Effectiveness of alectinib and osimertinib in a brain metastasized lung adenocarcinoma patient with concurrent EGFR mutations and DCTN1-ALK fusion
Qiang Yin, Taiyan Guo, Yangyang Zhou contributed equally to this study.
Funding information: National Natural Science Foundation of China, Grant/Award Number: 81702481; Young Innovative Talents Training Program of Tianjin Medical University Cancer Institute and Hospital, Grant/Award Number: TMUCIH-2019-1-9
Abstract
The echinoderm microtubule associated protein-like 4 gene (EML4) encodes the predominant anaplastic lymphoma kinase (ALK) fusion partner in non-small-cell lung cancer (NSCLC); however, the dynactin subunit 1 (DCTN1)-ALK rearrangement is extremely rare. The co-occurrence of primary epidermal growth factor receptor (EGFR) T790M mutation with EGFR exon 19 deletion (del) in patients with NSCLC is uncommon. Here we report a female lung adenocarcinoma patient with brain metastases and possible coexistence of primary EGFR T790M mutation/EGFR exon 19 del/DCTN1-ALK translocation. The patient received multiline treatment including chemotherapy, antivascular, and targeted therapies. To overcome developed resistance to chemotherapy or targeted therapy to prolong overall survival, the patient's circulating tumor DNA (ctDNA) was dynamically monitored. The patient responded to successive osimertinib and alectinib treatment, and alectinib achieved a nearly complete response for lung and brain lesions after she acquired osimertinib resistance. Furthermore, we summarize 22 published cases of patients with lung adenocarcinoma with concurrent EGFR mutation and ALK rearrangement, including details of clinical characteristics, natural history, and pertinent therapy of this uncommon tumor subtype. This literature review shows that EGFR inhibition was an indispensable aspect of the treatment of patients with EGFR/ALK co-alterations in the pre-alectinib era and that ALK inhibition with crizotinib did not show more eye-catching therapeutic results. Considering the effectiveness achieved by alectinib, this case study provides a new perspective for the treatment of lung cancer brain metastasis patients with concurrent EGFR/ALK mutations.
INTRODUCTION
Most epidermal growth factor-receptor (EGFR)-positive patients with non-small-cell lung cancer (NSCLC) will acquire resistance within 1 year after initial EGFR-tyrosine kinase inhibitor (TKI) treatment. Multiple mechanisms are involved in acquired resistance to EGFR-TKIs, including emergence of an EGFR T790M mutation, mutations in KRAS, BRAF or PIK3CA, MET amplification, and histological transformation.1-3 Furthermore, primary resistance to EGFR-TKIs occurs in approximately 5–10% of the Asian population.4 Anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (ALK-TKIs) are recommended for treating NSCLCs with sensitizing mutations. Here we report a female patient with lung adenocarcinoma harboring an EGFR exon 19 deletion (del), a primary EGFR T790M mutation, and a rare ALK fusion who achieved a good response to multiline treatment including osimertinib and alectinib. Moreover, we performed a literature review of the rare DCTN1-ALK cases and current treatment strategies for patients with the co-alteration of EGFR mutation and ALK translocation.
CASE PRESENTATION
The patient was a 60-year-old female nonsmoker who presented with a cough and blood-tinged sputum. Chest computed tomography (CT) revealed a space-occupying lesion in the right upper lobe (Figure 1a, 2016 April). A percutaneous lung biopsy performed on the right lung indicated a pathological diagnosis of T3N2M1a (IVA) lung adenocarcinoma, accompanied by multiple metastases in the right pleura, right hilum, and mediastinal lymph node. EGFR mutations (exons 18–21) and EML4-ALK were undetectable by direct sequencing in 2016. Chemotherapy comprising six cycles of pemetrexed and carboplatin was administered as first-line treatment. The patient experienced stable disease (SD) 8 months later. However, disease progressed after 14 months of chemotherapy. The patient then received docetaxel and carboplatin as second-line treatment. Unfortunately, after 9 months of chemotherapy, tumor progression slowly developed and she presented with hemoptysis as well as brain metastases (Figure 1a, 2018 March). The patient was then switched to antivascular endothelial growth factor receptor 2 (VEGFR2) therapy with apatinib. As we expected, apatinib effectively reduced peritumor edema in the brain 3 months later. However, she discontinued apatinib treatment because of severe adverse reactions. Two months after apatinib was withdrawn, the follow-up images showed that although the primary lung tumor was slightly reduced, new intrapulmonary metastases were present and the intracranial tumor exhibited a significant enhancement ring detected using MRI (Figure 1a, 2018 August).
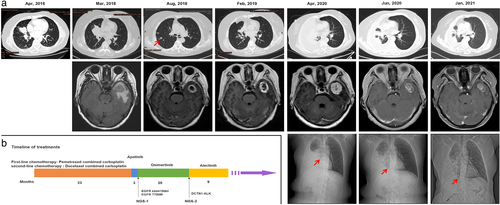
To seek potential therapeutic regimens, next-generation sequence (NGS) analysis of the patient's circulating tumor (ct)DNA was performed. This analysis revealed an EGFR exon 19 deletion (level, plasma 0.6%) and a primary EGFR T790M mutation (level, plasma 0.1%). The patient then started oral osimertinib and after 8 weeks an SD period was observed for the brain tumor. Imaging scans performed after 6 months showed a partial response (PR) of the lung mass and intracranial tumors (Figure 1a, 2019 February).
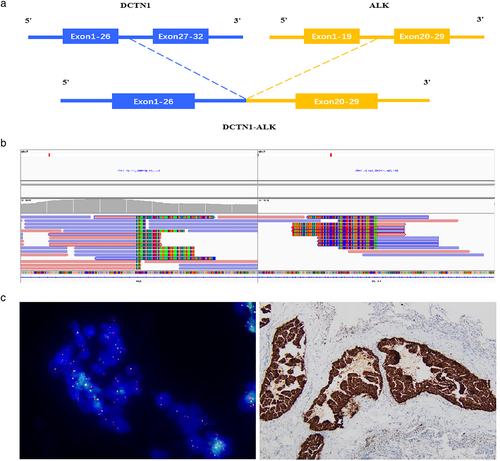
After progression-free survival (PFS) for approximately 11 months, although the intracranial tumor continued to exhibit a PR state, the lung lesions progressed, and pleural fluid seemed to accumulate during the 16th month of osimertinib treatment. Four months later, she began to suffer from breath-holding, headaches, dizziness, and speech problems. Chest CT, chest x-ray, and brain MRI indicated that the patient's disease progressed to a life-threatening state, particularly with severe cerebral edema and midline displacement (Figure 1a, 2020 April). A repeat liquid biopsy was then performed. NGS analysis of ctDNA revealed a rare ALK oncogenic fusion involving DCTN1 exon 26 and ALK exon 20 (level, plasma 0.23%), and no detectable T790M mutation.
The patient was therefore treated with alectinib, and her symptoms significantly improved after 1 month with almost no noticeable side effects. Follow-up images in June 2020 showed marked pulmonary improvement, resolution of pleural effusion, dramatically decreased brain tumor volume, and significant amelioration of cerebral edema, as well as relief from midline displacement (Figure 1a, 2020 June). The patient was evaluated with a PR, nearly complete response (CR), as revealed by chest CT, chest x-ray, and brain MRI 6 months later (Figure 1a, 2021 January). The timeline of the overall treatment process is summarized in Figure 1b. Detection of the DCTN1-ALK fusion, including detailed breakpoint identification of the 2020 ctDNA NGS analysis and fluorescence in situ hybridization (FISH) plus immunohistochemistry (IHC) result of the 2016 primary lung tumor tissue, is illustrated in Figure 2. Long-term follow-up of this patient and the evaluation of the efficacy of alectinib will be continued, and we will pay close attention to whether she develops alectinib resistance in the future.
DISCUSSION
The EGFR T790M mutation, which is one of the common mechanisms of acquired resistance to EGFR inhibitors, is detected in 50–60% of EGFR-TKI therapy-resistant cases.5, 6 Recently, primary EGFR T790M mutation was identified by routine molecular testing in a minor subgroup of TKI-naive NSCLC patients.7, 8 Generally, there are differences in clinical and molecular characteristics between primary and acquired T790M mutations.9 However, studies are inconsistent because of their different sample sizes. The primary EGFR T790M mutation always coexists with L858R, whereas the acquired T790M is more likely to coexist with EGFR exon 19 del.9 Both primary and acquired EGFR T790M mutations respond well to osimertinib. Patients with an acquired T790M mutation experience longer overall survival during the entire course of clinical treatment. However, patients with a primary T790M mutation may experience greater benefits from osimertinib.10
Previous studies showed that ALK rearrangements and EGFR mutations were mutually exclusive.11, 12 Nevertheless, some recent studies have shown that EGFR mutations may coexist with ALK fusions in patients with lung adenocarcinoma.13-17 With the popularization and advancement of NGS technology, there will be more and more reports about EGFR/ALK co-mutations.
For therapy of patients with concurrent acquisition of EGFR mutations and ALK rearrangements, Kuo et al. described a favorable response to gefitinib of a patient with lung adenocarcinoma with EML4-ALK/EGFR co-alterations.18, 19 However, Lee et al. reported the opposite, in which the patient did not respond to EGFR-TKI treatment but responded to crizotinib.20 Another study suggested that dual-TKI treatment (EGFR-TKI plus ALK-TKI) may be more effective than single TKI (EGFR-TKI or ALK-TKI) for EML4-ALK/EGFR co-altered patients.21
We reviewed 22 NSCLC cases with the EGFR and EML4-ALK mutations and the clinical outcomes of EGFR TKIs and ALK TKIs (Table 1).13, 14, 16-19, 21-24 Although the total PFS achieved using dual-TKI/triple-TKI treatment seemed longer than single-TKI treatment for these co-altered patients, the difference was not statistically significant (p = 0.85). Yang et al. reported that the diverse response to EGFR-TKIs, ALK-TKIs, or both, may be associated with diverse phospho-ALK and phospho-EGFR levels.16 Unfortunately, the ALK inhibitor used in the 22 EGFR/ALK co-altered cases was crizotinib, not alectinib, partially explained by later marketing approval and application of alectinib.
| Case/reference | Age/sex | Histology | EGFR and ALK alterations | Targeted agents (treatment lines) | EGFR TIKs treatment PFS (months) | EGFR TIKs treatment AEs | ALK TIKs treatment PFS (months) | ALK TIKs treatment AEs | Total PFS (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1/Tanaka et al.13 | 39/M | Ad | EGFR L858R/EML4-ALK | Erlotinib (first) | 1 | NA | NA | NA | 1 |
| 2/Xu et al.14 | 71/F | Ad | EGFR exon19del/EML4-ALK | Gefitinib (first) | 8 | NA | NA | NA | 8 |
| 3/Yang et al.16 | 44/F | Ad | EGFR exon19del/EML4-ALK | Gefitinib (first) | 9 | NA | NA | NA | 9 |
| 4/Yang et al.16 | 56/F | Ad | EGFR L858R/EML4-ALK | Gefitinib (first) | 11.2 | NA | NA | NA | 11.2 |
| 5/Yang et al.16 | 59/M | Ad | EGFR L858R/EML4-ALK | Erlotinib (first) | 13 | NA | NA | NA | 13 |
| 6/Yang et al.16 | 70/M | Ad | EGFR L858R/EML4-ALK | Erlotinib (first) | 27.4 | NA | NA | NA | 27.4 |
| 7/Yang et al.16 | 40/M | Ad | EGFR exon19del/EML4-ALK | Erlotinib (first) | 17.5 | NA | NA | NA | 17.5 |
| 8/Yang et al.16 | 60/F | Ad | EGFR exon19del/EML4-ALK | Afatinib (first) | 7 | NA | NA | NA | 7 |
| 9/Yang et al.16 | 66/F | Ad | EGFR L858R/EML4-ALK | Gefitinib (first) | 24.5 | NA | NA | NA | 24.5 |
| 10/Kuo et al.18 | 72/F | Ad | EGFR exon19del/EML4-ALK | Gefitinib (first) | 7 | NA | NA | NA | 7 |
| 11/Shin et al.19 | 77/F | Ad | EGFR L858R/EML4-ALK | Osimertinib (first) | 5.1 | NA | NA | NA | 5.1 |
| 12/Popat et al.23 | 65/F | Ad | EGFR exon19del/EML4-ALK | Erlotinib (first) | 25 | NA | NA | NA | 25 |
| 13/Santelmo et al.24 | 52/F | Ad | EGFR exon19del/EML4-ALK | Gefitinib (first) | 7 | NA | NA | NA | 7 |
| 14/Yang et al.16 | 54/F | Ad | EGFR exon19del/EML4-ALK | Erlotinib (first) Crizotinib (second) |
12 | NA | 2.7 | NA | 14.7 |
| 15/Yang et al.16 | 45/F | Ad | EGFR exon19del/EML4-ALK | ND (first) Crizotinib (second) |
NA | NA | 15.1 | NA | 15.1 |
| 16/Baldi et al.17 | 68/M | Ad | EGFR L858R/EML4-ALK | Erlotinib (second) Crizotinib (third) |
7 | skin toxicity | 11 | Visual toxicity | 18 |
| 17/Chen et al.22 | 56/M | Ad | EGFR exon19del/EML4-ALK | Erlotinib (second) Crizotinib (third) |
8 | rash | ~1.5 | ~9.5 | |
| 18/Shin et al.19 | 57/F | Ad | EGFR L858R/EML4-ALK | Gefitinib (first) Crizotinib (second) Osimertinib (third) |
7.7; 9.5 | NA | 0.75 | NA | 17.95 |
| 19/Shin et al.19 | 32/M | Ad | EGFR L858R/EML4-ALK | Osimertinib (first) Crizotinib (second) |
0.75 | chest pain; fever | 1 | NA | 1.75 |
| 20/Lee et al.20 | 73/M | Ad | EGFR exon19del/EML4-ALK | Gefitinib (first) Crizotinib (second) Osimertinib (third) |
0.5 | NA | 9 | NA | 9.5 |
| 21/Liu et al.21 | NA/NA | NA | EGFR exon19del/T790M/CEBPZ-ALK | Erlotinib (first) Osimertinib (second) Crizotinib (third) |
NA | NA | NA | NA | 13.2 |
| 22/Liu et al.21 | NA/NA | NA | EGFR exon19del/EML4-ALK | Erlotinib (first) Osimertinib (second) Osimertinib+Crizotinib (third) |
NA | NA | NA | NA | 18.6 |
- Abbreviations: Ad, adenocarcinoma; NA, not available; ND, not done; PFS, progression-free survival.
In our case, possibly due to the limitation of the relatively low sequencing depth achieved by the local molecular testing center (in 2018), ALK fusion was not discovered along with EGFR mutation. Thus, the patient was not administered dual-TKI treatment. Whether dual-TKI therapy is the best strategy for these co-altered patients, or if alectinib is better administered before an EGFR inhibitor, remains to be determined. Furthermore, long-term follow-up of this patient is in progress, and we will pay close attention to whether alectinib resistance develops.
ACKNOWLEDGMENTS
We thank the patient and her family. Informed consent was obtained from the patient for publication. We thank the staff at Tianjin Medical University Cancer Institute and Hospital. We thank Dr. Guoqiang Chang and Madison Ann Ward of Virginia Commonwealth University for their kind help in the process of language editing. This research is partly funded by National Natural Science Foundation of China (81702481) and the Young Innovative Talents Training Program of Tianjin Medical University Cancer Institute and Hospital (TMUCIH-2019-1-9).
CONFLICT OF INTEREST
The authors declare that there are no potential conflicts of interest.