Sarcoidosis-like reaction after neoadjuvant pembrolizumab combined with chemotherapy mimicking disease progression of NSCLC induced encouraging discovery of pathological complete response
Abstract
Neoadjuvant chemoimmunotherapy has demonstrated improved efficacy and prognosis in stage IIa–IIIb patients with non-small cell lung cancer (NSCLC). Drug-induced sarcoidosis-like reaction (DISR), an autoimmune reaction, has been reported as a type of immune-related adverse event that may mimic disease progression. Here, we report the case of patient with NSCLC who developed DISR during neoadjuvant chemoimmunotherapy and finally achieved pathological complete response after surgery.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are indicated for use as neoadjuvant treatment of non-small cell lung cancer (NSCLC), as they have demonstrated improved efficacy and prognosis in several ongoing trials (NADIM, NCT02716038, NEOMUN, and NCT03197467, to name a few). Drug-induced sarcoidosis-like reaction (DISR), a type of immune-related adverse event reported in patients receiving ICIs, is a systematic granulomatous reaction that may be misinterpreted on imaging studies as tumor progression.1 Here, we present a patient with NSCLC who developed DISR while being treated with neoadjuvant pembrolizumab combined with chemotherapy. Although the occurrence of DISR post therapy mimicked disease progression, a complete pathological response after surgery was induced, which is an encouraging finding.
CASE REPORT
A 68-year-old woman presented to Peking Union Medical College Hospital on May 28, 2020 with a history of several months of cough and blood in her sputum. Baseline computed tomography (CT) and fluorodeoxyglucose positron-emission tomography combined with computed tomography (FDG PET/CT) revealed a left hilar mass measuring 80 mm × 20 mm, with left pulmonary artery involvement (Figure 1a–d). Squamous cell carcinoma was confirmed through a bronchoscopic biopsy. Epidermal growth factor receptor sensitive mutations, anaplastic lymphoma kinase rearrangement, and c-ros oncogene 1 receptor kinase rearrangement were negative. According to the staging system of the eighth edition of the American Joint Committee on Cancer Tumor Node Metastases the clinical stage of the patient was IIIA (T4N0M0). She received pembrolizumab (200 mg) combined with paclitaxel (175 mg/m2) and carboplatin (area under curve = 5) on day 1 of each 21-day period as neoadjuvant treatment from July 16, 2020. However, contrast-enhanced CT scan showed enlarged N7, 10L, and 10R lymph nodes (Figure 1e–h) after two cycles of treatment. To confirm whether this was due to disease progression, an endobronchial ultrasound-guided transbronchial needle aspiration of the 10R lymph node was performed. Histopathological examination showed inflammatory cells and cellulose exudation with carbon dust deposition, with no sign of tumor cells.
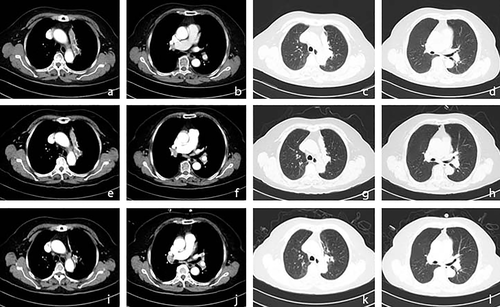
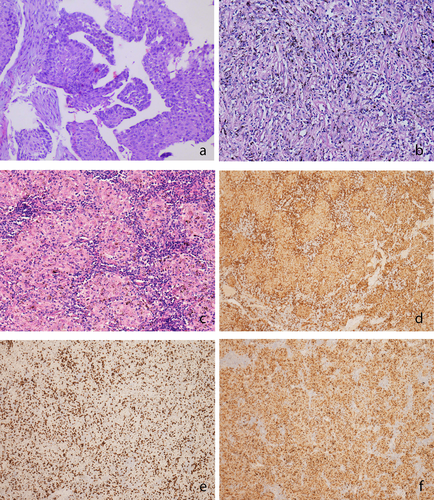
Another two cycles of pembrolizumab combined with paclitaxel and carboplatin at the same dose were administered. The CT scan is shown in Figure 1i–l. The patient then underwent video-assisted left upper lung lobectomy plus radical mediastinal lymph node dissection on October 20, 2020. Notably, the post-surgical pathological evaluation revealed complete pathological response, with focally clustered tissue cells forming a sarcoidosis-like reaction. Lymph nodes at 4L, 5, 7, 9, 10L, 12L, and 13L also showed sarcoidosis-like reactions with lymphocytic infiltration, with no sign of tumor cells. Furthermore, cluster of differentiation CD3/CD4/CD8/CD68 staining of the primary tumor bed and N5 lymph node was performed, which showed abundant lymphocytic and histiocytic infiltration (Figure 2). We performed further peripheral blood 12 cytokine tests at three timepoints, including pretreatment (July 14, 2020), first detection of onset of DISR (August 31, 2020), and post-surgery as well as before adjuvant treatment (November 19, 2020) (Table 1). We noticed that at the timepoint of onset of DISR, negative prognostic factors including interleukin-6 and interleukin-8 presented at a relatively low level, which may help clinicians determine whether it is real or pseudoprogression.
| Date (pg/ml) | IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-8 | IL-10 | IL-12p70 | IL-17 | TNF-α | IFN-α | IFN-γ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment July 14, 2020 | 0.355 | 0.388 | 9.421 | 0.301 | 3.351 | 9.507 | 1.464 | 0.334 | 1.121 | 0.518 | 0.684 | 3.43 |
| Onset of DISR August 31, 2020 | 0.752 | 0.076 | 15.132 | 0.061 | 2.25 | 6.125 | 1.52 | 0.066 | 1.247 | 0.397 | 0.125 | 6.391 |
| Post-surgery November 19, 2020 | 0.052 | 0.039 | 10.648 | 0.067 | 5.182 | 3.587 | 1.185 | 0.114 | 0.067 | 0.268 | 0.212 | 5.275 |
- Abbreviations: DISR, drug-induced sarcoidosis-like reaction; IFN-α, interferon-α; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-4, interleukin-4; IL-5, interleukin-5; IL-6, interleukin-6; IL-8, interleukin-8; TNF-α, tumor necrosis factor-α; IL-10, interleukin-10; IL-12p70, interleukin-12pro70; IL-17, interleukin-17.
The patient was started on pembrolizumab (200 mg on day 1, every 21 days) as adjuvant treatment starting 1 month after surgery from November 25, 2020. She was still receiving ongoing adjuvant treatment until the last follow up on August 31, 2021.
DISCUSSION
Neoadjuvant antiprogrammed cell death protein-1 (PD-1) therapy is provided to increase excision rates, improve pathological response rates, reduce recurrences, and improve survival outcomes for NSCLC patients.2 However, tumor progression or pseudoprogression can keep bewildering surgeons on the decision whether a patient should undergo further treatment. The most frequently affected sites of DISR are the mediastinal and hilar lymph nodes and lungs, followed by the skin and bone, which might mimic disease progression.1, 3, 4 Physicians and radiologists have to be aware of the possibility of DISR in patients receiving neoadjuvant ICIs. A thorough multidisciplinary workup should be performed, including biopsy, if necessary, to discriminate it from true disease progression.5 Moreover, monitoring long-term cytokine levels may help clinicians discriminate whether there is tumor progression or not.
Studies have shown high pretreatment levels of IL-6 (>11.4 pg/ml) may be related to shorter survival in melanoma patients.6 Consistently, the pre-ICI-treatment IL-6 level of our patient was 3.351 pg/ml, which is lower than the negative prognostic IL-6 level previously reported. Moreover, decreased serum IL-8 levels have been reported to indicate true response in NSCLC patients presenting pseudoprogression.7 In our case, when DISR occurred, the on-treatment IL-8 level of the patient decreased from 9.507 to 6.125 pg/ml, which is in accordance with the literature that although radiologically presenting as progression, a decrease in serum IL-8 may reflect a true response. It is interesting to note that patients who develop DISR after ICI treatment have an increased treatment response rate.8 To the best of our knowledge, this is the first report of complete pathological response after the occurrence of DISR mimicking radiographic disease progression induced by neoadjuvant anti-PD-1 therapy in patients with NSCLC. Prompt investigation of DISR in patients receiving neoadjuvant ICIs is necessary to avoid delaying surgery. A complete pathological response might be the potential reward for a clinician's carefully considered differential diagnosis of NSCLC progression.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest for this article.