A phase II study of a doublet metronomic chemotherapy regimen consisting of oral vinorelbine and capecitabine in Chinese women with HER2-negative metastatic breast cancer
Registration number: NCT05747326 (www.clinicaltrials.gov).
Abstract
Background
This single-arm prospective phase II trial was performed to assess the efficacy and safety of the dual oral metronomic vinorelbine and capecitabine (mNC) regimen in women with HER2-negative metastatic breast cancer (MBC) in China.
Methods
The mNC regimen was administered to the enrolled cases, including oral vinorelbine (VNR) 40 mg three times weekly (on days 1, 3 and 5 every week) and capecitabine (CAP) 500 mg three times a day, until disease progression or intolerable toxicity. The primary endpoint was the 1-year progression-free survival (PFS) rate. Secondary endpoints included objective response rate (ORR), disease control rate (DCR), clinical benefit rate (CBR) and treatment-related adverse events (TRAEs). Stratified factors included treatment lines and hormone receptor (HR) status.
Results
Between June 2018 and March 2023, 29 patients were enrolled into the study. The median follow-up time was 25.4 months (range, 2.0–53.8). In the entire group, the 1-year PFS rate was 54.1%. ORR, DCR and CBR were 31.0%, 96.6% and 62.1%, respectively. The mPFS was 12.5 months (range, 1.1–28.1). Subgroup analysis revealed that ORRs were 29.4% and 33.3% in first- and ≥second-line chemotherapy, respectively. ORRs were 29.2% (7/24) and 40.0% (2/5) for HR-positive MBC and metastatic triple-negative breast cancer (mTNBC), respectively. Grade 3/4 TRAEs were neutropenia (10.3%) and nausea/vomiting (6.9%).
Conclusions
The dual oral mNC regimen showed very good safety features and improved compliance without loss of efficacy in both first- and second-line treatments. The regimen also reached an excellent ORR in the mTNBC subgroup.
INTRODUCTION
Based on worldwide cancer statistics, breast cancer (BC) has emerged as the most frequent malignancy and the leading cause of cancer-related mortality among women.1-3 HER2-negative BC comprises two subtypes, which are distinguished by hormone receptor (HR) status of estrogen and progesterone as biomarkers. HR-positive/HER2-negative BC accounts for 63%–75% of all HER2-negative BC cases.4, 5 After receiving effective treatment, HR-positive/HER2-negative BC cases achieved a notable 5-year overall survival (OS) rate of 85.4%. In contrast, patients with distant metastases exhibited a comparatively lower 5-year OS rate of approximately 30.1%.6 Triple-negative breast cancer (TNBC) accounts for approximately 10%–20% of all BC cases.7, 8 TNBC is characterized by a relatively young onset age, an aggressive biological behavior, a high risk of early recurrence, and a high rate of distant metastasis including visceral and brain metastases.9, 10 The median OS (mOS) of patients with TNBC after metastasis approximated 13.3 months.11 In total, 40% of TNBC cases died during the first five years of diagnosis.12
Chemotherapy remains the standard treatment option for HER2-negative BC. For patients with HR-positive BC and failed prior endocrine therapy or TNBC cases with failed anthracycline or taxanes, or individuals who are intolerant to these therapies, salvage therapy options include monotherapy with capecitabine (CAP), gemcitabine, vinorelbine (VNR), and eribulin, as well as combination therapy with platinum-based chemotherapy regimens.13 Metronomic chemotherapy is a therapeutic approach characterized by the administration of cytotoxic drugs at lower doses, but with high frequency and continuous dosing schedule. A phase I/II VICTOR-1 trial showed the combination of oral metronomic CAP and VNR had high antitumor activity and favorable tolerability in patients with locally advanced or metastatic breast cancer (MBC).14 The VICTOR-2 study was a validation phase II trial based on the VICTOR-1 trial, which assessed the efficacy of a dual chemotherapy regimen comprising VNR and CAP in patients with HER2-negative BC. The VICTOR-2 study revealed a median progression-free survival (PFS) of 6.7 and 7.2 months for first- and ≥second-line treatments, respectively, confirming the efficacy of this treatment approach.15 However, the VICTOR study mainly included Italian patients, thus the long-term effectiveness of oral mNC in Chinese women with HER2-negative MBC remains unclear. Therefore, this single-arm, prospective phase II trial aimed to specifically evaluate the efficacy and safety of oral mNC in Chinese women with HER2-negative MBC.
METHODS
This was a prospective, single-arm, open-label phase II clinical trial performed at the National Cancer Center in China. The trial was registered in the clinical trial registry (www.clinicaltrials.gov, NCT05747326) and had approval from the Ethics Committee of the National Cancer Center in China. Prior to enrollment, the patients were provided with comprehensive information regarding the potential toxicities associated with the treatment protocol, and each patient provided informed consent.
Patients
The main inclusion criteria were: (1) female gender; (2) age between 18 and 75 years; (3) histologically proven HER2-negative MBC, with HER2-negative status determined by fluorescence in situ hybridization (FISH) or immunohistochemistry (IHC) (IHC 0, 1+ or 2+ and/or FISH HER2-negative); (4) at least 1 measurable or evaluable lesion according to RECIST 1.1 criteria; (5) estimated life expectancy ≥3 months; (6) normal heart, liver and kidney functions; (7) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; (8) signed informed consent. Exclusion criteria were: (1) neoadjuvant or adjuvant therapy containing VNR or CAP within one year prior to treatment initiation, (2) participation in other clinical trials involving new drugs within four weeks before enrollment, (3) inflammatory BC, (4) symptomatic visceral disease, (5) second primary malignancy and (6) mental disorder.
Treatment schedule
The enrolled patients meeting the eligibility criteria were administered oral VNR at a dose of 40 mg on days 1, 3 and 5 every week, along with CAP at a dose of 500 mg three times daily (t.i.d.) after meals every 3 weeks. Until disease progression, unacceptable toxicity or consent withdrawal, VNR and CAP were administered continuously without drug-free periods over 21-day cycles. Patients with HR-positive BC were not allowed to receive endocrine therapy during the treatment period.
Upon the first occurrence of grade 3 neutropenia or thrombocytopenia, VNR dose was temporarily adjusted to 30 mg and administered three times a week. In case of complete recovery of symptoms at the initiation of the next cycle, the dose was escalated back to the previous level of 40 mg and administered three times a week. In case grade 3 neutropenia or thrombocytopenia recurred, VNR dose was kept at 30 mg throughout the duration of the study. In case of grade 3 nonhematological toxicity (diarrhea or hand-foot syndrome) or grade 4 hematological toxicity (neutropenia or thrombocytopenia), CAP dose was reduced to 1000 mg daily until the toxicity recovered to grade 1. In case of any other grade 3 nonhematological or grade 4 hematological toxicities, both VNR and CAP treatments were halted until recovery to a lower grade. Patients with grade 4 neutropenia or thrombocytopenia lasting for ≥14 days or required 2 dose adjustments for any drug in the VNR + CAP metronomic chemotherapy regimen were excluded from the study.
Outcomes
The primary endpoint was 1-year PFS. Secondary endpoints included: objective response rate (ORR), which encompassed complete and partial responses (CR + PR); disease control rate (DCR), which encompassed CR + PR + stable disease (SD); clinical benefit rate (CBR), which was defined as CR + PR + SD lasting for ≥6 months; and adverse effects. PFS was defined as the time from treatment initiation to disease progression or cancer-related death. Overall survival (OS) was defined as the time from the date of diagnosis to death or last visit. Metastases to liver, lung, brain and other organs were considered a visceral disease.
Disease evaluation
Baseline assessment comprised a comprehensive medical history, a thorough physical examination, hematological tests (routine blood and urine tests, and liver and renal function tests), evaluation of tumor markers (CEA and CA153), electrocardiography, breast ultrasound, brain and breast magnetic resonance imaging (MRI) scans, and neck, chest, abdomen, pelvic and bone computed tomography (CT) scans. All participants were advised to undergo positron emission tomography-computed tomography (PET/CT). BC staging was performed in accordance with the eighth edition of the TNM staging system.
Tumor responses were evaluated based on Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) criteria before treatment and every two chemotherapy cycles until disease progression, death, consent withdrawal or lost to follow-up. Treatment efficacy assessments were carried out by physical examinations, evaluation of tumor markers (CEA and CA153), and radiological imaging tests (breast ultrasound, brain MRI and neck, chest, abdomen and pelvic CT scans). Routine blood tests (detecting hemoglobin, platelets and neutrophils) and liver and kidney function tests were performed before the next chemotherapy cycle. Toxicity was recorded and evaluated every week during chemotherapy. Adverse events were recorded per the Common Terminology Criteria for Adverse Effects Version 5.0 (CTCAE 5.0).
Statistical analysis
Analyses were performed in the intention-to-treat (ITT) population utilizing a diverse array of statistical methods. Response rates between two groups or subgroups were evaluated by the chi-squared test. The Kaplan–Meier method was employed to generate median PFS (mPFS) and mOS, and differences in survival rates between the groups or subgroups were assessed by the log-rank test. Furthermore, the impact of clinical factors on the survival endpoint in the study population was assessed by the Cox proportional hazards model, with results exhibited as hazard ratios (HRs) and the respective 95% confidence intervals (CIs).
SPSS (version 22.0) was utilized for statistical analysis. Survival curves were graphed with GraphPad Prism (version 8.0). Two-sided p < 0.05 indicated statistical significance.
RESULTS
Patient characteristics
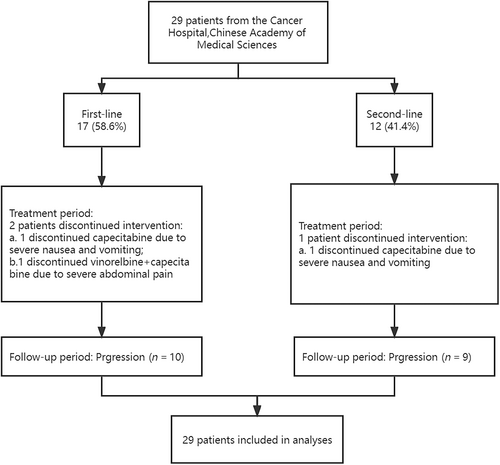
From June 2018 to March 2023, a total of 29 patients with HER2-negative MBC in the Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China) were enrolled. The study flow chart (Figure 1) shows a visual overview of the study design. The baseline characteristics of patients are listed in Table 1. The median age of the entire cohort was 56 years (range, 29–71). Of the 29 patients, 24 (82.8%) had HER2-negative tumors, while 5 (17.2%) were diagnosed with TNBC. Visceral involvement was observed in 21 patients (72.4%). Totally 12 patients (76.9%) had metastases in two or more sites.
| Variable | First-line chemotherapy (Group1) | Second-line chemotherapy (Group2) | Overall |
|---|---|---|---|
| (n = 17) | (n = 12) | (n = 29) | |
| Age (range) | |||
| ≤ 50 years | 7 (41.2) | 4 (33.3) | 11 (37.9) |
| >50 years | 10 (58.8) | 8 (66.7) | 18 (62.1) |
| ECOG score | |||
| 0 | 6 (35.3) | 3 (25.0) | 9 (31.0) |
| 1 | 11 (64.7) | 9 (75.0) | 20 (69.0) |
| Receptor status | |||
| HR-positive | 24 (82.8) | ||
| ER+/PR+ | 1 (5.9) | 0 | 1 (3.4) |
| ER+/PR- | 14 (82.4) | 9 (75.0) | 23 (79.3) |
| Triple-negative | 2 (11.8) | 4 (33.3) | 5 (17.2) |
| HER2 statusa | |||
| HER2− | 6 (35.3) | 5 (41.7) | 11 (37.9) |
| HER2+ | 7 (41.2) | 4 (33.3) | 11 (37.9) |
| HER2++ | 4 (23.5) | 3 (25.0) | 7 (24.1) |
| Visceral metastasis | |||
| Yes | 13 (76.5) | 8 (66.7) | 21 (72.4) |
| No | 4 (23.5) | 4 (33.3) | 8 (27.6) |
| Number of metastatic sites | |||
| 1 | 10 (58.8) | 7 (58.3) | 17 (58.6) |
| 2 | 4 (23.5) | 3 (25.0) | 7 (24.1) |
| >2 | 3 (17.6) | 2 (16.7) | 5 (17.2) |
| Prior adjuvant therapy | |||
| None | 1 (5.9) | 1 (8.3) | 2 (6.9) |
| Chemotherapy | 6 (35.3) | 4 (33.3) | 10 (34.5) |
| Endocrine therapy | 0 | 1 (8.3) | 1 (3.4) |
| Both | 10 (58.8) | 6 (50.0) | 16 (55.2) |
| Prior therapy for metastatic disease | |||
| None | 5 (29.4) | 0 | 5 (17.2) |
| Chemotherapy | 0 | 6 (50.0) | 6 (20.7) |
| Endocrine therapy | 12 (70.6) | 0 | 12 (41.4) |
| Both | 0 | 6 (50.0) | 6 (20.7) |
| Prior treatments | |||
| Only anthracyclines | 0 | 1 (8.3) | 1 (3.4) |
| Only taxanes | 3 (17.6) | 1 (8.3) | 4 (13.8) |
| Both | 14 (82.4) | 10 (83.3) | 24 (82.8) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; HER2, human epithelial growth factor receptor 2; HR, hormone receptor.
- a According to immunohistochemistry.
A total of 17, 11 and one patient underwent mNC as first-, second- and >second-line chemotherapy, respectively. For prior interventions regarding neoadjuvant/adjuvant therapy, 24 patients (82.8%) were administered chemotherapy regimens containing both anthracyclines and taxanes, while one (3.4%) was subjected solely to anthracycline-based chemotherapy, and four (13.8%) underwent only taxane-based chemotherapy. Additionally, five patients (17.2%) had no history of chemotherapy or endocrine therapy for advanced-stage disease. Of the remaining patients, 10 (34.5%) were treated with chemotherapy regimens, one (3.4%) was administered endocrine therapy, and 16 (55.2%) had both chemotherapy and endocrine therapy.
Efficacy
Of the 29 patients administered oral metronomic combined CAP with VNR, nine (31.0%) achieved PR, 19 (65.5%) achieved SD and one (3.4%) achieved PD (Table 2). The CBR, ORR and DCR for the entire cohort were 62.1%, 31.0% and 96.6%, respectively. In the first- and ≥second-line subgroups, CBRs were 64.7% and 58.3%, respectively. ORRs were 29.4% and 33.3% in the first- and ≥second-line chemotherapy subgroups, respectively. DCRs were 94.1% and 100.0% in the first- and ≥second-line subgroups, respectively.
| Variable | First-line (group 1) | Second-line (group 2) | Overall |
|---|---|---|---|
| N = 17 (%) | N = 12 (%) | N = 29 (%) | |
| Best response | |||
| CR | 0 | 0 | 0 |
| PR | 5 (29.4) | 4 (33.3) | 9 (31.0) |
| SD | 11 (64.7) | 8 (66.7) | 19 (65.5) |
| PD | 1 (5.9) | 0 | 1 (3.4) |
| ORR | 5 (29.4) | 4 (33.3) | 9 (31.0) |
| DCR | 16 (94.1) | 12 (100.0) | 28 (96.6) |
| CBR | 11 (64.7) | 7 (58.3) | 18 (62.1) |
- Abbreviations: CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Regarding receptor status, in patients with HR-positive and triple-negative MBC, CBRs were 62.5% (15/24) and 60.0% (3/5), respectively. ORRs were 29.2% (7/24) for HR-positive MBC and 40.0% (2/5) for triple-negative MBC. DCRs were 100.0% (24/24) for HR-positive MBC and 80.0% (4/5) for triple-negative MBC. Based on the metastatic site, patients without visceral involvement demonstrated a CBR of 87.5% (7/8), while those with visceral involvement had a CBR of 52.4% (11/21). Furthermore, patients without visceral involvement had an ORR of 37.5% (3/8) compared to 28.6% (6/21) in patients with visceral involvement. DCRs were 87.5% (7/8) in patients without visceral involvement and 100.0% (21/21) in the visceral involvement group.
Survival
In the final analysis, out of 29 patients initially included in the study, 22 had discontinued treatment, with the majority due to disease progression or death (n = 19, 65.5%), and a smaller proportion due to toxicity (n = 3, 10.3%). During a median follow-up of 25.4 months, ranging from 2.0 to 53.8 months, 19 patients showed signs of disease progression and 8 died.
The 1-year PFS rate was 54.1% and the mPFS was 12.5 months (range, 1.1–28.1). According to stratification factor, 1-year PFS rates were 65.2% in the first-line subgroup and 41.6% in the ≥second-line subgroup, demonstrating no apparent difference between the two subgroups (log rank p = 0.245). The mPFS durations were 15.5 months (range, 1.1–28.1) for the first-line subgroup and 11.2 months (range, 1.2–19.2) for the ≥second-line subgroup (Figure 2).
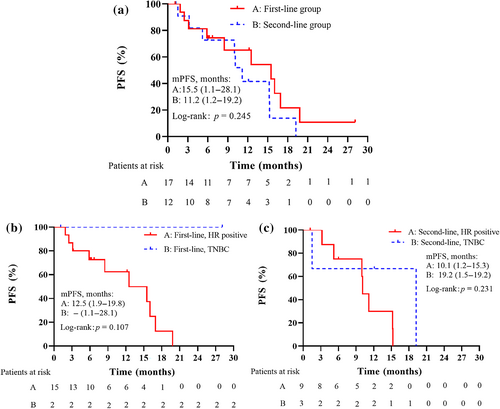
Subgroup analyses of PFS based on patient characteristics are presented in Table 3. The mPFS for patients ≤50 years old was 12.5 months (range, 1.5–19.8) versus 15.2 months (range, 1.1–28.1) in patients over 50 years old. The mPFS for HR-positive patients was 11.2 months (range, 1.2–19.8) versus 19.2 months (range, 1.1–28.1) for TNBC patients. In patients without visceral metastasis, mPFS was 12.5 months, ranging from 1.2 to 28.1 months, while in patients with visceral metastasis, it was 15.2 months, ranging from 1.1 to 19.8 months. The mPFS for patients with one metastatic site was 12.5 months (range, 1.1–28.1), versus 10.1 months (range, 1.2–16.9) in those with two or more metastatic sites. No significant differences in PFS were found based on age, biological subtype, visceral metastasis, and metastatic site (Table 3).
| Variable | N | Median PFS (range, months) | 1-year PFS rate, % | p-value | HR (95% CI) |
|---|---|---|---|---|---|
| Overall | 29 | 12.5 (1.1–28.1) | 54.1 | – | |
| Age (range) | 0.870 | 1.080 (0.428–2.728) | |||
| ≤ 50 years | 11 | 12.5 (1.5–19.8) | 51.9 | ||
| >50 years | 18 | 15.2 (1.1–28.1) | 55.5 | ||
| Hormone receptor | 0.115 | 3.410 (0.743–15.650) | |||
| Positive | 24 | 11.2 (1.2–19.8) | 49.0 | ||
| Negative | 5 | 19.2 (1.1–28.1) | 75.0 | ||
| Visceral metastasis | 0.693 | 1.232 (0.438–3.469) | |||
| Yes | 21 | 15.2 (1.1–19.8) | 53.9 | ||
| No | 8 | 12.5 (1.2–28.1) | 57.1 | ||
| Number of metastatic sites | 0.446 | 0.688 (0.263–1.801) | |||
| 1 | 17 | 12.5 (1.1–28.1) | 60.0 | ||
| ≥2 | 12 | 10.1 (1.2–16.9) | 43.0 |
- Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
Safety profile
In the first-line subgroup, two patients discontinued the treatment due to toxicities. Specifically, one patient discontinued CAP for severe nausea and vomiting, while the other discontinued VNR + CAP due to severe abdominal pain. In the subgroup of patients administered second-line or later treatment, one patient discontinued CAP for severe nausea and vomiting. The adverse events experienced by the patients are summarized in Table 4. The most frequent grade 1–2 adverse event was nausea/vomiting (41.4%), followed by increased levels of AST/ALT (31.0%), leucopenia (24.1%), fatigue (17.2%), neutropenia (17.2%) and anemia (17.2%). The commonest grade 3/4 adverse events were neutropenia (10.3%) and nausea/vomiting (6.9%).
| Variable | First-line (n = 17) | Second-line (n = 12) | Overall (n = 29) | |||
|---|---|---|---|---|---|---|
| Grade 1/2 (%) | Grade 3/4 (%) | Grade 1/2 (%) | Grade 3/4 (%) | Grade 1/2 (%) | Grade 3/4 (%) | |
| Nonhematological | ||||||
| Nausea/vomiting | 10 (58.8) | 1 (5.9) | 2 (16.7) | 1 (8.3) | 12 (41.4) | 2 (6.9) |
| Fatigue | 3 (17.6) | 0 | 2 (16.7) | 0 | 5 (17.2) | 0 |
| Diarrhea | 2 (11.8) | 0 | 0 | 0 | 2 (6.9) | 0 |
| Abdominal pain | 1 (5.9) | 1 (5.9) | 0 | 0 | 1 (3.4) | 1 (3.4) |
| Hand and foot syndrome | 1 (5.9) | 0 | 1 (8.3) | 0 | 2 (6.9) | 0 |
| Mucositis | 1 (5.9) | 0 | 0 | 0 | 1 (3.4) | 0 |
| Increased AST/ALT | 5 (29.4) | 0 | 4 (33.3) | 0 | 9 (31.0) | 0 |
| Hematological | ||||||
| Leucopenia | 6 (35.3) | 1 (5.9) | 1 (8.3) | 0 | 7 (24.1) | 1 (3.4) |
| Neutropenia | 2 (11.8) | 2 (11.8) | 3 (25.0) | 1 (8.3) | 5 (17.2) | 3 (10.3) |
| Thrombocytopenia | 1 (5.9) | 0 | 0 | 0 | 1 (3.4) | 0 |
| Anemia | 4 (23.5) | 0 | 1 (8.3) | 0 | 5 (17.2) | 0 |
DISCUSSION
Breast cancer is an increasingly prevalent malignancy with high incidence in women. Despite the development of medical science, patient survival in MBC remains unsatisfactory. At the same time, different populations have distinct levels of response and tolerance to similar regimens. Thus, developing effective therapies for specific patients with MBC remains a significant challenge requiring close attention. Metronomic chemotherapeutic regimens are considered a possible treatment alternative in patients with MBC.16 To the best of our knowledge, this is the first prospective, open-label, phase 2 study examining the effectiveness of two metronomic agents (VNR and CAP) orally administered in Chinese women with HER-2-negative MBC. It was found that mNC is a promising therapeutic option for Chinese patients with HER2-negative MBC. Furthermore, we conducted subgroup analyses based on treatment-line, age, biological subtype, visceral metastasis and metastatic site, to provide a more comprehensive overview of the efficacy and survival outcomes of the mNC regimen in different subpopulations, offering additional therapeutic data for treating these specific patients.
Endocrine therapy is the standard initial treatment option for advanced HR-positive, HER-2-negative BC cases, except those with visceral crisis or primary endocrine resistance.17 In patients with TNBC, chemotherapy is the main treatment option.18, 19 Nevertheless, resistance invariably emerged among patients with HR-positive, HER-2-negative BC or TNBC, and patients exhibited reduced tolerance to the treatment drugs with increasing number of treatment lines.20 Therefore, determining an effective and low-toxicity treatment regimen is crucial to addressing this issue. Metronomic chemotherapy is an alternative strategy to conventional chemotherapy, which involves administering low doses of chemotherapeutic drugs at frequent intervals, over an extended period of time, without prolonged treatment interruptions.21, 22 This approach is thought to offer several benefits, including antiangiogenic effects, immune modulation and reduced toxicity.23-25 The detailed mechanisms underlying the antitumor effects of metronomic chemotherapy remain incompletely elucidated. Possible mechanisms may involve the inhibition of tumor angiogenesis, an induction of tumor dormancy, and the modulation of immune responses.26-29 A recent study showed that metronomic chemotherapy (5-fluorouracil + VNR) cotargeting TNBC cells and endothelial cells effectively inhibits cell regrowth and migration via FAK/VEGFR2/VEGF downregulation and autophagy/apoptosis activation.30 Owing to the diverse antitumor mechanisms of metronomic chemotherapy, a growing number of studies are currently endeavoring to integrate metronomic chemotherapy with other interventions, including targeted therapy and immunotherapy, among others, for the purpose of treating tumors.31-35 In recent years, metronomic chemotherapy has gained widespread application in a range of cancers, including BC,36, 37 esophageal and gastroesophageal cancer,38 lung cancer,39 pancreatic cancer,40 ovarian cancer,41 and others. The current regimen utilized the oral metronomic chemotherapy approach with a combination of drugs, with the advantages of convenient drug administration and higher pharmacoeconomic efficiency compared with intravenous administration. Considering that numerous metronomic schedules involved the oral route, it could also serve as a therapeutic approach to ensure the continuity of cancer care during the COVID-19 pandemic.42
VNR and CAP are commonly used in HER-2-negative MBC. CAP is an antimetabolite that inhibits DNA synthesis, and VNR is a microtubule inhibitor that strongly suppresses microtubule dynamics and affects endothelial cell function at very low concentrations.17, 43 VNR is one of the representative drugs suitable for metronomic chemotherapy.44, 45 Low-dose continuous administration of CAP is also safe and well-tolerated.26, 46-48 The combination of oral VNR and CAP has been studied in several clinical trials.49-51 A phase II clinical trial revealed that the metronomic combination regimen encompassing VNR, cyclophosphamide and CAP displayed remarkable activity and favorable tolerability in patients with HR-positive MBC. The median time to progression (TTP) was 25.1 months in treatment-naive cases and 11.2 months in pretreated cases.51 Another open-label, international, multicenter, phase II study enrolled 40 MBC cases who were administered oral VNR at 60 mg/m(2) on days 1, 8 and 15, along with CAP at 1000 mg/m(2) bid from day 1 to day 14. The treatment cycles were 3 weeks, and the response rate was 23.5% in the 34 evaluable cases.50 The VICTOR-2 study examined the efficacy of the mNC regimen in patients with HER-2-negative MBC. The VICTOR-2 trial enrolled 80 patients who were administered mVNR 40 mg three times a week along with mCAP 500 mg three times a day on a continuous basis. Of these patients, 65% were HR+ BC and 35% were TNBC. In HR+ patients, the CBR for the combination metronomic chemotherapy regimen was 55.8%, with CBRs of 50.0% and 60.0% in patients receiving first- and second-line treatments, respectively. In TNBC patients, the CBR for the combination metronomic chemotherapy regimen was 35.7%, with CBRs of 38.5% and 33.3% in patients receiving first- and second-line treatments, respectively.15
This study showed a CBR of 62.1% for the entire cohort, with 64.6% and 58.3% in the first- and ≥second-line subgroups, respectively. In patients with HR-positive and triple-negative MBC, CBRs were 62.5% (15/24) and 60.0% (3/5), respectively. The possible reasons for the higher CBR (62.1%) in this study compared with the VICTOR-2 study (48.8%) could be that we included a higher percentage of HR-positive, HER-2-negative cases (82.8%), who might have a better response to chemotherapy, compared with the VICTOR2 study (65.0%). Moreover, this study enrolled a higher proportion of first-line cases (58.6% vs. 43.8% in VICTOR-2 study), who might achieve enhanced clinical benefit than those administered mNC in later lines. In addition, this study showed a relatively longer PFS in TNBC patients with an mPFS of 19.2 months (range, 1.1–28.1). This observation might be affected by the limited number of patients with TNBC included in this study (n = 5). Further multicenter larger sample-size studies are required to confirm this finding.
In this study, subgroup analysis revealed that while the mNC regimen displayed favorable clinical efficacy in the entire group, no obvious difference in mPFS was observed across different subgroups. This observation could be attributed to the uniform impact of the treatment in patients across diverse subgroups. Moreover, the small number of participants in this study may have decreased our ability to identify minor differences. Furthermore, differences in baseline patient characteristics among various subgroups may potentially affect the mPFS. To address this, future studies should consider enlarging the sample size, adopting more refined subgroup stratifications and controlling for potential confounders to better understand the efficacy of the mNC regimen in specific patient subgroups.
In terms of safety, the most frequently reported adverse events were grade 1/2 gastrointestinal and hematological toxicities, which were manageable with dose adjustment, supportive care or prophylactic medications (such as granulocyte colony-stimulating factor or antiemetic drugs). Additionally, 31.0% of patients had increased AST/ALT levels, suggesting that close monitoring of the liver function and timely administration of hepatoprotective medications should be considered during treatment with this regimen. Overall, the doublet metronomic chemotherapeutic regimen with oral VNR and CAP showed relatively low toxicity, which may help increase treatment compliance, reduce adverse reactions and enhance the patient's quality of life.
There were limitations in this study. First, the sample size was relatively small, including only 29 patients with HER2-negative MBC. A larger, randomized controlled trial with a control group is warranted to verify these findings. Second, only five patients with TNBC were enrolled in the current study, which might reduce our ability to draw conclusions about the application of oral metronomic VNR + CAP in this patient population. Future studies with larger sample sizes specifically focusing on patients with TNBC are needed to further assess the effectiveness of the mNC regimen in this subgroup of patients with MBC.
In conclusion, the dual oral mNC regimen showed very good safety features and high compliance maintaining efficacy in both first- and second-line HER2-negative MBC treatments in Chinese women. It also achieved an excellent ORR in the mTNBC subgroup. Due to the limited sample size, the findings should be interpreted with caution, but these interesting conclusions are worth verifying in larger trials.
AUTHOR CONTRIBUTIONS
Concept and design, Yue Chai, Fei Ma, Binghe Xu, and Qiao Li; data collection and assembly, Yue Chai, Jiaxuan Liu, Mingxia Jiang, Maiyue He, Zijing Wang, Fei Ma, Jiayu Wang, Peng Yuan, Yang Luo, Binghe Xu, and Qiao Li; data analysis and interpretation, Yue Chai, Jiaxuan Liu, Mingxia Jiang, Maiyue, He, Zijing Wang, Fei Ma, Jiayu Wang, Peng Yuan and Yang Luo; writing–original draft preparation, Yue Chai, Jiaxuan Liu, Mingxia Jiang, Maiyue He, and Zijing Wang; final approval, Binghe Xu and Qiao Li. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Yujie Chen for critical reading of the manuscript.
FUNDING INFORMATION
This study was funded by the CICAMS-MOTRP2022004, CAMS Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-014.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets developed and analyzed during this study are available from the corresponding author on reasonable request.




