Restin protein expression in non-small cell lung cancer
Abstract
Background
Restin is a member of the melanoma-associated antigen (MAGE) superfamily. Its expression has been reported to be up- or downregulated in cancer. Preclinical data suggest it is a tumor suppressor. In this study, we aimed to evaluate restin expression and prognostic value in non-small cell lung cancer (NSCLC).
Methods
Restin expression was analyzed by immunohistochemistry in three tissue microarrays consisting of formalin-fixed/paraffin-embedded NSCLC specimens from 113 patients, represented in triplicate. Restin staining H-score was the result of the staining intensity (0-no, 1-weak, 2-moderate, and 3-strong) multiplied by the percentage of stained tumor cells; it was defined as low if 1–100, moderate if 101–200, and strong if 201–300. Haverage-score was the average H-score in the triplicate. Restin Haverage-scores were tested for correlations with clinical and pathological characteristics and patient outcome.
Results
Restin expression was localized to the cytoplasm, with nuclear enhancement, of 112/113 (99.1%) NSCLCs. Restin Haverage-scores were 0 in 1/113 (0.88%), low in 15/113 (13.3%), moderate in 48/113 (42.5%), and strong in 49/113 (43.4%) NSCLCs. Restin Haverage-scores did not correlate with NSCLC histological subtype, disease stage, recurrence/progression-free, or overall survival.
Conclusion
Restin is moderately to strongly expressed in the majority of NSCLC tumors but its expression has no prognostic value in patients with NSCLC.
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality worldwide.1 Non-small cell lung cancer (NSCLC), which represents 85% of all lung cancers, has a 5-year overall survival of 92% in stage IA1 but this drops to 10% in stage IVA and 0% in stage IVB.2 Considerable progress has been made these last years in the treatment strategies proposed to patients with advanced-stage NSCLC after the discovery of oncogenic abnormalities sensitive to targeted therapies (e.g., EGFR mutations and ALK rearrangements)3 and dysregulations in the immune system sensitive to immune checkpoint inhibitors (e.g., PD-L1 overexpression).4 However, new biomarkers and therapeutic targets are still needed to improve the treatment and ultimately the prognosis of this aggressive cancer.
Restin is a protein belonging to the melanoma-associated antigen (MAGE) superfamily, also known as MAGEH15. The MAGE family is divided into types I and II according to protein expression patterns and functions.5 Type I genes are expressed in cancer but not in normal cells, except in male germ cells, placenta, and developing embryos. Their expression is correlated with poor prognosis. Type II genes are universally expressed in normal tissues but only in some types of cancer cells. Their expression is mainly associated with inhibition of cell proliferation, cell cycle arrest, induction of apoptosis, and cell differentiation. Restin is a type II MAGE gene, identified in 2002 from the HL-60 promyelocytic leukemia cell line induced by all-trans retinoic acid (ATRA).6
In previous studies, restin overexpression in vitro induced apoptosis in HeLa cervical carcinoma7 and in melanoma8 cell lines. It also inhibited proliferation and induced cell cycle arrest in G2/M phase in a cholangiocarcinoma cell line.9 Moreover, restin overexpression inhibited epithelial-mesenchymal transition and tumor metastasis in breast cancer cell lines in vitro and in mouse models.10 Consistently, restin expression was found to be low in breast cancer cell lines as opposed to high in normal breast epithelial cell lines, and higher in low-metastatic than in high-metastatic breast cancer cell lines.10 Finally, restin overexpression inhibited proliferation, migration, and invasion in hepatocarcinoma cell lines.11 These in vitro and in vivo studies suggest that restin is a tumor suppressor but the knowledge about its expression and role in human cancers remains extremely limited. While restin expression has been reported to be downregulated in some cancers, accordingly to its potential tumor suppressor role, its overexpression has also been observed in other cancers.9, 11-15
We therefore aimed to evaluate the protein expression of restin by immunohistochemistry (IHC) in NSCLC tissues and correlate it to pathological and clinical characteristics, including prognosis.
METHODS
Experimental subjects
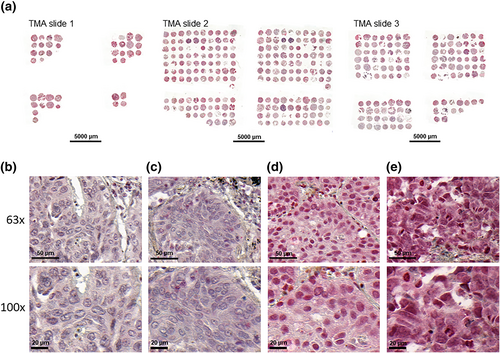
Three tissue microarrays (TMAs) made of NSCLC specimens were prepared from formalin-fixed paraffin-embedded (FFPE) tissue blocks following previously reported methods.16 Pathology blocks were retrieved from the archives of the Department of Pathology at CHU UCL Namur (Godinne Site), Yvoir, and Cliniques Universitaires Saint-Luc, Brussels, Belgium. Tissue blocks were surgically collected between 2011 and 2014 from 113 patients suffering from NSCLC. Histological diagnosis was confirmed on hematoxylin and eosin-stained sections by experienced lung cancer pathologists (D.H. and C.P.S.). The average percentage of tumor cells in all the samples was 60% (95% confidence interval [CI]: 57–63).
In addition to surgery, some patients received chemotherapy or radiotherapy as an adjuvant or palliative treatment, while we excluded patients who received a neoadjuvant treatment in case this influenced restin expression. Treatment was decided on an individualized basis according to disease stage and patient performance status (PS) as per standard of care. All patients were followed thanks to their medical records until death or until data analysis of this manuscript. The study was approved by Institutional Review Boards at Cliniques Universitaires Saint-Luc, Brussels, and CHU UCL Namur (Godinne Site), Yvoir, Belgium (reference 68/2015). The study conforms with The Code of Ethics of the World Medical Association (Declaration of Helsinki), printed in the British Medical Journal (July 18, 1964).
Immunohistochemistry
Staining
After deparaffinization in toluene and rehydration through graded series from methanol to water, 5 μm tissue sections were placed in antigen retrieval reagent (10 mmol/L of citrate buffer [pH 6.0 containing 0.1% triton]) and heated using microwave treatment (3 min 45 s at 800 Watts [100% power] and then 15 min à 160 Watts [20% power]). Endogenous peroxidase activity was blocked by incubation in Bloxall (SP-6000, Vector Laboratories Inc.) for 15 min and 0.3% hydrogen peroxide in 5% horse serum (C09SA, Bio-Rad Laboratories) for 30 min. Tissue sections were incubated at room temperature (RT) for 1 h with a rabbit anti-MAGEH1 antibody (40 μg/mL; PA5-13166, Thermo Fisher Scientific) in a humidified chamber with 5% normal horse serum. Afterwards, the sections were washed in Tris-buffered saline with Tween (TBST) and incubated with a horse anti-Rabbit Poly Horseradish peroxidase (HRP) secondary antibody (MP-7401, Vector Laboratories Inc.) at RT for 40 min. The sections were then washed in TBST and peroxidase activity was revealed using VECTOR NovaRED Peroxidase Substrate Kit (SK-4800, Vector Laboratories Inc.). Sections were counterstained with hematoxylin and mounted in Permount. Negative controls were established by adding nonspecific isotype controls as primary antibodies.
Scoring
The staining intensity of restin in the NSCLC specimens represented in triplicate on the three TMAs was evaluated by two independent observers (DH, VL) as previously described:17 0-no staining, 1-weak, 2-moderate, and 3-strong. The staining intensity was then multiplied by the percentage of stained tumor cells to obtain the final staining H-score (range: 0–300). For each triplicate, we considered the average staining H-score (Haverage-score).
Statistical analysis
Spearman and Pearson's correlation coefficients were used to assess monotonic or linear relationships, respectively. Since the use of time-dependent receiver operating characteristic (ROC) analysis did not identify a better threshold, we used the median of restin staining Haverage-scores to separate low (<200) and high (≥200) levels of restin staining. Welch's two sample t-test was used to compare means of FEV1 and DLCO in patients with low versus high level of restin staining. Pearson's chi-squared test with Yate's continuity correction or Fisher's exact test for count data when at least one expected frequency is ≤5 were used to test the association between restin levels (low vs. high) and the following variables: gender, race, smoking status, age (≤60 or >60 year-old), histology (adenocarcinoma [ADC] vs. squamous cell carcinoma [SCC]), disease stage (according to the seventh edition of the TNM classification), T, N, and M.
Kaplan–Meier method was used to compute overall survival (OS) and recurrence/progression-free survival (R/PFS). OS was calculated from date of diagnosis to date of death, or last date of contact for those alive at the time of analysis in September 2017. R/PFS was calculated from date of diagnosis to date of recurrence/progression, or last date of contact for those who did not recur/progress at the time of analysis. The log-rank test was used to compare OS and PFS according to restin staining level (low vs. high). Three Cox proportional hazards regression models were used to predict OS or R/PFS: (1) a model with restin staining level (low vs. high) only, (2) a model with four covariables: age at diagnosis (≤60 vs. >60 year-old), sex, smoking (current vs. other), and disease stage (I to III vs. IV), and (3) a model with covariables and restin staining level. No evidence of multicolinearity was detected since the variance inflation factors were all <2. Time-dependent ROC curves were estimated using the nearest neighbor estimation method.18 Statistical analyses were performed in R 4.1.1 (R Foundation for Statistical Computing) using ggplot2, survival and survivalROC.
RESULTS
Patient characteristics
A total of 113 patients diagnosed with NSCLC were included in this retrospective study based on the availability of archival surgical pathology specimens. Patient characteristics are summarized in Table 1. Out of the 113 patients, 36 (31.9%) were women and 77 (68.1%) were men. The median age at diagnosis was 65 years-old (interquartile range [IQR]: 60–74). A total of 111 (98.2%) patients were Caucasian, one (0.9%) was Asian, and one (0.9%) was North African. Ten (8.9%) patients had never smoked and 101 (89.4%) patients were current or ex-smokers, while the smoking status was unknown for two (1.8%) patients. Among smokers and ex-smokers, the pack-years information was available for 96 (85.0%) patients and the median pack-years was 38 (IQR: 20–50). At the respiratory functional level, 69 (61.1%) patients had an obstructive pulmonary disease (forced expiratory volume in 1 s [FEV1]/forced vital capacity [FVC] <0.7), the median FEV1 was 84% (IQR: 69–95) and the median diffusing capacity for carbon monoxide (DLCO) was 69% (IQR: 58–78) of the predicted normal value. Seventy-one (62.8%) patients had a lung ADC and 42 (37.2%) had a lung SCC. Based on the seventh edition of lung cancer TNM classification, 107 patients (94.7%) had a nonmetastatic stage (IA to IIIB) and six patients (5.3%) had a stage IV NSCLC.
| Variable | N | Restin Haverage staining score | p-value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | <200 (%) | ≥200 (%) | ||||
| Age, year-old | ≤60 | 28 | 188 | 57 | 50 | 50 | 0.87 |
| >60 | 85 | 188 | 64 | 45.88 | 54.12 | ||
| Gender | Female | 36 | 177 | 61 | 61.11 | 38.89 | 0.06 |
| Male | 77 | 193 | 63 | 40.26 | 59.74 | ||
| Race | Caucasian | 111 | 187 | 63 | 47.75 | 52.25 | 0.50 |
| Other | 2 | 220 | 28 | 0 | 100 | ||
| Smoking status | Current | 42 | 191 | 59 | 47.62 | 52.38 | 0.15 |
| Ex | 59 | 193 | 61 | 40.68 | 59.32 | ||
| Never | 10 | 159 | 72 | 70 | 30 | ||
| Unknown | 2 | 97 | 52 | 100 | 0 | ||
| NSCLC histological subtype | SCC | 42 | 177 | 70 | 52.38 | 47.62 | 0.48 |
| ADC | 71 | 194 | 57 | 43.66 | 56.34 | ||
| Pathological stage | I | 59 | 190 | 62 | 45.76 | 54.24 | 0.86 |
| II | 28 | 186 | 61 | 42.86 | 57.14 | ||
| III | 20 | 177 | 67 | 55 | 45 | ||
| IV | 6 | 212 | 55 | 50 | 50 | ||
| Pathological T | T1 | 0 | 0 | 0 | 0 | 0 | 0.12 |
| T1a | 26 | 200 | 78 | 34.62 | 65.38 | ||
| T1b | 20 | 200 | 48 | 45 | 55 | ||
| T2a | 31 | 189 | 52 | 41.94 | 58.06 | ||
| T2b | 9 | 214 | 55 | 33.33 | 66.67 | ||
| T3 | 24 | 159 | 57 | 70.83 | 29.17 | ||
| T4 | 3 | 140 | 95 | 66.67 | 33.33 | ||
| Pathological N | N0 | 80 | 187 | 62 | 47.5 | 52.5 | 0.68 |
| N1 | 16 | 176 | 70 | 50 | 50 | ||
| N2 | 14 | 211 | 55 | 35.71 | 64.29 | ||
| N3 | 0 | 0 | 0 | 0 | 0 | ||
| Clinical M | M0 | 108 | 187 | 62 | 47.22 | 52.78 | 0.47 |
| M1b | 4 | 221 | 69 | 25 | 75 | ||
| Mx | 1 | 177 | NA | 100 | 0 | ||
- Note: The p-value tested the association between restin staining Haverage-score levels (low [<200] vs. high [≥200]) and each of the variables (Pearson's chi-squared test with Yate's continuity correction or Fisher's exact test for count data when appropriate).
- Abbreviations: ADC, adenocarcinoma; NSCLC, non-small cell lung carcinoma; SCC, squamous cell carcinoma; SD, standard deviation.
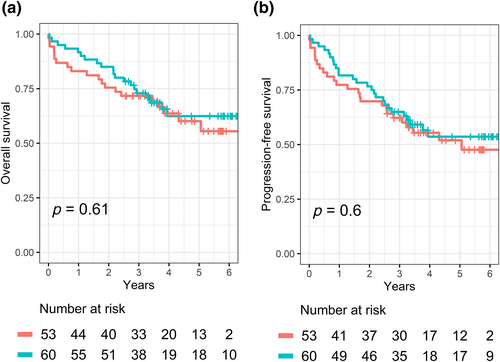
Out of these 113 patients, 81 (78%) were disease-free at the last date known alive, while 32 (28%) suffered from recurrence/progression. The recurrence/progression dates were available for 26 of the 32 patients who recurred/progressed. The OS (95% CI) at 1, 3, and 5 years was 88% (95% CI: 82%–94%), 72% (95% CI: 64%–81%), and 61% (95% CI: 52%–72%), respectively. The median follow-up in surviving patients was 4.1 years. The R/PFS at 1, 3, and 5 years was 80% (95% CI: 73%–87%), 64% (95% CI: 55%–73%), and 53% (95% CI: 44%–64%), respectively.
Restin is expressed in the majority of NSCLCs
Restin was expressed in the cytoplasm, with nuclear enhancement, of 112/113 (99.1%) NSCLC, including 70/71 (98.6%) ADCs and 42/42 (100%) SCCs. The staining pattern was homogeneous within the tumor. Restin staining Haverage-scores (average staining scores of triplicate samples) were 0 in 1/113 (0.9%), 1–100 (low) in 15/113 (13.3%), 101–200 (moderate) in 48/113 (42.5%), and 201–300 (strong) in 49/113 (43.4%) NSCLC samples. Restin expression was low (staining Haverage-score <200) in 53/113 (46.9%) and high (staining Haverage-score ≥200) in 60/113 (53.1%) NSCLC samples. Representative images of NSCLC with absent, low, moderate, and strong restin staining scores are displayed in Figure 1.
Restin staining scores do not correlate with clinicopathological characteristics and prognosis
Samples represented in the TMAs were linked to a clinical database, allowing correlation analyses between various pathological and clinical characteristics and restin staining scores. Restin staining levels (low vs. high) were not associated with gender, race, smoking status, age at the time of NSCLC diagnosis, disease stage (Table 1), or FEV1 (Haverage-score <200 vs. ≥200: 82% ± 20% vs. 82% ± 19%, p = 0.92) and DLCO (Haverage-score <200 vs. ≥200: 72% ± 19% vs. 69% ± 20%, p = 0.47).
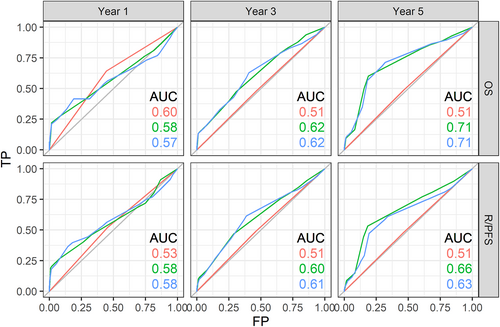
Restin staining levels (low vs. high) were not associated with OS or R/PFS in univariate (Figure 2) or multivariate analyses including age, sex, smoking status, and disease stage as covariables (Table 2). In a complementary analysis, we attempted to measure how well a model predicts death or relapse using time-dependent ROC curves. Models based solely on restin level (≥200 vs. <200) did not predict OS or R/PFS: the AUC at 1, 3, and 5 years were 0.60, 0.51, and 0.51 for OS and 0.53, 0.51, and 0.51 for R/PFS (Figure 3, red lines). Models based on age, sex, smoking status, and disease stage predicted survival and relapse to some extent: the AUC at 1, 3, and 5 years were 0.58, 0.62, and 0.71 for OS and 0.58, 0.60, and 0.66 for R/PFS (Figure 3, green lines). The addition of the restin level in the multivariate model did not improve the prediction of OS or R/PFS: the AUC at 1, 3, and 5 years were 0.57, 0.62, and 0.71 for OS and 0.58, 0.61, and 0.63 for R/PFS (Figure 3, blue lines).
| OS | R/PFS | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age >60 year-old | 0.88 (0.42–1.87) | 0.75 | 0.89 (0.46–1.71) | 0.72 |
| Sex male | 2.70 (1.16–6.25) | 0.02 | 1.80 (0.93–3.52) | 0.08 |
| Current smoking status | 1.23 (0.72–2.31) | 0.53 | 1.29 (0.72–2.31) | 0.39 |
| Stage I–III cancer | 0.22 (0.10–0.88) | 0.01 | 0.30 (0.10–0.88) | 0.03 |
| Haverage restin staining score ≥200 | 0.81 (0.43–1.53) | 0.52 | 0.83 (0.47–1.47) | 0.53 |
- Note: The HR, 95% CI and p-values were computed through Cox proportional hazard regression models.
- Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; R/PFS, recurrence/progression-free survival.
DISCUSSION
Because restin is suggested to be a tumor suppressor based on preclinical data showing antitumoral effects of its overexpression in various cancer cell lines,6, 9-11 in this study we investigated whether restin expression in NSCLC is downregulated and correlated with patient prognosis. Restin expression, analyzed by IHC in three TMAs made of NSCLC specimens from 113 patients, was found in the cytoplasm (with nuclear enhancement) of all NSCLC tissues except those from a single patient, and the staining score was high or moderate in the majority of them. Moreover, restin staining scores did not independently correlate with R/PFS and OS. Therefore, our research hypotheses were not verified in this study.
Knowledge about restin expression and its role in human cancer is extremely limited. While restin expression has been reported to be downregulated in some cancers, accordingly to its potential tumor suppressor role, its overexpression has also been observed in other cancers. To the best of our knowledge, only one study has previously analyzed restin expression in lung cancer but at the mRNA instead of the protein level. This study, which analyzed 52 NSCLC tissues by membrane array, showed that restin mRNA was overexpressed in 36/52 NSCLCs as compared to matched normal lung tissues.15 This is consistent with our study, even though overexpression at the mRNA level does not always translate into overexpression at the protein level because of post-translational modifications. However, while we did not find any difference in restin protein expression between lung ADCs and SCCs, the previous study showed that restin mRNA overexpression was mainly observed in lung ADCs (27/38 of them).15 A small study using microarray gene expression analysis also reported restin mRNA expression in 10/10 (100%) renal cell carcinomas, 1/2 (50%) glioblastomas, and 6/7 normal human tissues.13 Similarly, a study of 100 colorectal cancer tissues from Taiwanese patients analyzed by chip array showed that restin mRNA was significantly overexpressed in 70% colorectal cancers and that it was statistically correlated to tumor depth.12
Recently, however, downregulation of restin expression was observed in four fresh-frozen cholangiocarcinoma tissues, as well as in six FFPE cholangiocarcinoma tissues as compared to matched tumor-adjacent tissue.9 In another study, restin expression, evaluated by quantitative real-time polymerase chain reaction (qRT-PCR), was also downregulated in 85% of 79 hepatocarcinoma tissues as compared to adjacent normal liver tissues, especially in those from patients with recurrence after curative-intent surgery.11 This was confirmed at the protein level by IHC on TMAs including 380 hepatocarcinoma tissues. Cytoplasmic restin expression was negatively correlated with tumor size and microvascular invasion, while nuclear restin expression was negatively correlated with TNM stage, tumor differentiation, and tumor number. Moreover, a correlation was found between restin expression and prognosis, patients with high restin expression having higher overall survival and lower recurrence rates after curative resection.11 Evaluation of restin mRNA and protein expression in 10 biliary tract carcinoma cell lines revealed a significant negative correlation with their sensitivity to gemcitabine.14 Restin expression evaluated by IHC was detected in 3/6 (50%) cell lines sensitive and 4/4 (100%) cell lines resistant to gemcitabine. In a validation cohort, restin protein expression was found in 2/5 (40%) cell lines from patients who presented a tumor response and in 4/4 (100%) cell lines from patients who did not present a tumor response to gemcitabine according to Response Evaluation Criteria in Solid Tumors (RECIST), suggesting that restin expression could be used as a negative predictor of response to gemcitabine.14
In the Human Protein Atlas, restin expression at the mRNA level is reported to be low in 679/994 (68%) and high in 315/994 (32%) NSCLC samples. When considering all NSCLCs in the Human Protein Atlas, restin expression was not correlated to OS. However, in lung adenocarcinoma, OS was better for patients with high restin expression in their tumor (N = 221) than those with low expression (N = 279).19 This is not consistent with our results but, as previously mentioned, we evaluated protein expression and not mRNA expression.
The different results described in the literature may be related to differences in methodologies (especially gene expression versus protein expression analyses and sensitivity or specificity of the antibody used) or truly be related to the specific cancer type. However, data currently available in literature are too limited to be able to draw any conclusion, with only a few cancer types and small sample sizes analyzed retrospectively.
Among the limitations of our study was its retrospective nature, the absence of comparison to normal lung because of the absence of adjacent normal tissues on the TMA biopsies, a small sample size, and a limited number of stage IV NSCLCs. As restin is suspected to have a role in the prevention of epithelial-mesenchymal transition, we may hypothesize that its expression would be lower in NSCLC samples from patients with a stage IV disease, a subpopulation that was underrepresented in our study.
In conclusion, the IHC analysis of 113 NSCLC tissues revealed that restin is moderately to strongly expressed in the majority of these tumors but failed to find a prognostic value of this expression. Evaluation of a larger cohort of NSCLC samples, with a good representation of all the disease stages, is required.
AUTHOR CONTRIBUTIONS
Conception: Frank Aboubakar Nana and Sebahat Ocak. Interpretation or analysis of data: Frank Aboubakar Nana, Virginie Lamberts, Delphine Hoton, Claudia Pop Stanciu, Marylène Lecocq, François M. Carlier, Fabrice Duplaquet, Lionel Pirard, Benoît Bihin, and Sebahat Ocak. Preparation of the manuscript: Frank Aboubakar Nana, Virginie Lamberts, and Sebahat Ocak. Revision for important intellectual content: François M. Carlier, Fabrice Duplaquet, Lionel Pirard, Charles Pilette, and Benoît Bihin. Supervision: Sebahat Ocak.
FUNDING INFORMATION
Frank Aboubakar Nana was supported by Télévie (Fonds National de la Recherche Scientifique [FNRS]) (7.4624.15), Fonds Spécial de Recherche (FSR) (Communauté Française de Belgique), and Fondation Willy and Marcy De Vooght, Belgium. Sebahat Ocak was supported by grants from Fondation Mont-Godinne (FMG-2011-BR-02, FMG-2013-BR-02, FMG-2014-BR-01, FMG-2015-BR-02, FMG-2016-BR-02, FMG-2017-BR-04, FMG-2018-BR-01, and FMG-2019-BR-01), Télévie (FNRS) (7.4588.10F and 7.4624.15), FSR, and Secteurs des Sciences de la Santé, Université catholique de Louvain (UCL), Belgium.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




