Stereotactic ablative radiotherapy as single treatment for early stage non-small cell lung cancer: A single institution analysis
Abstract
Background
Stereotactic ablative radiotherapy (SABR) is the current standard-of-care in cases of inoperable early stage non-small cell lung cancer (ES-NSCLC). This study aimed to assess the survival outcomes and recurrence patterns after SABR for ES-NSCLC in a hospital setting.
Methods
A single-institution retrospective study was performed which included 109 patients who had undergone SABR. The main study endpoints were overall survival (OS), cancer specific survival (CSS), local recurrence-free survival (LRFS), regional recurrence free survival (RRFS) and distant metastasis-free survival (DMFS). Univariate and multivariate analysis were conducted to explore the potential factors which might be related to patient survival.
Results
A total of 109 patients were enrolled into the study. Median follow-up was 44 months (range: 2–93 months). (i) Recurrence results: Among 45 patients with recurrence, 30 patients (28%) had distant metastasis (DM), 17 patients (16%) had local recurrence (LR), 10 patients (9%) had regional recurrence (RR) of lymph nodes and two patients (2%) had second primary lung cancer (SPLC). (ii) Survival results: Median OS, CSS, PFS was 78 months, 78 and 40 months. Two-year OS, CSS, PFS, LRFS, RRFS and DMFS was 84.7%, 87.1%, 69.2%, 86.8%, 92.7% and 78.0%, respectively. Four-year OS, CSS, PFS, LRFS, RRFS and DMFS was 55.6%, 60.7%, 37.3%, 76.3%, 88.4% and 59.4%, respectively. (iii) Univariate and multivariate analyses indicated that age was a prognostic factor of CSS in patients aged <75 years (P = 0.04 HR 2.12 95% confidence interval [CI]: 1.04–4.33).
Conclusions
Although high survival rates can be achieved in ES-NSCLC patients treated with SABR, using SABR on its own may not be enough. Prolonged surveillance and adjuvant therapy is therefore needed.
Introduction
Lung cancer is a commonly diagnosed malignancy and a major cause of oncologic morbidity and mortality. Although lung cancer historically has a poor prognosis, early stage NSCLC (ES-NSCLC) generally has more favorable outcomes.1 Stereotactic ablative radiotherapy (SABR), also known as stereotactic body radiotherapy (SBRT), has been defined by the American Society of Radiation Oncology and the American Society of Radiology as an external beam radiation therapy method used to very precisely deliver a high dose of radiation to an extracranial target within the body, using either a single dose or a small number of fractions.2 SABR involves the use of multiple conformal radiation beams that deliver high doses of radiation and are individually tailored to avoid radiosensitive organs in the proximity of the tumor.
Clinical use of SABR has increased dramatically in the past 20 years, The NRG Oncology Radiation Therapy Oncology Group (RTOG) 0236 trial,3 as well as several other international cooperative group trials4, 5 has shown that SBRT affords high rates of tumor control while avoiding severe toxic effects in the majority of patients who are unable to tolerate surgical resection, such as the elderly, those who are unwilling to accept the risks of surgical resection, and those with severe chronic obstructive pulmonary disease. This local control has translated into a survival advantage over conventional radiotherapy.6 SABR is the current standard-of-care in cases of inoperable ES-NSCLC.
In recent years, there have been many studies on SABR in ES-NSCLC,4, 5, 7 but there are few reports on its prognostic factors and failure patterns.8 Herein, our study evaluated the survival outcomes, and reports on the detailed recurrence patterns of ES-NSCLC patients treated with SABR at a single institution.
Methods
Patient population
From January 2011 to December 2018, we identified 109 patients who received SABR for inoperable T1-2aN0M0 NSCLC (American Joint Committee on Cancer, seventh edition, guidelines)9 in Henan cancer hospital.
Radiotherapy treatment
The SABR treatment methods and tumor volume delineation for lung cancer were performed as previously described.10 All patients underwent four dimensional (4D) computed tomography (CT) simulation (4DCT). Target delineation was based on standard International Commission on Radiation Units and Measurements (ICRU) definitions. The gross target volume (GTV) was delineated on the 3DCT images. IGTV was contoured on the 4DCT MIP scans. Internal target volume (ITV) was generated by combining gross tumor volume (GTV) and internal gross tumor volume (IGTV). Planning target volume (PTV) had to be created to account for both set-up error and error related to motion followed by a 5 mm circumferential ITV expansion. Three-dimensional conformal radiation therapy (3D-CRT), intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) were used (True Beam SN1403 accelerator, Varian Medical Systems) in the study.11Cone-beam CT (CBCT) was performed for optimal alignment during treatment set-up. The SABR regimens were adapted and depended on the tumor size and location. The SABR fractionation schedule was 48 Gy/4f or 50 Gy/5f or 55 Gy/5f for peripheral lung cancer and 60 Gy/8f for tumors located centrally or closer to the chest wall. Dosimetry required that the 100% isodose line cover 95% of the PTV. However, a PTV under dosage was permitted in order to protect the organ at risk. SABR was delivered every day or every other day.
Follow-up
After the treatment, computed tomography imaging (CT) was performed every three months for the first two years and at six month intervals thereafter. Magnetic resonance imaging (MRI) of the brain was performed once at three months or when clinical signs and symptoms suspicious for brain involvement were present. Recurrences were documented by biopsy unless there was clear evidence of metastatic disease on CT or positron emission tomography (PET)/CT scan.
Endpoints of the study
Patterns of failure were categorized into local, regional and metastatic recurrence. Local recurrence (LR) was defined as the local failure, which was any of the following: primary enlargement confirmed by CT or biopsy, or involved lobe failure. Regional recurrence (RR) referred to the ipsilateral hilus, mediastinum, or supraclavicular fossa lymph nodes. Distant metastasis (DM) was defined as recurrence in other sites.5 Second primary lung cancer (SPLC) was defined as a new pulmonary malignancy occurring in a different lobe or lung than the first tumor with no intervening lymph nodes and no evidence of metastases, using all available radiological and pathological information.12 Cancer-specific survival (CSS) was defined as from the date of SABR to the date of death from cancer, or at the date of the last follow-up. Progression-free survival (PFS) was defined as the time from the date of SABR until the earliest signs of disease progression or death from any cause or last follow-up. Local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS) and distant metastasis-free survival (DMFS), were calculated from the first day of treatment to local or regional recurrence, or distant metastasis.
Statistical analysis
Statistical analysis was calculated using the SPSS version 24.0 (IBM Software Group, Chicago, USA). Survival analysis was evaluated via the Kaplan-Meier method, and the Log-rank test was used to detect potential differences. Cox regression analysis was performed to determine whether any tumor characteristics and treatment-related variables were predictors of OS, CSS, PFS and LRFS. Candidate factors with P < 0.05 on univariate analysis were incorporated into a multivariate model. All statistical tests were two-sided, and the level of significance was set to 5% (P < 0.05) for all statistical analyses.
Results
Patient characteristics
There were 109 medically inoperable ES-NSCLC patients who were treated with SABR and were eligible for analysis in our institution. A total of 85 (78%) were male and 24 (22%) were female, with a median age of 73 years (range: 33–86 years), 77 (71%) patients had clinical T1 disease, and 32 (29%) had clinical T2a disease. Among 109 patients, 89 (81%) patients had histologically confirmed disease, and 20 (18%) patients did not. All 20 patients were examined by PET/CT and carefully reviewed by a lung cancer multidisciplinary team. The median lesion diameter was 2.2 cm (range: 1–5 cm). Indicators defining a patient to be medically inoperable included baseline forced expiratory volume in the first second of expiration (FEV1) of less than 40% predicted, severe pulmonary hypertension, severe cardiovascular, or severe chronic heart disease. The median biologically effective dose (BED) was 100 Gy (100–119 Gy). During the study period, two SABR planning and delivery techniques were used. Initially, 3D-CRT was used in 37 patients and IMRT or VMAT was used in 72 patients. A total of 92 patients (84%) had a good performance status with Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 at diagnosis. The main characteristics of the selected patients are illustrated in Table 1.
| Patients (n = 109) | ALL (n = 109,%) | Patients (n = 109) | ALL (n = 109,%) | ||
|---|---|---|---|---|---|
| Age (years) | BED10 (Gy) | ||||
| Median | 73 (33–86) | Median | 100 (100–119) | ||
| <75 | 64 | 59 | SABR regimens | ||
| >75 | 45 | 41 | 10 Gy × 5f | 65 | 60 |
| Sex | 11 Gy × 5f | 12 | 10 | ||
| Male | 85 | 78 | 12 Gy × 4f | 15 | 14 |
| Female | 24 | 22 | 7.5 Gy × 8f | 12 | 11 |
| ECOG PS | 8 Gy × 8f | 1 | 1 | ||
| 0/1 | 92 | 84 | 7 Gy × 8f | 2 | 2 |
| 2 | 17 | 16 | 7 Gy × 10f | 2 | 2 |
| Smoking history | inoperable cause | ||||
| Yes | 70 | 64 | FEV1<40% predicted | 35 | 32 |
| No | 39 | 36 | Pulmonary hypertension | 13 | 11 |
| Tumor diameter | Cardiovascular | 15 | 14 | ||
| Median | 2.2 (1–5) | chronic heart disease | 40 | 37 | |
| Tumor location | Diabetes | 6 | 5 | ||
| Peripheral | 94 | 86 | SABR delivery technique | ||
| Central | 15 | 14 | 3DCRT | 37 | 34 |
| TNM | IMRT/VMAT | 72 | 66 | ||
| T1 | 77 | 71 | IDT (day) | ||
| T2a | 32 | 29 | ≥30 | 35 | 32 |
| Histology | <30 | 74 | 68 | ||
| Squamous cell carcinoma | 37 | 34 | |||
| Adenocarcinoma | 52 | 48 | |||
| Not attained | 20 | 18 |
- FEV1, forced expiratory volume in 1 second; IDT, interval from diagnosis to treatment.
Recurrence patterns
Median follow-up was 44 months (range: 2–93 months). Table 2 summarizes the patterns of failure. Among the 109 patients, 45 (41%) failures were observed. The one-, two-, three- and four-year recurrence rates were 6%, 19%, 28% and 38%, respectively. A total of 30 patients (28%) developed DM, which was the most frequently seen pattern of failure, and 17 patients (16%) developed LR including four patients with primary recurrence and 13 patients with recurrence in the same pulmonary lobe. RR rate was as low as 9% and two (2%) patients developed SPLC. The most common site of initial DM was pulmonary (37%); other common sites were brain (37%), bone (17%), adrenal (10%), pleura (7%), retroperitoneal lymph node (3%) and pancreas (3%). The failure patterns are illustrated in Table 2.
| Total (n = 109) | Total (%) | Total recurrence (n = 45, %) | Distant metastasis (n = 30, %) | |
|---|---|---|---|---|
| Total recurrences | 45 | 41% | 100% | |
| LR | 17 | 16% | 38% | |
| Primary | 4 | 4% | 9% | |
| Involved lobe | 13 | 12% | 29% | |
| Solitary LR | 8 | 7% | 17% | |
| LR + RR | 1 | <1% | 2% | |
| RR + DM | 7 | 6% | 16% | |
| LR + RR + DM | 1 | <1% | 2% | |
| RR | 10 | 9% | 22% | |
| Solitary RR | 4 | 4% | 9% | |
| RR + DM | 4 | 4% | 9% | |
| DM | 30 | 28% | 67% | |
| Solitary DM | 18 | 17% | 40% | |
| SPLC | 2 | 2% | 4% | |
| DM site | ||||
| Lung | 11 | 37% | ||
| Ipsilateral different lobes | 6 | 20% | ||
| Bilateral lung | 5 | 17% | ||
| Pleural | 2 | 7% | ||
| Brain | 11 | 37% | ||
| Bone | 5 | 17% | ||
| Adrenal | 3 | 10% | ||
| Retroperitoneal lymph node | 1 | 3% | ||
| Pancreas | 1 | 3% | ||
- DM, distant metastasis; LR, local recurrence; RR, regional recurrence; SPLC, second primary lung cancer.
Survival and prognostic factors
During the follow-up period, 37 patients died; 31 of cancer, and six of other causes. Among them, two patients died of cerebral hemorrhage, one patient died of a traffic accident, two patients died of heart disease and one patient died of massive hemoptysis induced by bronchiectasis.
The survival results calculated by Kaplan-Meier method showed that the median OS, CSS and PFS was 78 months, 78 months and 40 months respectively. Two-year OS, CSS, PFS, LRFS, RRFS, DMFS was 84.7%, 87.1%, 69.2%, 86.8%, 92.7% and 78.0%, respectively. Four-year OS, CSS, PFS, LRFS, RRFS, and DMFS was 55.6%, 60.7%, 37.3%, 76.3%, 88.4% and 59.4%, respectively, as shown in Figs 1 and 2.
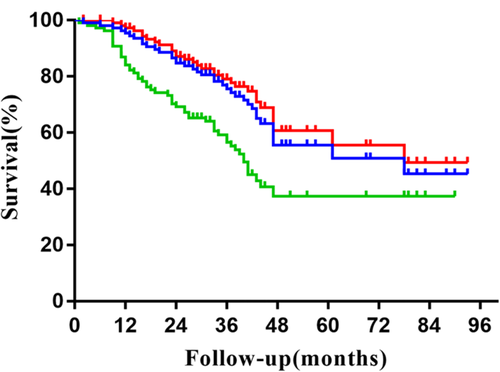
 , OS;
, OS;  , CSS;
, CSS;  , PFS.
, PFS.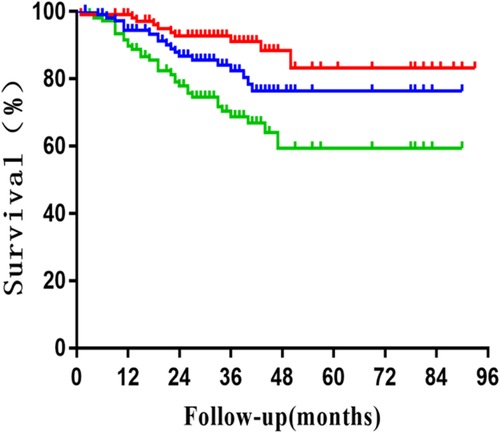
 , LRFS;
, LRFS;  , RRFS;
, RRFS;  , DMFS.
, DMFS.On univariate analysis predictors for OS, CSS, PFS and LRFS included age, sex, ECOG PS, histology, T stage, smoking history, tumor location and interval from diagnosis to treatment (IDT). The univariate and multivariate analyses showed that age was a prognostic factor of CSS in patients aged <75 years. The two- and four-year CSS was 90.1% and 76.2%, respectively whereas in patients aged ≥75 years was 82.6% and 32.6%, respectively (P = 0.04 HR 2.12 95% confidence interval [CI]: 1.04–4.33), as shown in Table 3.
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
| Clinical factors | P-value | Hazard ration (HR) (95% CI) | P-value |
| OS | |||
| Age (<75 vs. ≥75) years | 0.02 | 0.13 | |
| Sex (male vs. female) | 0.09 | ||
| ECOG PS (0–1 vs. 2) | 0.02 | 0.35 | |
| Smoking history (Yes vs. No) | 0.04 | 0.08 | |
| Location (peripheral vs. central) | 0.79 | ||
| T stage (T1 vs. T2a) | 0.15 | ||
| Histology (squamous vs. adenocarcinoma vs. none) | 0.78 | ||
| IDT (<30 days vs. ≥30 days) | 0.44 | ||
| EGFR mutation (Yes vs. No) | 0.47 | ||
| CSS | |||
| Age (<75 vs. ≥75) years | 0.02 | 2.12 (1.04–4.33) | 0.04 |
| Sex (male vs. female) | 0.09 | ||
| ECOG PS (0–1 vs. 2) | 0.09 | ||
| Smoking history (Yes vs. No) | 0.03 | 0.05 | |
| Location (peripheral vs. central) | 0.89 | ||
| T stage (T1 vs. T2a) | 0.32 | ||
| Histology (squamous vs. adenocarcinoma vs. none) | 0.7 | ||
| IDT (<30 vs. ≥30) days | 0.46 | ||
| EGFR mutation (Yes vs. No) | 0.76 | ||
| PFS | |||
| Age (<75 vs. ≥75) years | 0.56 | ||
| Sex (male vs. female) | 0.07 | ||
| ECOG PS (0–1 vs. 2) | 0.11 | ||
| Smoking history (Yes vs. No) | 0.12 | ||
| Location (peripheral vs. central) | 0.45 | ||
| T stage (T1 vs. T2a) | 0.28 | ||
| Histology (squamous vs. adenocarcinoma vs. none) | 0.63 | ||
| IDT (<30 vs. ≥30) days | 0.3 | ||
| EGFR mutation (Yes vs. No) | 0.18 | ||
| LRFS | |||
| Age (<75 vs. ≥75) years | 0.94 | ||
| Sex (male vs. female) | 0.24 | ||
| ECOG PS (0–1 vs. 2) | 0.95 | ||
| Smoking history (Yes vs. No) | 0.07 | ||
| Location (peripheral vs. central) | 0.66 | ||
| T stage (T1 vs. T2a) | 0.19 | ||
| Histology (squamous vs. adenocarcinoma vs. none) | 0.61 | ||
| IDT (<30 vs. ≥30) days | 0.05 | ||
| EGFR mutation (Yes vs. No) | 0.78 | ||
- CSS, cancer specific survival; ECOG PS, Eastern Cooperative Oncology Group Performance Status; IDT, interval from diagnosis to treatment; LRFS, local recurrence-free survival; OS, overall survival; PFS, progression-free survival.
Discussion
Over the last decade, SABR has gained ground and has become a valuable addition in the treatment of early stage NSCLC, especially for elderly patients and patients whose conditions are inoperable due to comorbid disease or who would rather not undergo surgery.13, 14 The RTOG0236 study7 reported that five-year local recurrence rate was 7% and five-year OS was 40%. Sun et al.15 reported that 65 cases of T1N0M0 NSCLC were followed-up for seven years after treatment with SABR (50 Gy/4f). Five- and seven-year PFS, OS were 49.5%, 38.2%, 55.7% and 47.5% respectively. The JCOG040316 study showed that the three-year OS of 100 inoperable and 64 operable T1N0M0 NSCLC patients treated with SABR (48 Gy/4f) was 59.9% and 76.5%, respectively. The RTOG0915 study17 evaluated 84 patients with T1-2N0M0, comparing the efficacy of 34 Gy/1f and 48 Gy/4f and median follow-up was 30.2 months. The two-year OS in the 34 Gy/1f group and the 48 Gy/4f group was 61.3% and 77.7%, respectively. At present, there is no unified standard scheme for SABR segmentation of lung cancer. In a multicenter retrospective study in Japan,18 BED <180 Gy was reported to be safe in 257 ES-NSCLC patients who received SABR treatment in 14 centers. In this range, the five-year OS of patients with BED ≥100 Gy reached 70.8%, which was significantly higher than that of the BED <100 Gy group. BED ≥100 Gy is currently the widely accepted total dose for SABR. Similarly, in our study, two-year OS, CSS, PFS, LRFS, RRFS and DMFS was 84.7%, 87.1%, 69.2%, 86.8%, 92.7% and 78.0%, respectively. Four year OS, CS, PFS, LRFS, RRFS and DMFS was 55.6%, 60.7%, 37.3%, 76.3%, 88.4% and 59.4%, respectively.
Univariate analysis showed that patients aged <75 years, ECOG PS 0–1 score and no smoking history were associated with OS (P = 0.02, 0.02, 0.04), However, these factors were not statistically significant in the multivariate analysis. Age was an independent prognostic factor of CSS in patients aged <75 years. The two- and four-year CSS was 90.1% and 76.2%, respectively, whereas in patients aged ≥75 years was 82.6% and 32.6%, respectively (P = 0.04). These results are inconsistent with previous reports. Brooks et al19 reported that there was no difference in CSS (P = 0.275) in patients aged <75 years when compared with patients aged ≥75 years. Mihai et al.20 reported that in 200 patients treated with SABR, the median OS was 31.6 months with one-, and three-year survival rates of 80.7%, and 44.4%, respectively. The possible reason for this is that when patients aged <75 years develop recurrence they are more likely to receive salvage treatment. However, older patients can successfully complete SABR for NSCLC, but might simultaneously require increased supportive care.
Recurrence patterns after SBRT vary but may involve the primary and lobe alone, involve the lobe and disseminated, lymph node alone, lymph node and disseminated, and disseminated alone. In the current study, the predominant failure pattern was distant recurrence. Isolated DM, LR and RR accounted for 40%, 17% and 9% of all recurrences, respectively and was similar to a previous study8 which reported that isolated distant recurrence accounted for 46% of all recurrences. RR was not the common pattern. It has also been previously reported that an incidental hilar dose greater than 20 Gy reduced ipsilateral hilar relapse.8
One-, two-, three- and four-year recurrence rates were 6%, 19%, 28% and 38%, respectively, almost 5% per patient per six months in the first three years. Hence, the subsequent three years provides the basis for identifying efficient follow-up schedules. When such recurrences develop after SABR, the use of salvage treatments can have a significant positive prognostic effect.21 It has been documented that the antitumor effects of immunotherapy and radiation are carried out by lymphocytes (specifically, CD8+ effector cells).22 Our previous study10 reported that the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and lymphocyte-monocyte ratio (LMR) could be considered useful prognostic indicators of OS following SABR. The exploratory results in this study require further validation and may potentially help to guide our future research direction.
Solitary lung recurrences or SPLCs have been the focus of recent interest. Similar to our study, the median time to diagnosis of metachronous solitary lung nodules was longer than for local, regional or distant recurrences in other reports.23 Verstegen et al.24 reported that SPLC was diagnosed after a median interval of 34 months. Wink et al.25 reported that the mean incidence of RR after SBRT was found to be almost 10%. Furthermore, second primary lung cancers (SPLC) have been reported to develop at an estimated crude rate of approximately 6%.8 In our study, the two SPLC patients were diagnosed at 34 and 44 months, respectively. These findings indicate the need for prolonged surveillance.
There were several limitations to this study. First, it was a retrospective study from a single institution with a limited follow-up period. Second, the radiation dose scheme of SABR was not unified. Hence further prospective investigation is needed to confirm the results.
In conclusion, although high survival rates can be achieved in ES-NSCLC patients treated with SABR, among the 109 patients in this study, 45 (41%) failures were observed. Treatment with SABR may not be enough on its own. Prolonged surveillance and adjuvant therapy is needed.
Acknowledgments
The study was funded by the Surface Project from National Natural Science Foundation of China (81373230).
Disclosure
The authors declare that they have no conflict of interest.




