EGFR-mutant lung adenocarcinoma associated with antisynthetase syndrome successfully treated with osimertinib
Abstract
Here, we report a rare case involving a 66-year-old man with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma and antisynthetase syndrome (ASS) treated with osimertinib. The patient presented with respiratory failure and bilateral pulmonary opacities; he was diagnosed with ASS accompanied by interstitial lung disease (ILD), consistent with paraneoplastic syndrome. After steroid pulse therapy, osimertinib was administered for lung adenocarcinoma without ILD exacerbation. Osimertinib could therefore be a treatment option for EGFR-mutant lung cancer with paraneoplastic ILD.
INTRODUCTION
For non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations, tyrosine kinase inhibitors (TKIs) are superior to cytotoxic agents, mainly in terms of progression-free survival.1-5 Osimertinib is a third-generation EGFR-TKI approved for the first-line treatment of EGFR-mutant NSCLC and later-line treatment for EGFR T790M-positive NSCLC.
Pre-existing interstitial lung disease (ILD) is a risk factor for TKI-related ILD.6 While studies have shown that cytotoxic agents are relatively safe for lung cancer patients with ILD,7 evidence on the safety of TKIs is scarce.
Antisynthetase syndrome (ASS) is characterized by the presence of serum anti-aminoacyl-transfer RNA synthetase (anti-ARS) antibodies, myositis, fever, polyarthritis, Raynaud's phenomenon, mechanic's hand, and ILD.8 ILD is observed in 75%–95% of ASS cases, sometimes being its only initial manifestation.9 Patients positive for anti-ARS antibodies could have complex cancers, with a reported prevalence rate of 12%.10 In such cases, ASS is considered a paraneoplastic syndrome.
Here, we report a case involving a 66-year-old man with lung adenocarcinoma (cT2aN3M1c, stage IVB, EGFR gene mutation exon 21 L858R) accompanied by ASS-ILD, who was successfully treated with osimertinib.
CASE REPORT
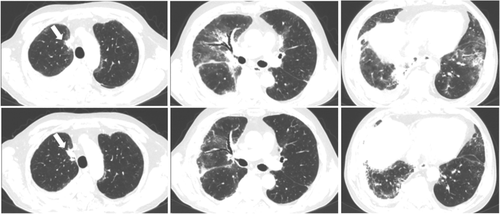
A 66-year-old man with dyspnea was admitted to Osaka Toneyama Medical Center (day 1). He was a former smoker. His body temperature was 36.6°C. He had tachypnea and percutaneous oxygen saturation of 95% (with oxygen supply via a nasal cannula at a flow rate of 2 L/min). Physical examination revealed Raynaud's phenomenon in his limbs and fine crackles in both lungs, but there was no cutaneous manifestation or muscle weakness. Blood samples showed elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), sialylated carbohydrate antigen (KL-6), carcinoembryonic antigen (CEA), and cytokeratin 19 fragment (CYFRA) levels. The creatine kinase (CK) level was normal, and antinuclear antibodies were not detected. The test for anti-ARS antibody was positive (anti-glycyl-tRNA synthetase [anti-EJ] antibody [3+] and anti-signal recognition particle [anti-SRP] [1+], EUROLINE Myositis Profile 3, EUROIMMUN, Germany) (Table 1). A computed tomography (CT) scan of the chest revealed bilateral ground-glass opacities (GGOs) with consolidations, right lung pleural effusion, a nodule in the upper lobe of the right lung, and mediastinal lymphadenopathies (Figure 1). Bronchoscopy was performed, and bronchoalveolar lavage fluid analysis at the right middle lobe (S4) showed that lymphocytes accounted for 80% of the total cells. Furthermore, histopathological examination of the mediastinal lymph node (4R) specimen revealed tumor cells with papillary structures that were positive for thyroid transcription factor-1 (TTF-1) and napsin A, determined by immunohistochemistry. He was diagnosed with adenocarcinoma (cT2aN3M1c, stage IVB, bone and brain metastases, and EGFR gene mutation exon 21 L858R), and ASS-ILD.
| Blood analysis | Arterial blood gas analysis | ||||
|---|---|---|---|---|---|
| WBC | 10 000/μl | CEA | 315 ng/ml | pH | 7.42 |
| Neu | 74.2% | CYFRA | 16.9 ng/ml | PaCO2 | 35.1 mmHg |
| Eos | 1.6% | BNP | 8.1 pg/ml | PaO2 | 91.9 mmHg |
| RBC | 495 × 104/μl | β-D-glucan | 17.7 pg/ml | HCO3− | 22.3 mmol/l |
| Hb | 14.8 g/dl | CMV antigen | (−) | ABE | −1.1 mmol/l |
| Plt | 34.3 × 104/μl | ANA | 80 | ||
| AST | 33 U/L | RF | 5 IU/ml | Respiratory function test | |
| ALT | 27 U/L | SS-A/Ro | (−) | FEV1 | 1.75 L |
| LDH | 338 U/L | PR3-ANCA | (−) | %FEV1 | 56.3 % |
| BUN | 20.1 mg/dl | MPO-ANCA | (−) | FEV1% | 84.5 % |
| Cre | 1.05 mg/dl | ARS | 120 | VC | 2.03 L |
| Na | 142 mEq/L | EJ | (3+) | %VC | 52.1 % |
| K | 5 mEq/L | SRP | (1+) | FVC | 2.07 L |
| Cl | 105.6 mEq/L | MDA-5 | (−) | %FVC | 54.6 % |
| Ca | 8.6 mg/dl | Scl-70 | (−) | ||
| TP | 6.8 g/dl | Centromere | (−) | ||
| Alb | 3.3 g/dl | RNP | (−) | ||
| CRP | 2.38 mg/dl | ss-DNA | (−) | ||
| CK | 249 U/ml | ds-DNA | (−) | ||
| KL-6 | 1903 U/ml | ||||
- Abbreviations: ABE, actual base excess; Alb, albumin; ALT, alanine transaminase; ANA, antinuclear antibody; ARS, anti-aminoacyl-tRNA synthetase antibody; AST, aspartate transaminase; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; Ca, calcium; CEA, carcinoembryonic antigen; centromere, anticentromere antibody; CK, creatine kinase; Cl, chloride; CMV, cytomegalovirus; Cre, creatinine; CRP, C-reactive protein; CYFRA, cytokeratin 19 fragment; ds-DNA, anti-double stranded DNA antibody; EJ, anti-glycyl-tRNA synthetase antibody; Eos, eosinophils; FEV1%, forced expiratory volume in 1 second percentage; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; Hb, hemoglobin; K, potassium; KL-6, sialylated carbohydrate antigen; LDH, lactate dehydrogenase; MDA-5, anti-melanoma differentiation-associated gene 5 antibody; MPO-ANCA, anti-myeloperoxidase-antineutrophil cytoplasmic antibody; Na, sodium; Neu, neutrophils; Plt, platelet count; PR3-ANCA, anti-proteinase 3-antineutrophil cytoplasmic antibody; RBC, red blood cell count; RF, rheumatoid factor; RNP, anti-ribonucleoprotein antibody; Scl-70, anti-scleroderma-70 antibody; SRP, anti-signal recognition particle antibody; ss-DNA, anti-single stranded DNA antibody; TP, total protein; VC, vital capacity; WBC, white blood cell count.

Methylprednisolone (1000 mg) was administered for three days, followed by oral prednisolone (60 mg/day) and tacrolimus (5 mg/day) (Figure 2). Subsequently, normalization of the CRP and LDH levels, decrease in the KL-6 and CEA levels, and radiological improvement on chest X-ray were observed. After stereotactic radiosurgery for brain metastases, osimertinib was initiated at 40 mg/day, and the patient was discharged. A CT scan was performed on day 133 (day 33 of osimertinib treatment), when the primary lesion showed a partial response, and bilateral interstitial opacities showed an improvement. Thereafter, he developed renal dysfunction attributable to osimertinib; and the osimertinib dose was subsequently decreased to 40 mg every other day. Tacrolimus was terminated on day 314, and prednisolone was discontinued on day 342 after tapering.
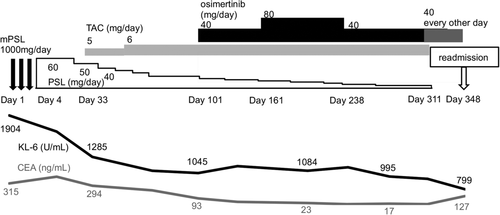
On day 348, the patient was readmitted with fever and desaturation. Bilateral lung opacities and enlarged primary lung tumors consistent with progressive disease were observed (Figure 3). A blood test revealed elevated levels of the following which reflected tumor progression and recurrence of inflammation: CRP, 4.07 mg/dl; LDH, 357 U/L; KL-6, 799 U/ml; and CEA 12.7 ng/ml. After steroid pulse therapy followed by oral prednisolone and tacrolimus, second-line chemotherapy was initiated with carboplatin and nab-paclitaxel. The patient is currently receiving treatment.
DISCUSSION
The rate of cancer occurrence in patients with ASS is 1.7%–19.4%,11, 12 which is equivalent to, or higher than, that in the general population. According to a retrospective analysis of 165 Japanese patients with ASS, 19 had cancer, of which three had lung cancer.10 In case reports of ASS patients with NSCLC, ASS behavior was in parallel with that of tumors.13-15 In these cases, systemic corticosteroids were first administered to stabilize ILD. NSCLC was subsequently treated, and both complete tumor resection and cytotoxic chemotherapy with or without radiotherapy were effective. In our case, ASS, which was detected simultaneously with lung cancer, was considered a paraneoplastic syndrome. The patient was also first treated for ASS with corticosteroids and subsequently with systemic chemotherapy. As ASS was expected to be controlled by effective lung cancer treatment, we treated the patient with osimertinib because of its efficacy in systemic lesions. Consequently, tumor shrinkage was achieved without apparent drug-induced ILD. Although ASS-ILD remained under control after the discontinuation of corticosteroids, ASS-ILD recurred simultaneously with tumor progression, supporting the paraneoplastic origin of ILD.
Contrarily, a fatal exacerbation of ASS-ILD following nivolumab administration has been previously reported,14 indicating that chemotherapeutic use with ILD risks, such as immune checkpoint inhibitors, requires caution. In patients with NSCLC treated with osimertinib, the frequency of drug-related ILD has previously been reported to be 2%–4%.16, 17 In our case, we chose osimertinib owing to its efficacy in systemic lesions, after informing the patient about potential drug-induced ILD exacerbation. The patient did not experience drug-induced ILD until ASS-ILD recurrence with tumor progression.
Although TKI use in patients with ILD requires careful consideration, our case suggests that osimertinib could be a treatment option for EGFR-mutant NSCLC with paraneoplastic ILD.
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with this manuscript. There was no support in the form of grants, gifts, equipment, or drugs for this study.