, and Roche-Genentech. Qui Tran, Joan Morris, and Janet Franklin are employees and stockholders of Amgen. Hitoshi Kiyoi received research funding from FUJIFILM, Otsuka, Chugai, Kyowa Kirin, Astellas, Zenoaq Kogyo, Nippon Shinyaku, Eisai, Takeda, Sumitomo Dainippon Pharma, Bristol Myers Squibb, Perseus Proteomics, and Daiichi Sankyo; and received honoraria from Pfizer Japan and Astellas. Hiroatsu Iida received research funding from Chugai. Iekuni Oh, Toshihiro Miyamoto, Won-Sik Lee, Jun Ho Jang, and Su-Peng Yeh have nothing to disclose.
ETHICS APPROVAL STATEMENT
The study protocols for both the studies were approved by the ethics committee or the institutional review board at each clinical site, and all patients provided a signed informed consent before the start of any study-related procedure. The studies were conducted in accordance with the International Council for Harmonisation Good Clinical Practice Guideline and conformed to the provisions of the Declaration of Helsinki
Open Research
DATA AVAILABILITY STATEMENT
The dataset(s) supporting the conclusions of this article is (are) available in the Amgen clinical studies repository, http://www.amgen.com/datasharing.




