Dynamic changes in pathology and PD-L1 expression in a patient with metastatic lung adenocarcinoma who received pembrolizumab therapy followed by two salvage surgeries two years later: A case report
Green Hong and Hee Sun Park contributed equally to this study.
Abstract
During chemotherapy, certain cancer cells undergo cell death, which alters the properties of remaining cells and leads to numerous changes in the constituent cells of lung cancer. Immunotherapy has been used as neoadjuvant therapy, and several studies have reported changes in lung cancer tissue following treatment with immuno-anticancer drugs in early stage disease. However, no research has currently discussed the pathological and PD-L1 expression changes in metastatic lung cancer. Here, we describe a patient with lung adenocarcinoma and multiple metastases who achieved complete remission after receiving initial carboplatin/pemetrexed followed by pembrolizumab treatment for 2-years. The initial biopsy revealed adenocarcinoma with high PD-L1 expression, and next-generation sequencing (NGS) identified KRAS, RBM10, and STAG2 mutations. After 2-years of treatment with pembrolizumab, the patient achieved complete response (CR). The patient underwent first salvage surgery for the oligo-relapse lesion, and the pathology result showed a large cell neuroendocrine tumor (NET) with adenocarcinoma and no PD-L1 expression. NGS revealed KRAS and TP53 mutations. After one year, a chest computed tomography (CT) scan revealed a small nodule in the right lower lobe, and the patient underwent second salvage surgery. Pathology results showed minimally invasive adenocarcinoma with no PD-L1 expression and no significant genetic mutations. This case report demonstrates the dynamic changes cancer cells undergo following pembrolizumab treatment and salvage surgeries and is the first report to compare pathological changes after immunotherapy and two subsequent salvage surgeries in metastatic lung adenocarcinoma. Clinicians must remain vigilant to these dynamic changes throughout treatment and consider salvage surgery for oligo-relapse lesions. By understanding these changes, new strategies can be developed to improve the long-term efficacy of immunotherapy.
INTRODUCTION
Cancer cells within a tumor exhibit significant heterogeneity, resulting in varying responses to chemotherapy.1, 2 Intrinsic and extrinsic factors include alterations in cellular properties, overexpression of multidrug resistance genes, impaired DNA repair mechanisms, and evasion of programmed cell death leading to drug resistance in cells, can operate independently or in combination.3 Among these factors, gene mutations are considered a primary cause of chemotherapy failure.4 Consequently, cancer can evolve during chemotherapy, potentially affecting its response to further treatment and clinical outcomes.
The advent of immune checkpoint inhibitors (ICIs), including nivolumab, pembrolizumab, atezolizumab, and durvalumab, has substantially improved treatment outcomes in patients with advanced non-small cell lung cancer (NSCLC).5 As a result, ICIs have become a standard treatment option for NSCLC, either alone or in combination with chemotherapy.6
PD-L1 expression plays an important role in immune evasion by cancer cells and is often used as a biomarker to predict response to immunotherapy.7 In contrast to generation alterations, PD-L1 expression is affected by numerous factors.8-14 Oncogenic signaling pathways such as JAK/STAT and RAS/ERK can affect PD-L1 expression. Additionally, transcriptional factors, such as hypoxia-inducible factor-1, nuclear factor kappa β can regulate PD-L1 transcription. Post-transcriptional regulation of PD-L1 can occur through specific miRNAs and proteins such as CSN5, CMTM6, and CDK4, and potentially unknown mechanisms.15
There have previously been reports about changes in PD-L1 expression, such as an increase after platinum based neoadjuvant chemotherapy, or an alteration in an autopsy case that deceased within a month after ICI treatment. To our knowledge, this is the first case report to reveal the dynamic changes in PD-L1, pathology and molecular findings after immunotherapy.
Here, we present the case of a patient whose pathology, next-generation sequencing (NGS) profile, and PD-L1 expression of cancer cells underwent significant changes after 2 years of pembrolizumab treatment and sequential salvage surgeries.
CASE REPORT
A 61-year-old man with a 40-pack-year smoking history underwent a biopsy for a 62 mm-sized mass in the right upper lobe (Figure 1a,b). The biopsy confirmed adenocarcinoma with multiple lymph nodes and lung-to-lung metastasis, malignant pleural effusion, and bony metastasis, resulting in a clinical stage of cT4N3M1c (Figure 2a,b). Biomarker analysis revealed negative results for EGFR and ALK, whereas PD-L1 expression was high (SP142 40%, SP263 90%, PharmDX High, Figure 2c). NGS-based testing of tissue biopsies revealed mutations in KRAS (G12D), RBM10 (E748*), and STAG2 (R953*), and the tumor mutation burden (TMB) assessed by targeted NGS was 91.29 mutations/Mb. The patient was administered four cycles of cisplatin and pemetrexed followed by seven cycles of maintenance therapy with pemetrexed alone. After disease progression, the patient was treated with pembrolizumab for 36 cycles, resulting in no detectable tumor.
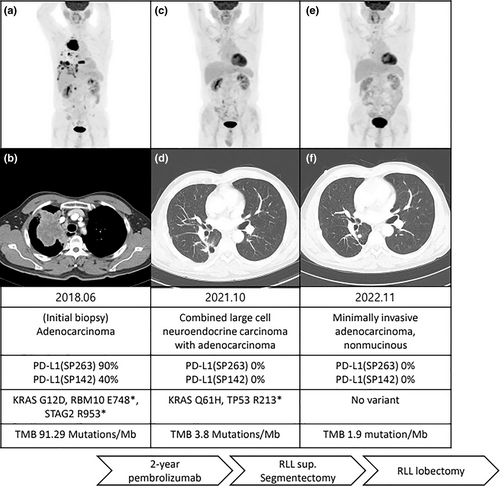

Six months after discontinuation of pembrolizumab, the patient exhibited an increase in the right lower lobe (RLL) nodule size from 4 to 13 mm, with no other lesions detected on positron emission tomography (PET) computed tomography (CT) scan (Figure 1c,d). Salvage surgery in the form of RLL segmentectomy was performed, and subsequent biopsy results confirmed combined large cell neuroendocrine tumor (NET) and adenocarcinoma (Figure 2d,e). Biomarker analysis revealed the absence of PD-L1 expression (Figure 2f), whereas CD56, thyroid transcription factor-1, and napsin-A were positive. KRAS (Q61H) and TP53 (R213*) mutations were detected, and the TMB was 3.8 mutations/Mb. After 1 year of follow-up, a nodule in the superior segment of the RLL had increased from 3 to 7 mm (Figure 1e,f). Lobectomy of the RLL was performed as a second salvage therapy, and biopsy results confirmed the presence of minimally invasive adenocarcinoma with no PD-L1 expression (Figure 2g–i). No mutations were detected, and the TMB was 1.9 mutations/Mb.
The patient is currently receiving adjuvant therapy consisting of carboplatin and vinorelbine.
DISCUSSION
In this case, we confirmed changes in the histological type of cancer, PD-L1 expression, mutation on molecular testing analyzed by NGS, and TMB after immunotherapy and two consecutive salvage surgeries.
In 2019, a case report demonstrated that treatment with immunotherapy led to a decrease in PD-L1 expression. However, these cases were based on autopsy findings, and the patients died on the ninth and 27th days, respectively. The report suggested that alteration of PD-L1 expression may be associated with ICI therapy failure.16 This is the first case report to demonstrate pathological changes, NGS profiles, and changes in PD-L1 expression following 2-years of treatment with pembrolizumab.
There is an issue of the possibility that the lesion we referred to in our case report as oligo-relapse may be another primary lesion. Since the patient had a highly widespread form of cancer from the beginning, it is difficult to know whether the new lesion was oligo-relapse or another primary lesion. However, considering the initial ipsilateral disseminated multiple lung to lung metastases, it seems more reasonable to think of it as recurrence rather than sequential primary lung cancer.
In general, immunotherapy is typically administered to patients with advanced NSCLC. However, it is difficult to confirm changes during disease progression histologically; therefore, only a few cases have been reported in clinical practice. In this case, salvage surgery was performed twice to treat oligo-recurrence regions and showed different pathology with negative PD-L1 expression.
PD-L1 expression is regulated by various factors.17 Although the exact mechanism by which PD-L1 expression decreased remains uncertain, a significant reduction in tumor size was observed coinciding with the decrease in PD-L1 expression. Therefore, clinicians treating lung cancer patients with immunotherapy must closely monitor changes in cancer and adopt an appropriate treatment strategy to improve treatment efficacy.
For patients with initially unresectable NSCLC who respond favorably to nonsurgical treatment, salvage surgery has shown low postoperative mortality rates and an acceptable complication rate.18 For oligo-recurrent lesions, intensive local therapies such as surgical resection and definitive radiotherapy can be considered.19 As cancer undergoes significant change, salvage surgery can offer histological confirmation, and complete excision can be therapeutic.
The dynamic changes in the histological type, PD-L1 expression, and molecular findings obtained through NGS suggest that reassessing PD-L1 status at different stages of treatment may be crucial in determining the most appropriate therapy for each patient.
In conclusion, this report highlights the dynamic changes that can occur in lung cancer during treatment with ICIs and salvage surgeries, including alterations in PD-L1 expression and histological and molecular findings. Clinicians must remain vigilant to these changes, including the development of resistance during lung cancer treatment, including immunotherapy.
Rebiopsy should be considered to guide treatment decisions for recurrence or disease progression. Salvage surgery may be a viable option for oligo-recurrent NSCLC, providing a means for histological confirmation and complete excision of tumors. Such strategies can help in developing optimal treatment regimens for lung cancer.
AUTHOR CONTRIBUTIONS
Chaeuk Chung: Conceptualization, data curation, writing—original draft. Green Hong, Hee Sun Park, and Min Kyung Yeo: Validation, writing—review and editing. Da-Hye Lee: data collection.
ACKNOWLEDGMENTS
The patient involved in this case report gave his informed consent authorizing use and disclosure of his health information.
FUNDING INFORMATION
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (no. NRF-2017R1A5A2015385, NRF-2022R1A2C2010148) and a grant of the Chungnam national university hospital Fund, 2021 (no. 2021-CF-051).
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.
Open Research
DATA AVAILABILITY STATEMENT
All images used in this article are uploaded as figures.




