Efficacy and safety of carboplatin and etoposide in older extensive-stage small-cell lung cancer patients with a poor performance status
Abstract
Carboplatin plus etoposide is a standard treatment for older extensive-stage small-cell lung cancer (ES-SCLC) patients with performance status (PS) 2. However, older patients often exhibit poor PS (3, 4), and the treatment effect in them is poorly understood. To determine the therapeutic efficacy and safety of carboplatin plus etoposide therapy for this population, we retrospectively analyzed 63 patients with ES-SCLC with PS ≥2, aged ≥71 years, who had received first-line carboplatin plus etoposide therapy. We compared the treatment efficacy and safety in patients with baseline PS 2 versus those with PS 3–4. In the PS 2 (38 patients) and PS ≥3 (25 patients) groups, the overall response rate was 71.1% and 72.0%, median progression-free survival was 4.6 and 3.1 months, and overall survival was 7.7 and 5.1 months, respectively. PS improved to 0–1 post-treatment in 65.8% and 48.0% of the patients in the PS 2 and PS ≥3 groups, respectively. Patients with PS ≥3 showing improved PS had a progression-free survival of 6.1 months. A higher incidence of grade ≥3 decreased neutrophil counts, febrile neutropenia, and treatment-related death was observed in the PS ≥3 group. The progression-free survival of patients administered prophylactic granulocyte colony-stimulating factor (G-CSF) was 5.2 and 6.1 months in the PS2 and PS ≥3 groups. Overall, carboplatin plus etoposide therapy provided comparable tumor shrinkage, but shorter progression-free and overall survival in older ES-SCLC patients with PS ≥3 than in those with PS 2. Thus, supportive care, such as prophylactic G-CSF administration, may be necessary to ensure safety and survival.
INTRODUCTION
Lung cancer is one of the most frequently diagnosed malignancies and is a leading cause of death worldwide.1 Small-cell lung cancer (SCLC) is a rapidly disseminated cancer that accounts for 14% of all lung cancers. It often presents as extensive-stage (ES) SCLC, which has an extremely poor prognosis, despite having the highest chemotherapy sensitivity among all solid tumors.2, 3
Before the era of immune checkpoint inhibitors (ICIs), platinum plus etoposide therapy was the global standard of care for patients with ES-SCLC for nearly 20 years. The objective response rate (ORR) of ES-SCLC to first-line platinum plus etoposide was 44–78%, the median progression-free survival (PFS) of patients was 4.3–5.7 months, the median overall survival (OS) was 7.5–10.9 months,4 and the 5-year survival rate was only 2.8%.5 As about half of SCLC cases are diagnosed at the age of 70 years or older,6 chemotherapy for patients with SCLC who are older or have a poor PS should be considered carefully. Older age and poor PS have been reported as risk factors for treatment-related mortality during chemotherapy for lung cancer.7
A previous phase III clinical trial (JCOG9702) indicated that carboplatin and etoposide (CE) combination therapy can be safe and effective for ES-SCLC patients with a poor PS (PS ≥3) or for those older than 70 years.8 Based on the results of the JCOG9702 trial, the Japan Lung Cancer Society guidelines recommend CE therapy for young (≤70 years old) ES-SCLC patients with a poor PS (≥3) or older (≥71 years old) ES-SCLC patients with a good PS (≤2) as the standard first-line therapy in Japan.
However, we often encounter ES-SCLC patients with two concurrent risk factors: poor PS (≥3) and advanced age (≥71 years). For these patients, the Japan Lung Cancer Society guidelines do not specify any recommended treatment regimens, such as platinum plus etoposide or those combined with ICIs.9 Furthermore, there are limited data on the therapeutic efficacy and safety of CE therapy in this population. Based on the above, we planned this retrospective study to clarify the therapeutic efficacy and safety of CE therapy for ES-SCLC patients who simultaneously have dual risk factors—advanced age (≥71 years) and poor PS (≥3)—compared with those for patients of a similar age with PS 2.
METHODS
Patients and data collection
We retrospectively collected data from the medical records of patients with ES-SCLC at Shizuoka Cancer Center between December 2002 and March 2018. This study involved patients diagnosed with ES-SCLC with PS ≥2, aged ≥71 years, and who had received at least one cycle of CE therapy as the first-line chemotherapy. Data of the following characteristics were collected for analysis: age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), smoking history, clinical stage at diagnosis according to the Union for International Cancer Control Tumor Node Metastasis classification, 8th edition (UICC-TNM), and baseline metastatic sites at diagnosis.
This study was conducted in accordance with the tenets of the Declaration of Helsinki. We provided the patients an opportunity to opt out of the study. This study was approved by the Institutional Review Board of Shizuoka Cancer Center (IRB number: J2019-30).
Treatment
All patients received at least one cycle of CE therapy. Etoposide dosage was calculated based on the body surface area and carboplatin dosage was calculated based on the area under the curve (AUC) using the Calvert formula.10 However, the actual dosages for individual patients were left to the discretion of the treating physician. The dosing schedule was fixed: etoposide was administered intravenously on days 1–3 of each cycle and carboplatin was administered intravenously on day 1 of each cycle. The treatment regimens were repeated in the clinical setting for up to six cycles, at the discretion of the treating physicians, depending on disease progression, unacceptable adverse events (AEs), or patient requests for discontinuation.
Treatment evaluation
Baseline lesions were evaluated using simple chest radiography, whole-body computed tomography (CT), positron emission tomography-CT, bone scintigraphy, brain CT, or magnetic resonance imaging (MRI). To assess treatment efficacy, patients were evaluated using whole-body CT and/or brain MRI as needed after at least one cycle of CE therapy. Tumor response was evaluated according to the Response Evaluation Criteria for Solid Tumors version 1.1.11 PFS was defined as the time from the start of CE therapy to disease progression, death, or the last follow-up visit. OS was defined as the time from the start of CE therapy to death or the last follow-up visit.
Toxicity assessment
Treatment-related AEs were evaluated according to the Common Terminology Criteria for Adverse Events version 5.0. We also collected data on the history of prophylactic recombinant human granulocyte colony-stimulating factor (G-CSF) administration during the first cycle of CE treatment.
Statistical analysis
Differences between groups were tested using Pearson's chi-square test or Fisher's exact test for categorical variables and an unpaired Student's t-test for continuous variables. PFS and OS were estimated using the Kaplan–Meier method and expressed as median and two-sided 95% confidence interval (95% CI). For PFS and OS analyses, the data of patients who survived without disease progression were censored at the date of the follow-up visit. All p values were two-sided, and results with p < 0.05 were considered statistically significant. All statistical analyses were performed using the statistical software JMP®13.2.1 (SAS Institute Inc.).
RESULTS
Patient characteristics
This study involved 63 patients with ES-SCLC who received CE therapy as the first-line therapy between December 2002 and March 2018. The baseline patient characteristics are shown in Table 1. The median age of the patients was 76 (range 71–86) years—24 (38.1%) patients were 75–79 years and 17 (27%) were ≥80 years of age. Thirty-eight (60.3%) patients with ECOG-PS 2, 24 (38.1%) with PS 3, and one (1.6%) with PS 4 were included. Male patients accounted for 79.4%; 96.9% of the patients had a smoking history and 92.1% were in clinical stage IV (UICC-TNM, 8th edition). At the baseline, metastases were observed in the brain in 18 patients (28.6%), lungs in 20 (31.7%), liver in 18 (28.6%), adrenal glands in 16 (25.4%), and the bone in 19 (30.2%); malignant pleural effusion was present in 18 patients (28.6%). Among 18 patients with brain metastases at the baseline, nine (14.3%) received cranial radiation therapy before chemotherapy. None of the baseline characteristics of the patients differed significantly between the PS 2 (N = 38) and PS ≥3 groups (N = 25).
| Characteristics | Overall N = 63 | ECOG-PS 2 N = 38 | ECOG-PS ≥3 N = 25 | p value |
|---|---|---|---|---|
| Age at diagnosis, median, years (range) | 76 years (71–86) | 76 years (71–86) | 75 years (71–84) | 0.578 |
| 71–74 years | 22 (34.9) | 12 (31.6) | 10 (40.0) | |
| 75–79 years | 24 (38.1) | 14 (36.8) | 10 (40.0) | |
| ≥80 years | 17 (27.0) | 12 (31.6) | 5 (20.0) | |
| Gender, n (%) | 0.592 | |||
| Male | 50 (79.4) | 31 (81.6) | 19 (76.0) | |
| Female | 13 (20.6) | 7 (18.4) | 6 (24.0) | |
| ECOG-PS, n (%) | ||||
| 2 | 38 (60.3) | 38 | 0 | |
| 3 | 24 (38.1) | 0 | 24 | |
| 4 | 1 (1.6) | 0 | 1 | |
| Smoking status, n (%) | 0.773 | |||
| Current | 35 (55.6) | 20 (52.6) | 15 (60.0) | |
| Former | 26 (41.3) | 17 (44.7) | 9 (36.0) | |
| Never | 2 (3.2) | 1 (2.6) | 1 (4.0) | |
| Clinical stage (UICC-TNM 8th), n (%) | 0.605 | |||
| III | 5 (7.9) | 4 (10.5) | 1 (4.0) | |
| IVA | 25 (39.7) | 14 (36.8) | 11 (44.0) | |
| IVB | 33 (52.4) | 20 (52.6) | 13 (52.0) | |
| Metastases at baseline, n (%) | ||||
| Brain | 18 (28.6) | 11 (28.9) | 7 (28.0) | 0.935 |
| Lung | 20 (31.7) | 14 (36.8) | 6 (24.0) | 0.284 |
| Liver | 18 (28.6) | 10 (26.3) | 8 (32.0) | 0.625 |
| Adrenal | 16 (25.4) | 8 (21.1) | 8 (32.0) | 0.329 |
| Bone | 19 (30.2) | 12 (31.6) | 7 (28.0) | 0.762 |
| Malignant pleural effusion | 18 (28.6) | 8 (21.1) | 10 (40.0) | 0.103 |
| Radiation therapy for baseline BM before systemic chemotherapy, n (%) | 9 (14.3) | 4 (10.5) | 5 (20.0) |
- Abbreviations: ECOG-PS, Eastern Cooperative Oncology Groups Performance Status; UICC-TNM 8th, Union International for Cancer Control-TNM 8th edition.
Details of treatments
Details of the actual treatments are summarized in Table 2. The carboplatin dose for individual patients was in the range of AUC of 4–5 and the etoposide dose was 20–100 mg/m2; the doses were adjusted and administered at the discretion of the treating physician. Fifty-two patients (82.5%) received carboplatin, with an AUC of 5, and etoposide at 80 mg/m2. The median number of cycles of CE therapy was four (range 1–6), and 40 patients (63.5%) could receive four or more cycles. Thirty patients (47.6%) received G-CSF from the first cycle of CE therapy, four (6.3%) received pegylated G-CSF (pegfilgrastim), and 26 (41.3%) received short-acting G-CSF (filgrastim or lenograstim). According to the PS, 16 patients (42.1%) in the PS 2 group (N = 38) and 14 patients (56.0%) in the PS ≥3 group (N = 25) received G-CSF during the first CE therapy cycle. These treatment details were also not significantly different between groups.
| Details of treatment | Overall N = 63 | ECOG-PS 2 N = 38 | ECOG-PS ≥3 N = 25 | p value |
|---|---|---|---|---|
| Chemotherapy dosage, n (%) | 0.813 | |||
| CBDCA (AUC 5) + ETP (80 mg/m2) | 52 (82.5) | 30 (78.9) | 22 (88.0) | |
| CBDCA (AUC 5) + ETP (100 mg/m2) | 2 (3.2) | 2 (5.3) | 0 | |
| CBDCA (AUC 5) + ETP (60 mg/m2) | 2 (3.2) | 1 (2.6) | 1 (4.0) | |
| CBDCA (AUC 5) + ETP (40 mg/m2) | 3 (4.8) | 2 (5.3) | 1 (4.0) | |
| CBDCA (AUC 5) + ETP (20 mg/m2) | 1 (1.6) | 1 (2.6) | 0 | |
| CBDCA (AUC 4) + ETP (80 mg/m2) | 1 (1.6) | 1 (2.6) | 0 | |
| CBDCA (AUC 4) + ETP (60 mg/m2) | 2 (3.2) | 1 (2.6) | 1 (4.0) | |
| Number of chemotherapy cycles, n (%) | 0.169 | |||
| Median (range) | 4 (1–6) | 4 (1–6) | 3 (1–6) | |
| 1 | 8 (12.7) | 3 (7.9) | 5 (20.0) | |
| 2 | 11 (17.5) | 4 (10.5) | 7 (28.0) | |
| 3 | 4 (6.3) | 2 (5.3) | 2 (8.0) | |
| 4 | 37 (58.7) | 27 (71.1) | 10 (40.0) | |
| 5 | 1 (1.6) | 1 (2.6) | 0 | |
| 6 | 2 (3.2) | 1 (2.6) | 1 (4.0) | |
| G-CSF administration during one cycle of CE, n (%) | 30 (47.6) | 16 (42.1) | 14 (56.0) | 0.280 |
| Pegylated G-CSF | 4 (6.3) | 2 (5.3) | 2 (8.0) | |
| Short-acting G-CSF | 26 (41.3) | 14 (36.8) | 12 (48.0) |
- Abbreviations: AUC, area under the curve; CBDCA, carboplatin; CE, carboplatin plus etoposide; ECOG-PS, Eastern Cooperative Oncology Groups Performance Status; ETP, etoposide; G-CSF, granulocyte colony-stimulating factor.
Efficacy
The treatment efficacy results are summarized in Table 3. Of the 63 patients, 45 showed a partial response, eight had stable disease, and 10 presented disease progression. The ORR was 71.4% (95% CI 59.3%–81.1%). The ORRs for the PS 2 and PS ≥3 groups were 71.1% and 72.0%, respectively, with no significant difference between groups (p = 0.558). The PFS and OS of all patients are shown in Figure 1. Among all patients, PFS events occurred in 57 patients, with a median PFS of 4.4 months (95% CI 3.6–5.2) (Figure 1a). The median PFS according to PS was 4.6 months (95% CI 3.8–5.6 months) for patients in the PS 2 group and 3.1 months (95% CI 1.9–4.7 months) for patients in the PS ≥3 group (Figure 2a). Although the PFS in the PS 2 group was longer than that in the PS ≥3 group, there was no significant difference between the groups (log-rank p = 0.946) (Table 3).
| Treatment outcome | Overall N = 63 | ECOG-PS 2 N = 38 | ECOG-PS ≥3 N = 25 | p value |
|---|---|---|---|---|
| Best response, n (%) | 0.558 | |||
| Complete response | 0 | 0 | 0 | |
| Partial response | 45 (71.4) | 27 (71.1) | 18 (72.0) | |
| Stable disease | 8 (12.7) | 6 (15.8) | 2 (8.0) | |
| Progressive disease | 10 (15.9) | 5 (13.2) | 5 (20.0) | |
| ORR (%) | 71.4% | 71.1% | 72.0% | |
| Median PFS, months (95% CI) | 4.4 (3.6–5.2) | 4.6 (3.8–5.6) | 3.1 (1.9–4.7) | 0.946 |
| Median OS, months (95% CI) | 6.5 (5.6–8.1) | 7.7 (5.8–9.7) | 5.1 (3.6–6.5) | 0.421 |
| Improvement to PS 1 or less after treatment of one cycle of CE, n (%) | 0.234 | |||
| Yes | 36 (57.1) | 24 (63.2) | 12 (48.0) | |
| Noa | 27 (42.9) | 14 (36.8) | 13 (52.0) | |
| Toxicity during first cycle of CE, n (%) | ||||
| Neutropenia of grade 3 or higher | 40 (63.5) | 22 (57.9) | 18 (72.0) | 0.255 |
| FN | 13 (20.6) | 6 (15.8) | 7 (28.0) | 0.241 |
| Treatment-related deaths associated with CEb, n (%) | 4 (6.3) | 2 (5.3) | 2 (8.0) | 0.663 |
| Modification of dosing plan due to side effects, n (%) | 24 (38.1) | 11 (28.9) | 13 (52.0) | 0.065 |
| Dose reduction | 14 (22.2) | 8 (21.1) | 6 (24.0) | |
| Discontinuation of administrationc | 10 (15.9) | 3 (7.9) | 7 (28.0) |
- Abbreviations: 95% CI, 95% confidence interval; CE, carboplatin plus etoposide; ECOG-PS, Eastern Cooperative Oncology Groups Performance Status; FN, febrile neutropenia; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
- a Including two deaths during one cycle of CE in the PS 2 group.
- b The causes of TRD were FN in two cases, aspiration in one case, and gastrointestinal bleeding in one case.
- c Including four treatment-related death cases during CE therapy.
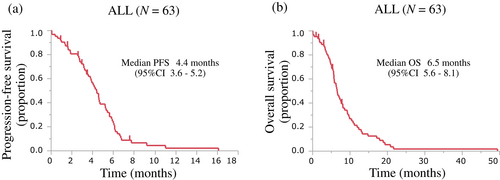
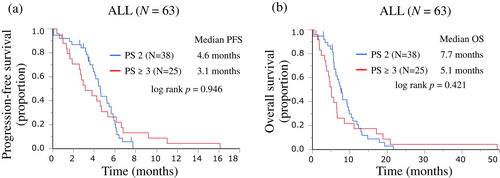
Among all patients, OS events occurred in 58 patients, with a median OS of 6.5 months (95% CI 5.6–8.1) (Figure 1b). The median OS according to the PS group was 7.7 months (95% CI 5.8–9.7 months) for patients in the PS 2 group and 5.1 months (95% CI 3.6–6.5 months) for patients in the PS ≥3 group (Figure 2b). The OS was not significantly different between the groups (log-rank p = 0.421), but there was a trend for a shorter OS in the PS ≥3 group (Table 3).
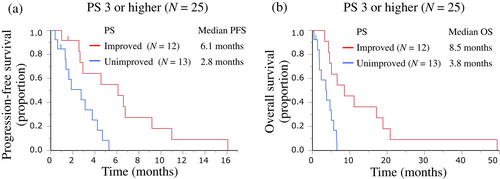
After the first cycle of CE therapy, 36 (57.1%) of the 63 patients improved to PS 1 or less, but the PS did not improve in 27 (42.9%) patients, including four patients who died during the first cycle of CE therapy (Table 3). PS improved from the baseline after the first cycle of CE therapy in 25 (65.8%) of the 38 patients in the PS 2 group and in 12 (48.0%) of the 25 patients in the PS ≥3 group. The PS improvement rate tended to be slightly lower in the PS ≥3 group, but the difference between the groups was not significant (p = 0.234). In the PS ≥3 group, PFS and OS in these 12 PS-improved patients and 13 PS-unimproved patients after the first cycle of CE therapy were 6.1 months (95% CI 2.6–9.2 months) and 2.8 months (95% CI 1.9–4.7 months), and 8.5 months (95% CI 4.3–18.9 months) and 3.8 months (95% CI 1.4–5.7 months), respectively (Figure 3a,b).
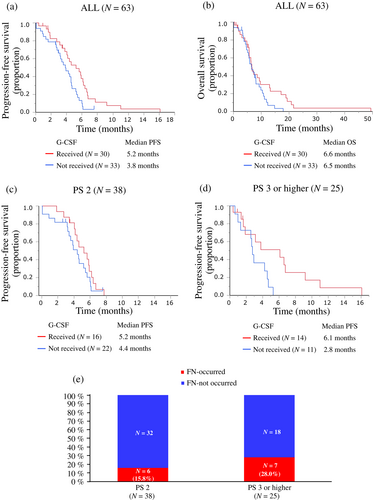
Among the 63 patients, PFS and OS in the 30 patients who received prophylactic G-CSF from the first cycle of CE therapy and those in the 33 patients who did not receive G-CSF were 5.2 months (95% CI 3.8–6.2 months) and 3.8 months (95% CI 2.9–4.6 months), and 6.6 months (95% CI 5.6–9.7 months) and 6.5 months (95% CI 4.7–8.1 months), respectively (Figure 4a,b). In the baseline PS 2 group (N = 38), PFS was 5.2 months (95% CI 4.0–6.2 months) and 4.4 months (95% CI 3.2–5.4 months) in 16 patients with G-CSF treatment and in 22 patients without G-CSF treatment, respectively (Figure 4c). In the PS ≥3 group (N = 25), PFS was 6.1 months (95% CI 1.6–9.2 months) and 2.8 (95% CI 1.0–4.6 months) in 14 patients with and 11 patients without G-CSF treatment, respectively (Figure 4d).
Toxicity assessment and treatment modification
The AEs are summarized in Table 4. All 63 patients experienced at least one adverse event; of these, 51 (81.0%) patients experienced grade 3 or higher AEs. The most common AEs were hematological toxicities, such as decreased neutrophil counts, decreased white blood cells, decreased platelet counts, and anemia. Forty (63.5%) patients developed grade ≥3 decreased neutrophil counts and 13 (20.6%) experienced febrile neutropenia (FN). According to the baseline PS, CE therapy resulted in grade ≥3 decreased white blood cells, decreased neutrophil counts, decreased platelet counts, and anemia in the PS 2 vs. PS ≥3 groups: 20 (52.6%) vs. 17 (68.0%), 24 (63.2%) vs. 19 (76.0%), eight (21.1%) vs. eight (32.0%), and 10 (26.3%) vs. four (16.0%), respectively. Furthermore, CE therapy resulted in FN in six (15.8%) and seven (28.0%) patients from the PS 2 and PS ≥3 groups, respectively (Table 3 and Figure 4e). Except for anemia, the incidence of grade ≥3 hematologic toxicity and FN tended to be higher in the PS ≥3 group than in the PS 2 group. Other grade ≥3 AEs observed were elevated aspartate aminotransferase/alanine aminotransferase, nausea, decreased appetite, oral mucositis, thromboembolic events, aspiration, and gastrointestinal bleeding. CE treatment-related deaths (TRD) accounted for four (6.3%) patients, two each in the PS 2 (5.3%) and PS ≥3 (8.0%) groups, respectively (Table 3). The causes of TRD were FN in two patients, aspiration in one patient, and gastrointestinal bleeding in one patient. The two patients with FN leading to TRD were in the PS ≥3 group.
| Event | Overall N = 63 | ECOG-PS 2 N = 38 | ECOG-PS ≥ 3 N = 25 | |||
|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any adverse event, n (%) | 63 (100) | 51 (81.0) | 38 (100) | 30 (79.0) | 25 (100) | 21 (84.0) |
| White blood cell decreased | 54 (85.7) | 37 (58.7) | 31 (81.6) | 20 (52.6) | 23 (92.0) | 17 (68.0) |
| Neutrophil count decreased | 54 (85.7) | 43 (68.3) | 31 (81.6) | 24 (63.2) | 23 (92.0) | 19 (76.0) |
| Platelet count decreased | 52 (82.5) | 16 (25.4) | 32 (84.2) | 8 (21.1) | 20 (80.0) | 8 (32.0) |
| Anemia | 58 (92.1) | 14 (22.2) | 34 (89.5) | 10 (26.3) | 24 (96.0) | 4 (16.0) |
| Febrile neutropeniaa | 13 (20.6) | 13 (20.6) | 6 (15.8) | 6 (15.8) | 7 (28.0) | 7 (28.0) |
| AST/ALT increased | 10 (15.9) | 2 (3.2) | 7 (18.4) | 0 | 3 (12.0) | 2 (8.0) |
| Creatinine increased | 4 (6.3) | 0 | 3 (7.9) | 0 | 1 (4.0) | 0 |
| Nausea | 8 (12.7) | 1 (1.6) | 3 (7.9) | 0 | 5 (20.0) | 1 (4.0) |
| Decreased appetite | 22 (34.9) | 3 (4.8) | 13 (34.2) | 1 (2.6) | 9 (36.0) | 2 (8.0) |
| Malaise | 6 (9.5) | 0 | 2 (5.3) | 0 | 4 (16.0) | 0 |
| Fatigue | 3 (4.8) | 0 | 1 (2.6) | 0 | 2 (8.0) | 0 |
| Cough | 6 (9.5) | 0 | 5 (13.2) | 0 | 1 (4.0) | 0 |
| Hiccups | 6 (9.5) | 0 | 4 (10.5) | 0 | 2 (8.0) | 0 |
| Mucositis oral | 5 (7.9) | 1 (1.6) | 2 (5.3) | 1 (2.6) | 3 (12.0) | 0 |
| Constipation | 21 (33.3) | 0 | 14 (36.8) | 0 | 7 (28.0) | 0 |
| Diarrhea | 5 (7.9) | 0 | 2 (5.3) | 0 | 3 (12.0) | 0 |
| Rash | 6 (9.5) | 0 | 5 (13.2) | 0 | 1 (4.0) | 0 |
| Vasculitis | 1 (1.6) | 0 | 0 | 0 | 1 (4.0) | 0 |
| Thromboembolic event | 1 (1.6) | 1 (1.6) | 1 (2.6) | 1 (2.6) | 0 | 0 |
| Aspirationb | 1 (1.6) | 1 (1.6) | 1 (2.6) | 1 (2.6) | 0 | 0 |
| Gastrointestinal hemorrhagec | 2 (3.2) | 1 (1.6) | 1 (2.6) | 1 (2.6) | 1 (4.0) | 0 |
| Atrial fibrillation | 1 (1.6) | 0 | 0 | 0 | 1 (4.0) | 0 |
| Pneumothorax | 1 (1.6) | 0 | 1 (2.6) | 0 | 0 | 0 |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CE, carboplatin plus etoposide; ECOG-PS, Eastern Cooperative Oncology Groups Performance Status.
- a Including two treatment-related death cases in the PS ≥3 group.
- b This event was one of the treatment-related deaths in the PS 2 group.
- c Including one treatment-related death case in the PS 2 group.
In total, 207 cycles of CE were administered to 63 patients during the treatment period. Of these, 24 (38.1%) patients required a change in the dosing plan after the second cycle because of toxicity. Carboplatin or etoposide dose reduction was required in 15 (23.8%) patients. Additionally, treatment was discontinued owing to AEs in nine patients (14.3%), including four patients who experienced TRD. In the PS 2 vs. PS ≥3 groups, changes in dosing plan, dose reductions, and treatment discontinuation after the first cycle because of toxicity occurred in 11 (28.9%) vs. 13 (52.0%), eight (21.1%) vs. six (24.0%), and three (7.9%) vs. seven (28.0%) patients, respectively (Table 3). These changes in dosing plan owing to toxicity tended to be more common in the PS ≥3 group, although there was no significant difference between groups.
Post-treatment outcomes after disease progression
Post-treatment outcomes after disease progression are summarized in Table 5. Twenty-two (34.9%) patients received second-line chemotherapy: 14 (36.8%) in the PS 2 group and eight (32.0%) in the PS ≥3 group. Of these, eight patients received platinum combination chemotherapy, 10 received amrubicin monotherapy, three received irinotecan monotherapy, and one received topotecan monotherapy. Furthermore, nine patients (14.3%) received third-line therapy and one patient received fourth-line therapy. Brain metastases occurred in 22 (34.9%) patients, 16 (42.1%) in the PS2 group, and six (24.0%) in the PS ≥3 group. Recurrence of brain metastases involved new lesions only in 17 patients, worsening of existing lesions in three patients, and both new and worsening of existing lesions in two patients. Ten patients received intracranial radiotherapy for brain metastases, of whom eight received whole-brain radiation therapy and two received stereotactic radiosurgery/stereotactic radiation therapy.
| Post-treatment | Overall N = 63 | ECOG-PS 2 N = 38 | ECOG-PS ≥3 N = 25 |
|---|---|---|---|
| Chemotherapy, n (%) | |||
| Received second-line chemotherapy | 22 (34.9) | 14 (36.8) | 8 (32.0) |
| Platinum-based doublet | 8 | 5 | 3 |
| Single agents | 14 | 9 | 5 |
| Amrubicin | 10 | 6 | 4 |
| Irinotecan | 3 | 2 | 1 |
| Topotecan | 1 | 1 | 0 |
| Received third-line chemotherapy | 6 (9.5) | 4 (10.5) | 2 (8.0) |
| Received fourth-line chemotherapy | 1 (1.6) | 1 (2.6) | 0 |
| BM recurrences, n (%) | 22 (34.9) | 16 (42.1) | 6 (24.0) |
| New lesions | 17 | 12 | 5 |
| Exacerbation of existing lesions | 3 | 2 | 1 |
| New + exacerbation | 2 | 2 | 0 |
| Received radiation therapy for BM recurrences, n (%) | 10 (14.3) | 7 (18.4) | 3 (12.0) |
| WBRT | 8 | 6 | 2 |
| SRT/SRS | 2 | 1 | 1 |
- Abbreviations: BM, brain metastases; ECOG-PS, Eastern Cooperative Oncology Groups Performance Status; SRS, stereotactic radiosurgery; SRT, stereotactic radiation therapy; WBRT, whole brain radiotherapy.
DISCUSSION
To our knowledge, no retrospective study has evaluated the efficacy and safety of CE therapy in chemotherapy-naive ES-SCLC patients with dual risk factors of advanced age and poor PS, compared with those in patients with PS 2. In patients with ES-SCLC aged ≥71 years and with PS ≥3, the first-line CE therapy yielded an ORR of 72.0%, median PFS of 3.1 months, and median OS of 5.1 months. PFS and OS were shorter in patients with PS ≥3 than in patients with PS 2, but there were no significant differences between the groups. We also demonstrated that in patients with these dual risk factors, patients whose PS had improved to 0 or 1 after the first cycle of CE therapy and those who had received prophylactic G-CSF during the first cycle might obtain therapeutic benefits comparable to those in patients with PS 2.
In a previous study (JCOG9702), the ORR was 73%, median PFS was 5.2 months, and median OS was 10.6 months in patients with ES-SCLC aged <70 years and with a poor PS (PS ≥3) or patients aged ≥70 years and with PS 0–1 receiving CE therapy.8 A comparison of the results of the JCOG9702 trial and baseline PS2 group in this study revealed that the patients with dual risk factors in our study tended to have a shorter PFS and OS with CE therapy, whereas the ORR was similar. There may be several reasons why the similar tumor shrinkage did not lead to similar prolongation of PFS and OS in these populations. First, in our study, patients with PS ≥3 required dose reduction or treatment discontinuation more frequently than those with PS 2 because of AEs (Table 3). Patients with PS ≥3 had a higher rate of decreased neutrophil counts (57.9%) and a higher incidence of FN (28.0%) than in those with PS 2 (Figure 4e). In our study, the G-CSF used was primarily short-acting G-CSF (87%) and pegylated G-CSF was used less frequently. Furthermore, primary prevention of myelosuppression with G-CSF during the first cycle of CE therapy was performed in only half of the patients with PS ≥3 (Table 3). In clinical practice, short-acting G-CSF is often used from the onset of decreased neutrophil counts, suggesting that in many cases it is used after the risk of developing FN is increased. Therefore, to prevent the development of FN more effectively, pegylated G-CSF should probably be used before the onset of decreased neutrophil counts. Furthermore, prevention of decreased neutrophil counts by G-CSF administration could prevent dose reduction or treatment discontinuation and maintain the dose intensity of CE therapy. Our data showed a trend toward longer PFS in the G-CSF received group compared to that in the G-CSF not received group (Figure 4a,c,d). Therefore, for frail patients with ES-SCLC in particular, more aggressive administration of G-CSF as a supportive therapy should be considered in clinical settings, as this may contribute to a longer response duration and longer life expectancy with CE therapy.
Second, a relatively small proportion of patients in this study received second-line treatment after disease progression compared with that in clinical trials. JCOG9702 reported that second-line treatment after CE therapy was administered to 62% of patients.8 However, even though more than 60% of the patients in our study received four or more cycles of CE therapy, only 34.9% of the 63 patients received second-line therapy. In the PS ≥3 group, only eight (32%) of the 25 patients received second-line therapy. It has been reported that post-progression survival after primary chemotherapy has a major effect on OS in older patients with ES-SCLC.12 Therefore, the shorter OS observed in this study may be partially due to the low percentage of patients who received second-line chemotherapy after disease progression. In the remaining 17 patients from the PS ≥3 group who did not receive second-line therapy, the reasons for not receiving second-line therapy were as follows: PS did not improve after initial therapy (N = 8), patient decision (N = 3), complications (N = 3), TRD caused by FN during initial therapy (N = 2), and impaired consciousness caused by symptomatic brain metastases (N = 1). Given these reasons, in ES-SCLC patients with the dual risk factors of advanced age (>70 years) and PS ≥3, it may be difficult to administer second-line treatment after CE therapy at a higher rate than that in this study.
In our study population, even in ES-SCLC patients with dual risk factors, the ORR of CE therapy was not inferior to that of the patient population without these risk factors in the historical data, and PS was improved in more than half of the patients after treatment. In general, the survival of patients with ES-SCLC who did not receive aggressive therapy has been reported to be 2–4 months,13 but in our study, the PFS was 6.1 months and OS was 8.5 months in the PS ≥3 patients with improved post-CE therapy PS (Figure 3). These results suggest that even a population with a poor PS at the baseline (PS ≥3) would have a better course than the populations in the historical data of best supportive care if the PS improved to 1 or better after CE therapy. In other words, CE therapy may reduce at least one poor prognostic factor, that is, poor PS, in this population.
The IMpower133 and CASPIAN trials have recently demonstrated improved survival with the addition of a programmed death ligand 1 (PD-L1) inhibitor to platinum plus etoposide therapy as first-line treatment for patients with ES-SCLC, which has become the standard of care for first-line treatment.14, 15 Clinical outcomes for treatment efficacy were reported as ORR 60.2%, median PFS 5.2 months, and median OS 12.3 months in the IMpower133 trial14 and ORR 68%, median PFS 5.1 months, and median OS 13.0 months in the CASPIAN trial.15 In both trials, addition of a PD-L1 inhibitor to standard chemotherapy with platinum plus etoposide did not improve the response rates, but did improve long-term survival. However, these clinical trials did not include patients with a poor PS (PS ≥2) and included only a small number of patients aged >70 years. Therefore, the benefit of adding ICIs to the platinum and etoposide combination therapy for patients with a poor PS or older age remains unclear. Based on these data, in clinical practice, we are hesitant to add ICIs to the combination of platinum and etoposide in patients with a poor PS (PS ≥2). Here, PS improved after the first cycle of CE therapy in 25 (65.8%) of 38 patients in the PS 2 group and in 12 (48.0%) of 25 patients in the PS ≥3 group, respectively. For patients who improved to PS 1 or better immediately after the start of chemotherapy, addition of ICIs to platinum-based chemotherapy in subsequent courses could improve survival. Further studies are thus warranted to clarify whether the addition of ICI to platinum-based chemotherapy for PS-improved older patients can improve survival.
This study had several limitations. First, because this study was a retrospective review and analysis of data from medical records, PS assessment details were extracted from the records of the treating physicians and may not be accurate. For example, patients with PS 2 may have actually included patients with PS 3. This is because assessments made by healthcare professionals tend to underestimate a poor PS, defined as ECOG-PS 2–4, as compared to self-assessments by patients with lung cancer.16 Second, because of the real-world clinical evaluation in a frail patient population, the timing of imaging evaluation was at the discretion of the physician in charge, and some patients were not adequately evaluated for intracranial lesions when determining treatment efficacy. Finally, the incidence of AEs may have been underestimated because of the differences in the timing of blood collection. Contrarily, this study included many patients from before the approval of pegylated G-CSF, and the incidence of FN and TRD associated with FN may have been overestimated compared to that in current real-world practice in the era of widespread pegylated G-CSF use. Despite these limitations, this study provides useful information because it is based on a population that is commonly encountered in clinical practice, with the dual risk factors of advanced age and poor PS. This population has not been included in prospective clinical trials. We believe that our data suggest the potential for improved prognosis in this population and support the consideration of chemotherapy with aggressive intensive supportive care.
In conclusion, this retrospective study demonstrates that the CE combination therapy provides comparable tumor shrinkage, but numerically shorter PFS and OS in chemotherapy-naïve ES-SCLC patients with the dual risk factors of advanced age (≥71 years) and PS ≥3 than in patients of similar age with PS 2. Supportive care, such as aggressive administration of pegylated G-CSF before the occurrence of decreased neutrophil counts, may be necessary to ensure safety and further therapeutic benefits. As almost half of the older ES-SCLC patients with PS 2 or PS ≥3 had improved to PS 1 or better after the first cycle of CE therapy, some of these patients may benefit from the addition of ICIs to CE therapy. Further development of effective treatment strategies in this population is thus warranted.
ACKNOWLEDGMENTS
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
DISCLOSURE STATEMENT
Toshiaki Takahashi, Hirotsugu Kenmotsu, and Haruki Kobayashi report honoraria for lectures from Bristol Myers Squibb. Haruyasu Murakami reports honoraria for lectures from Bristol Myers Squibb and Nippon Kayaku. The other authors have no conflicts of interest to declare for this article.
ETHICS APPROVAL STATEMENT
This study was conducted in accordance with the tenets of the Declaration of Helsinki. This study was approved by the Institutional Review Board of Shizuoka Cancer Center (IRB number: J2019-30).
PATIENT CONSENT STATEMENT
We provided the patients an opportunity to opt out of the study.




