Usefulness of serum S100A4 and positron-emission tomography on lung cancer accompanied by interstitial pneumonia
Funding information: Japan Society for the Promotion of Science KAKENHI, Grant/Award Number: JP20K17749
Abstract
Background
The S100 calcium-binding protein A4 (S100A4) and the accumulation of [18F]-fluoro-2-deoxy-D-glucose (FDG) in noncancerous interstitial pneumonia (IP) area are predictors of postoperative acute exacerbation (AE) of IP after pulmonary resection for lung cancer with IP. However, the significance of combining these markers for predicting short-term outcome and long-term prognosis is not known.
Methods
Patients diagnosed with IP on preoperative high-resolution computed tomography and who had undergone pulmonary resection for primary lung cancer between April 2010 and March 2019 at Hiroshima University were included in this study. Predictive factors for the cumulative incidence of death from other than lung cancer (CIDOL) were investigated using the Fine and Gray model. CIDOL, perioperative outcome, and cumulative incidence of all death (CIAD) were retrospectively compared based on serum S100A4 and FDG accumulation.
Results
A total of 121 patients were included in this study. High S100A4 (hazard ratio [HR], 2.541; p = 0.006) and FDG accumulation (HR, 3.199; p = 0.038) were significant predictors of CIDOL. AE of IP occurred only in patients with high S100A4/FDG (+). CIDOL of patients with high S100A4/FDG (+) was higher than those with high S100A4/FDG (−) or low S100A4/FDG (+) (p < 0.001), and CIAD of patients with high S100A4/FDG (+) was also higher than those with high S100A4/FDG (−) or low S100A4/FDG (+) patients (p = 0.021).
Conclusions
Serum S100A4 and FDG accumulation in the noncancerous IP area were significant predictors of CIDOL after lung resection for lung cancer with IP and may help decide the treatment strategy.
INTRODUCTION
Interstitial pneumonia (IP), mostly idiopathic pulmonary fibrosis, is associated with an increased risk of lung cancer.1, 2 Approximately 4%–6% of resected lung cancer specimens show some type of interstitial lung disease.3 IP can sometimes be exacerbated acutely after lung resection with a reported incidence of 9.3%.4 Previous studies have investigated the risk factors for acute exacerbation (AE) of IP. Decline of vital capacity (VC), male sex, preoperative steroid use, history of AE of IP, usual IP (UIP) pattern, and extent of resection were identified as predictors of AE of IP; however, their predictive ability is insufficient because the mortality rate of AE of IP remains high, and IP is the major cause of perioperative death after lung resection.4, 5 Moreover, the long-term prognosis of lung cancer with IP has been reported to be poorer than that without IP.6 Therefore, risk factors for AE of IP and predictors of long-term prognosis should be identified in lung cancer complicated by IP.
The S100 calcium-binding protein A4 (S100A4) is a member of the S100 protein family and is a known marker of tissue fibrosis.7 Recently, we reported the usefulness of serum S100A4 as a novel predictor of postoperative AE of IP after lung cancer resection.8 We also showed the usefulness of [18F]-fluoro-2-deoxy-D-glucose (FDG) accumulation on positron emission tomography/computed tomography (PET/CT) in predicting AE of IP.9 However, the significance of S100A4 and FDG accumulation in long-term survival after lung resection remains unknown. Moreover, there was a weak correlation between FDG accumulation and serum S100A4 in our data. This indicates that using a combination of S100A4 and FDG accumulation may potentially improve the accuracy of predicting short-term outcomes. Therefore, this study aimed to investigate the usefulness of combining serum S100A4 and FDG accumulation for predicting long- and short-term outcomes after lung resection for lung cancer with IP.
METHODS
Patients
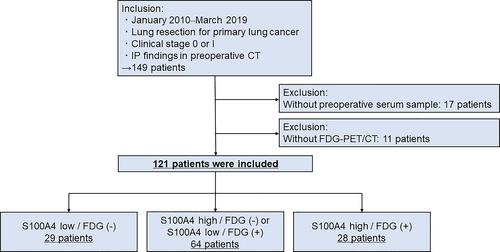
This retrospective study was approved by the Ethics Committee of Hiroshima University (approval numbers Gen-38 and E-2098). Patient consent was obtained using informed consent documents with the opt-out process. Patients diagnosed with IP on preoperative high-resolution computed tomography (HRCT) and who had undergone pulmonary resection for clinical stage 0 or I lung cancer between April 2010 and March 2019 were included in this study. Patients with unavailable preoperative serum samples and FDG-PET/CT data were excluded from the study. The flowchart of patient recruitment is shown in Figure 1. Included patients were divided into three groups based on the serum S100A4 levels and FDG accumulation: low S100A4/FDG (−), high S100A4/FDG (−) or low S100A4/FDG (+), and high S100A4/FDG (+). The cutoff S100A4 (17.13 ng/ml) value determined in the previous study was used.8
Preoperative examination, lung cancer staging, and pathological diagnosis
Preoperative evaluations, including chest HRCT, whole-body FDG-PET/CT, brain magnetic resonance imaging, and respiratory function tests, were performed to determine treatment strategies. The clinical and pathological stages were determined based on the eighth edition of the TNM classification of malignant tumors.10 The histological subtype was determined based on the World Health Organization classification.11
Radiological findings
The imaging protocol for HRCT and FDG-PET/CT has been described in our previous study.9 IP was defined radiologically based on the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association classification, and the patterns were classified into the usual IP (UIP), possible UIP pattern, and inconsistent with UIP12 based on preoperative HRCT findings.
Serum S100A4 level measurement
Serum samples were obtained one day before surgery. The samples were stored at −80°C until S100A4 measurement. To measure S100A4, a commercially available enzyme-linked immunosorbent assay kit was used (CircuLex S100A4 ELISA Kit version 2; MBL Co., Ltd.). ELISA for S100A4 was performed twice using the same sample from a patient, and the median value was used as the value of the patient.
FDG-PET/CT evaluation
The evaluation of FDG accumulation was performed by comparing the background normal lung field as performed in our previous study.9 If the uptake of the noncancerous IP area was higher than the background normal lung field, patients had a positive FDG accumulation. When distinguishing FDG accumulation in lung cancer and IP is difficult, the scores were determined on the contralateral side of lung cancer and assessed and shared among thoracic surgeons of the authors' group. Representative images of the FDG-PET/CT are shown in Figure S1.
Surgical approach and evaluation of complications
Postoperative complications were evaluated using the Clavien-Dindo classification.13 AE of IP is defined based on the following clinical characteristics: (i) worsening or onset of dyspnea, (ii) deterioration of the interstitial shadow on CT, (iii) decreased SpO2 or PaO2 severe than the preoperative value, and (iv) no evidence of other causes presenting these findings.12 Respiratory adverse events were as follows: AE of IP, bacterial pneumonia, bronchopleural fistula, pulmonary fistula that lasted >7 days, or pleurodesis. Pleural effusion after chest tube removal was also included.
Statistical analysis
Data are presented as the median and interquartile range (IQR) for continuous variables and n (%) for categorical variables. Categorical and continuous variables were compared using a Chi-square test and Student's t-test, respectively. Non-normally distributed continuous variables were analyzed using Wilcoxon's rank-sum test.
Cumulative incidence of death other than lung cancer (CIDOL) was defined as the interval from the date of surgery to the date of death from other than lung cancer. Cumulative incidence of all death (CIAD) was defined as the time interval from the date of surgery to the date of death from any cause or the last follow-up visit. Recurrence free survival (RFS) was defined as the time interval from the date of surgery until the date of recurrence, death, or the last follow-up visit.
Univariable and multivariable analyses for CIDOL were performed using Fine and Gray models. In the univariable analysis, high serum S100A4 level (≥17.13 ng/ml) and FDG accumulation, age (>70 years), sex (male or female), percent predicted VC (%VC; ≤80%), percent diffusing capacity for carbon monoxide (%DLCO; ≤40%), serum Krebs von den Lungen-6 level (KL-6; ≥1000), the radiological pattern of IP (UIP pattern or not), pathological stage (≥stage 2), histology of lung cancer (adenocarcinoma or not), and surgical procedure (lobectomy or sublobar resection) were included as variables. Multivariable analysis was performed including variables identified as significant predictors (p-value < 0.05) in univariable analysis.
CIDOL was analyzed by competing risk analysis using a cumulative incidence function that accounted for death from lung cancer as a competing event. CIAD and RFS were calculated using the Kaplan–Meier method and compared using the log-rank test.
All statistical analyses were performed with EZR version 1.51 (Saitama Medical Center, Jichi Medical University, Saitama, Japan),14 a graphical user interface for R (The R Foundation for Statistical Computing).
RESULTS
Multivariable analysis
A total of 121 patients were included in the present study. The characteristics of the study population are presented in Table S1. In the univariable analysis of CIDOL, high S100A4 (hazard ratio [HR], 2.532; 95% confidence interval [CI]: 1.325–4.873; p = 0.005), FDG accumulation (HR, 4.428; 95% CI: 1.580–12.410; p = 0.005), %VC (HR, 3.272; 95% CI: 1.720–6.223; p < 0.001), and %DLCO (HR, 2.274; 95% CI: 1.081–4.783; p = 0.030) were found to be significant predictors. In multivariable analysis, high S100A4 (HR, 2.541; 95% CI: 1.308–4.934; p = 0.006), FDG accumulation (HR, 3.199; 95% CI: 1.066–9.605; p = 0.038), and %VC (HR, 2.545; 95% CI: 1.181–5.488; p = 0.017) were significant predictors of CIDOL (Table 1).
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| High S100A4 | 2.532 (1.325–4.837) | 0.005 | 2.541 (1.308–4.934) | 0.006 |
| FDG accumulation | 4.428 (1.580–12.410) | 0.005 | 3.199 (1.066–9.605) | 0.038 |
| Male | 1.904 (0.707–5.129) | 0.200 | - | - |
| Age (≥70) | 1.178 (0.591–2.349) | 0.640 | - | - |
| %VC (≤80%) | 3.272 (1.720–6.223) | <0.001 | 2.545 (1.181–5.488) | 0.017 |
| DLCO (≤40%) | 2.274 (1.081–4.783) | 0.030 | 1.103 (0.465–2.619) | 0.820 |
| Radiological UIP pattern | 1.192 (0.598–2.376) | 0.620 | - | - |
| KL-6 (≥1000) | 1.182 (0.428–3.262) | 0.750 | - | - |
| Pathological stage (≥stage 2) | 0.843 (0.317–2.240) | 0.730 | - | - |
| Histology (other subtypes/adenocarcinoma) | 1.595 (0.808–3.142) | 0.180 | - | - |
| Procedure (lobectomy/sublobar resection) | 0.803 (0.425–1.519) | 0.500 | - | - |
- Abbreviations: AE, acute exacerbation; CI, confidence interval; DLCO, diffusing capacity for carbon monoxide; FDG, [18F]-fluoro-2-deoxy-D-glucose; HR, hazard ratio; IP, interstitial pneumonia; KL-6, Krebs von den Lungen-6; S100A4, S100 calcium-binding protein A4; UIP, usual interstitial pneumonia; VC, vital capacity.
Cumulative incidence of death from other than lung cancer
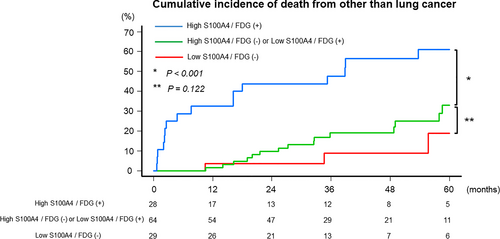
Based on the multivariable analysis results, patients were divided into three groups: 29 (24.0%) with low S100A4/FDG (−), 64 (52.9%) with high S100A4/FDG (−) or low S100A4/FDG (+), and 28 (23.1%) with high S100A4/FDG (+). Their characteristics are shown in Table 2. The incidence of radiological UIP pattern (p = 0.007) and clinical stage (p = 0.016) was higher in patients with high S100A4/FDG (+). The incidence of adenocarcinoma was higher in patients with low S100A4/FDG (−) (p = 0.005). No significant differences were observed in other characteristics (Table 2). The median follow-up period was 43 (IQR, 32–69) months. No significant difference in CIDOL was observed between patients with low S100A4/FDG (−) (5-year CIDOL rate: 18.9%; 95% CI: 3.3–44.1) and high S100A4/FDG (−) or low S100A4/FDG (+) (33.0%; 95% CI: 18.4–48.3; p = 0.122). CIDOL was higher in patients with high S100A4/FDG (+) (61.0%; 95% CI: 38.3–77.5; p < 0.001) than in those with high S100A4/FDG (−) or low S100A4/FDG (+) (Figure 2).
| Variables | Low S100A4 / FDG (−) n = 29 (24.0%) | High S100A4 / FDG (−) or low S100A4 / FDG (+) n = 64 (52.9%) | High S100A4 / FDG (+) n = 28 (23.1%) | p-value |
|---|---|---|---|---|
| Age, years (IQR) | 74 (71–80) | 73 (68–77) | 72 (64–76) | 0.385 |
| Sex, male (%) | 21 (72.4%) | 56 (87.5%) | 23 (82.1%) | 0.222 |
| Respiratory function | ||||
| %VC (%) (IQR) | 93.5 (89.0–101.6) | 90.6 (80.3–102.0) | 83.8 (73.6–95.4) | 0.137 |
| %VC (≤80%) | 4 (13.8%) | 15 (23.4%) | 12 (42.9%) | 0.039 |
| %DLCO (%) (IQR) | 61.1 (49.4–76.4) | 52.4 (43.9–67.7) | 49.5 (37.4–67.1) | 0.095 |
| DLCO (≤40%) | 3 (10.3%) | 9 (14.5%) | 8 (28.6%) | 0.164 |
| Serum KL-6 (U/ml) (IQR) | 395 (282–478) | 464 (317–682) | 512 (285–972) | 0.056 |
| Radiological IP pattern | 0.007 | |||
| UIP pattern (%) | 3 (10.3%) | 24 (37.5%) | 14 (50.0%) | |
| Possible UIP pattern (%) | 18 (62.1%) | 27 (42.2%) | 12 (42.9%) | |
| Inconsistent with UIP pattern (%) | 8 (27.6%) | 13 (20.3%) | 2 (7.1%) | |
| Preoperative steroid use | 1 (3.5%) | 3 (4.7%) | 4 (14.3%) | 0.219 |
| Preoperative antifibrotic agent use | 0 (0%) | 2 (3.1%) | 1 (3.6%) | 0.432 |
| Clinical stage | 0.016 | |||
| 0 (%) | 2 (6.9%) | 1 (1.6%) | 0 (0%) | |
| IA1 (%) | 7 (24.1%) | 3 (4.7%) | 1 (3.6%) | |
| IA2 (%) | 4 (13.8%) | 26 (40.6%) | 10 (35.7%) | |
| IA3 (%) | 11 (37.9%) | 23 (35.9%) | 8 (28.6%) | |
| IB (%) | 5 (17.2%) | 11 (17.2%) | 9 (32.1%) | |
| Histology | 0.005 | |||
| Adenocarcinoma (%) | 20 (69.0%) | 20 (31.3%) | 10 (35.7%) | |
| Squamous cell carcinoma (%) | 8 (31.3%) | 27 (42.2%) | 12 (42.9%) | |
| Others (%) | 1 (3.5%) | 17 (26.6%) | 6 (21.4%) | |
| Surgical procedure | 0.561 | |||
| Wedge resection (%) | 8 (27.6%) | 21 (32.8%) | 9 (32.1%) | |
| Segmentectomy (%) | 9 (31.0%) | 12 (18.8%) | 9 (32.1%) | |
| Lobectomy (%) | 12 (41.4%) | 31 (48.4%) | 10 (35.7%) | |
| Pathological stage | 0.212 | |||
| IA1 (%) | 6 (20.7%) | 7 (10.9%) | 1 (3.6%) | |
| IA2 (%) | 6 (20.7%) | 14 (21.9%) | 3 (10.7%) | |
| IA3 (%) | 4 (13.8%) | 10 (15.6%) | 3 (10.7%) | |
| IB (%) | 8 (27.6%) | 25 (39.1%) | 15 (53.6%) | |
| IIA (%) | 1 (3.5%) | 0 (0%) | 2 (7.1%) | |
| IIB (%) | 2 (6.9%) | 6 (9.4%) | 1 (3.6%) | |
| IIIA (%) | 2 (6.9%) | 2 (3.1%) | 2 (7.1%) | |
| IV (%) | 0 (0%) | 0 (0%) | 1 (3.6%) | |
| Recurrence | 7 (24.1%) | 17 (26.6%) | 4 (14.3%) | 0.407 |
- Abbreviations: DLCO, diffusing capacity for carbon monoxide; FDG, [18F]-fluoro-2-deoxy-D-glucose; IP, interstitial pneumonia; IQR, interquartile range; KL-6, Krebs von den Lungen-6; S100A4, S100 calcium-binding protein A4; UIP, usual interstitial pneumonia; VC, vital capacity.
Postoperative AE of IP and short-term outcomes
The short-term outcomes are shown in Table 3. AE of IP occurred only in patients with high S100A4/FDG (+). The incidence of any grade respiratory complications (p < 0.001), and respiratory complications more severe than grade III (p = 0.004) were higher in patients with high S100A4/FDG (+). Death within 30 and 90 days from surgery also occurred only in patients with high S100A4/FDG (+) (Table 3).
| Low S100A4 / FDG (−) n = 29 (24.0%) | High S100A4 / FDG (−) or low S100A4 / FDG (+) n = 64 (52.9%) | High S100A4 / FDG (+) n = 28 (23.1%) | p-value | |
|---|---|---|---|---|
| AE of IP+ (any grade) | 0 (0%) | 0 (0%) | 9 (32.1%) | <0.001 |
| AE of IP + (grade IIIa) | 0 (0%) | 0 (0%) | 7 (25.0%) | <0.001 |
| Respiratory complications+ (any grade) | 6 (20.7%) | 9 (14.1%) | 15 (53.6%) | <0.001 |
| Respiratory complications+ (grade IIIa) | 6 (20.7%) | 7 (10.9%) | 12 (42.9%) | 0.004 |
| 30-days mortality rate | 0 (0%) | 0 (0%) | 3 (10.7%) | 0.011 |
| 90-days mortality rate | 0 (0%) | 0 (0%) | 7 (25.0%) | <0.001 |
- Abbreviations: AE, acute exacerbation; FDG, [18F]-fluoro-2-deoxy-D-glucose; IP, interstitial pneumonia; S100A4, S100 calcium-binding protein A4.
Cumulative incidence of all death and recurrence free survival
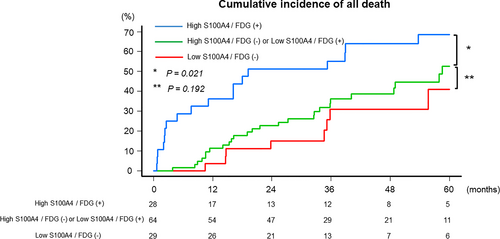
No significant difference was observed in CIAD between patients with low S100A4/FDG (−) (5-year CIAD rate: 40.9%; 95% CI: 21.2–68.6) and high S100A4/FDG (−) or low S100A4/FDG (+) (52.6%; 95% CI: 38.0–68.8; p = 0.192). CIAD was higher in patients with high S100A4/FDG (+) (68.5%; 95% CI: 50.2–85.2; p = 0.021) than those with high S100A4/FDG (−) or low S100A4/FDG (+) (Figure 3). No significant difference was observed in RFS between patients with S100A4/FDG (−) (5-year RFS rate: 56.0%; 95% CI: 30.5–75.4) and high S100A4/FDG (−) or low S100A4/FDG (+) (45.2%; 95% CI: 29.5–59.7; p = 0.358). There was also no significant difference in RFS between patients with high S100A4/FDG (−) or low S100A4/FDG (+) and patients with high S100A4/FDG (+) (36.4%; 95% CI: 17.7–55.6; p = 0.066; Supporting Information, Figure S2).
DISCUSSION
In the present study, AE of IP occurred only in patients with high S100A4/FDG (+). CIAD and CIDOL were significantly worse in patients with high S100A4/FDG (+). High S100A4 and FDG accumulation were also significant predictors of CIDOL in multivariable analysis. These results implicate that serum S100A4 and FDG accumulation are useful in predicting not only short-term outcomes, such as AE of IP, but also long-term prognosis after lung resection for lung cancer accompanied with IP.
S100A4, which is known to play a key role in lung fibrosis, was shown to activate fibrogenic mesenchymal progenitor cells (MPCs),15 and confer fibrogenicity upon MPCs.16 Extracellular S100A4 was also shown to activate lung fibroblasts,15 and the main activation mechanism is assumed to be αSMA and type I collagen upregulation due to increased sphingosine-1-phosphate level.7, 17 Macrophages have been suggested as the origin of extracellular S100A4.18 There are no studies using cell line or animals that directly compared the serum S100A4 values with the situation in tissues. However, Akiyama et al. showed that serum S100A4 is useful in predicting the long-term prognosis of IP,19 and our previous study showed its usefulness in predicting AE of IP8; in addition, these studies showed numerous S100A4 expressing cells not present in the normal lung area but are found in the IP area. Although the significance of serum S100A4 as a predictive factor of long-term survival has been already reported in patients with IP, its significance in predicting long-term survival in patients with lung cancer with IP after pulmonary resection remains unknown, and we believe that this is a novel point of this article. FDG accumulation in the noncancerous IP area is also known as an AE predictor of IP.9, 20 The FDG accumulation on PET/CT is based on a metabolic shift to glycolysis; thereby, FDG of the noncancerous IP area may reflect the IP activity. Using the combination of serum S100A4 and FDG accumulation helped improve the accuracy of predicting short-term outcomes such as AE of IP. Looking at the survival curve, short-term postoperative mortality affects the long-term prognosis. On the other hand, S100A4 and FDG accumulation were also significant prognostic factors in the multivariate analysis for long-term prognosis. This finding may indicate the importance of preventing short-term complications in lung cancer with IP. In our multivariable analysis for CIDOL, %VC was also a significant prognostic factor. To confirm the significance of S100A4 and FDG accumulation, analysis among patients with low %VC is needed; however, we could not perform this analysis because of the small sample size. This issue should be investigated in a larger prospective study.21
Among the deaths of patients with IP and lung cancer, lung cancer is responsible for approximately 50%, and the remaining deaths were caused by other factors, such as respiratory failure.3, 22 Predictors of long-term prognosis in lung cancer complicated by IP have not yet been fully evaluated. Several studies have investigated the predictors of survival;3, 23-27 however, only one study reported the cause of death3 and most of these studies had a small sample size. Therefore, we believe that the present study, which analyzed prognosis by dividing the causes of death into two categories, that is, lung cancer and other causes, is meaningful. In our cohort, the incidence of death from lung cancer was lower than CIDOL, and high S100A4 and FDG accumulation and %VC were significant predictors of CIDOL. These results suggest that there is a subset of patients with lung cancer accompanied with IP in whom preventing death from factors other than lung cancer, including AE of IP, should be prioritized over oncological factors. Indeed, there were no significant differences among the groups in terms of the number of patients who developed recurrence and RFS, although RFS of patients with high S100A4 / FDG (+) tended to be worse. Patients who are at high risk of death from factors other than lung cancer may be suitable candidates for sublobar resection, which is known to be associated with a low incidence of AE of IP.3 A larger prospective study is required to confirm these findings.21 Effectiveness of perioperative pharmacotherapy such as pirfenidone28 should also be investigated.
This study has several limitations. First, it was a retrospective study from a single institution with a very small sample size. Due to the small sample size, we could not perform propensity score matching to adjust the difference in characteristics among patients with low S100A4/FDG (−), patients with high S100A4/FDG (−) or low S100A4/FDG (+), and patients with high S100A4/FDG (+). The small sample size may also explain why already known prognostic factors were not significant in the multivariable analysis of the present study. Next, at present, S100A4 cannot be easily measured in daily practice. However, several studies on long-term prognostic factors of lung cancer with IP are limited, and the results of this study will help decide the treatment strategy.
In conclusion, using the combination of S100A4 and FDG accumulation helped improve the predictive ability for postoperative short-term outcomes after lung resection for lung cancer with IP. S100A4 and FDG accumulation were significant predictors of CIDOL. Larger studies are required to confirm the significance of our findings in deciding a suitable treatment strategy for patients and selecting candidates suitable for sublobar resection or perioperative treatment for IP.
AUTHOR CONTRIBUTIONS
Atsushi Kagimoto: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Visualization, and Writing – Original Draft Preparation. Yasuhiro Tsutani: Conceptualization, Data Curation, Methodology, and Writing – Review & Editing. Kei Kushitani: Conceptualization and Writing – Review & Editing. Takahiro Kambara: Writing – Review & Editing. Takahiro Mimae and Yoshihiro Miyata: Data Curation and Writing – Review & Editing. Yukio Takeshima: Writing – Review & Editing. Morihito Okada: Writing – Review & Editing and Supervision.
ACKNOWLEDGMENT
The authors would like to thank Enago (www.enago.jp) for the English-language review.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
All data is original from our study. No data from public or shared database was utilized.