Matrix metalloproteinase-14 (MMP-14) downregulation inhibits esophageal squamous cell carcinoma cell migration, invasion, and proliferation
Abstract
Background
Matrix metalloproteinase-14 (MMP-14) is known to be a key regulator of oncogenesis and tumor progression. The present study was designed to assess the relationship between the downregulation of MMP-14 and the in vitro proliferative, migratory, and invasive activity of esophageal squamous cell carcinoma (ESCC) cells.
Methods
MMP-14 expression in human ESCC and paracancerous normal esophageal tissue samples was evaluated via immunohistochemistry, and correlations between MMP-14 staining and patient clinicopathological features were examined. In addition, siRNA was used to knockdown MMP-14 in ESCC cells, and the proliferation and invasive activity of these cells were then evaluated via MTT and Transwell assays, respectively. Flow cytometry was additionally used to assess cell cycle progression, while Western blotting was employed to measure protein levels within these cells.
Results
ESCC samples were found to exhibit MMP-14 overexpression relative to paracancerous tissue samples, and this overexpression was positively correlated with tumor T classification (T1-2 vs. T3; P < 0.05), N classification (negative vs. positive; P < 0.001), degree of differentiation (G1 vs. G3, P < 0.05; G2 vs. G3, P < 0.05) and clinical stage (I–IIA vs. IIB–III; P < 0.05). When MMP-14 was knocked down in ESCC cells, this induced cell cycle arrest, impairing their proliferative and invasive activity.
Conclusions
MMP-14 is a key regulator of the proliferation and invasion of ESCC cells, making it a viable therapeutic target for the treatment of this cancer.
Introduction
Esophageal cancer (EC) remains the sixth leading cause of cancer-associated death globally, with esophageal squamous cell carcinoma (ESCC) being the primary form of EC that is observed in China and other Asian countries. While therapeutic advances in recent years have improved the prognosis of this disease, ESCC patients still have a relatively low five-year survival rate,1 primarily due to the fact that this cancer is often not diagnosed until at a relatively advanced stage and is prone to metastasis.2 As such, it is crucial that new diagnostic biomarkers and therapeutic targets associated with ESCC be identified so as to improve patient detection and treatment.
Matrix metalloproteinase-14 (MMP-14) is a membrane-bound proteinase that is capable of cleaving extracellular proteins and that has been shown to be of key importance for the invasion and metastasis of cancer cells.3 While MMP-14 expression is linked with poor prognosis in patients with different cancers,4 the prognostic relevance of MMP-14 in ESCC patients remains to be fully explored.
To examine the impact of MMP-14 expression of ESCC development and progression, in the present study we assessed the association between MMP-14 levels and clinicopathological findings in ESCC patients. In addition, we assessed the functional importance of this protein in vitro by employing an siRNA-based approach to achieve MMP-14 knockdown in ESCC cells.
Methods
Patients and tumor samples
A tissue microarray (TMA) (NO. BS17017b) containing ESCC patients and corresponding key clinicopathological data was obtained from Alenabio Biotechnology LTD (Xi'an, China). This TMA included 120 total EC samples and 30 paracancerous normal esophageal tissue samples. Demographic and clinicopathological findings pertaining to patients included in this TMA are compiled in Table 1.
| Clinicopathological features | No. of patients (%) | |
|---|---|---|
| Gender | Male | 84 (70.0%) |
| Female | 36 (30.0%) | |
| Age | ≤60 | 79 (65.8%) |
| >60 | 41 (34.2%) | |
| T classification | T1–2 | 31 (25.8%) |
| T3 | 89 (74.2%) | |
| N classification | Negative | 72 (60.0%) |
| Positive | 48 (40.0%) | |
| Differentiated degree | G3 | 37 (30.8%) |
| G2 | 43 (35.8%) | |
| G1 | 40 (33.4%) | |
| Clinical stage | I–IIA | 72 (60.5%) |
| IIB–III | 47 (39.5%) | |
| MMP-14 expression | High | 40 (33.3%) |
| Low | 80 (66.7%) | |
Immunohistochemistry (IHC)
IHC was conducted via a streptavidin-peroxidase (SP) approach using standard protocols. Tissue section staining was conducted with rabbit anti-human MMP-14 (clone no. EP1264Y, dilution 1:100, Abcam, MA, USA), while control samples were stained with a nonspecific IgG. Two pathologists blinded to patient clinical parameters then independently evaluated and scored tissue sections as in prior studies.5 Briefly, MMP-14 expression was scored based upon both staining intensity and the proportion of positively stained tumor cells. The staining index was calculated as follows: staining index = staining intensity × proportion of positively stained tumor cells. Using this method of assessment, we evaluated the expression of MMP-14 by staining index (scored as 0, 1, 2, 3, 4, 6, or 9). Final staining scores of 0–5 and 6–9 were indicative of low and high MMP-14 expression, respectively.
Cell culture
Human esophageal carcinoma cell line Eca-109 was obtained from Cell Bank, Chinese Academy of Sciences (Shanghai, China) and maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, USA) containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA) at 37°C with 5% CO2.
Cellular transfection
siRNA targeting human MMP14 and nontargeted siRNA were purchased from by Shanghai GenePharma Co (Shanghai, China). Sequences of the siRNAs are as follows: 5′-AACAGGCAAAGCUGAUGCAGAdTdT-3′ (sense) and 5′-AAUCUGCAUCAGCUUUGCCUGdTdT-3′ (antisense) for MMP14 siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense) for non-targeted siRNA as a negative control. Cells were transfected based on provided directions.
Reverse transcription polymerase chain reaction (RT-PCR)
An RNAfast200 Total RNA Extract Kit (Fastgene, Shanghai, China) was used to isolate total RNA from cell samples, after which cDNA was generated via reverse transcribing 2 μg of RNA per sample with a RevertAid First Strand cDNA Synthesis Kit (Fermentas, MBI, Lithuania). Primers used in this analysis included MMP-14-F: 5′-CGAGGTGCCCTATGCCTAC-3′, MMP-14-R: 5′-CTCGGCAGAGTCAAAGTGG-3′, GAPDH-F: 5′-GGTATCGTGGAAGGACCATGAC-3′, and GAPDH-R: 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′. All PCR reactions were performed in a 25 μL total volume and were resolved using 2% agarose gels.
Cell proliferation assay
An MTT assay was used to assess cell proliferation as in prior studies.6 Briefly, cells were seeded at 5 × 103 per well in 96-well flat-bottom plates one day before transfection. The MTT assay was performed just before transfection as well as 24, 48, and 72 hours after transfection using a microplate reader (Bio-Rad, Hercules, CA, USA).
Cell cycle analysis
Flow cytometric analyses (FACSCalibur, BD, CA, USA) of cells that had been stained with propidium iodide (PI) were used to assess cell cycle progression in triplicate experiments, as in prior reports.7 Briefly, the cells were fixed with cold 70% ethanol for at least 1 hour at 4°C after cell collection. Before flow cytometric analysis, the cells were incubated with RNase at 0.01 mg/mL and PI at 50 μg/mL in the dark at room temperature for 30 minutes. A total of 104 cells were analyzed for each sample. Cell cycle analysis was performed by ModFit LT version 3.0 software (Verity Software House, Topsham, ME, USA). Experiments were done in triplicate.
Western blotting
Western blotting was conducted as in prior reports.8 Briefly, RIPA buffer supplemented with protease inhibitors was used to lyse cells, after which equivalent protein amounts from each sample were separated via SDS-PAGE and transferred to nitrocellulose membranes. Blots were then incubated with appropriate primary antibodies overnight at 4°C, followed by secondary antibody incubation. An ECL detection system (Pierce, IL, USA) was then used to detect protein bands, with GAPDH being used for normalization purposes.
Cell migration assay
Wound healing assays were used to evaluate ESCC cell migration as in prior reports.9 Briefly, cells were plated in six-well plates and grown to confluence, at which time a sterile micropipette tip was used to generate a scratch wound. Detached cells were then removed via washing with PBS, and fresh DMEM containing 10% FBS was added. Cell migration was evaluated at 0 and 24 hours post-wounding with a Nikon Eclipse TE300 microscope, with ImageJ being used to measure wound closure. The wound closure percentage was the ratio of the final wound width to the initial wound width.
Cell invasion assay
For invasion assays, transwell chambers (Millipore, MA, USA) (8 μm pore size) were coated using Matrigel (15 μg/filter), and experiments were then conducted in triplicate as in prior studies.10
Statistical analysis
Data are means ± SD or percentages and are representative of the results of three or more experimental repetitions. Data were compared between subgroups via Student's t-tests, while chi-squared tests were used for evaluating the association between MMP-14 expression and patient clinicopathological characteristics. SPSS v13.0 (SPSS, IL, USA) was used for statistical testing, with P < 0.05 serving as the significance threshold.
Results
MMP-14 upregulation is commonly observed in human ESCC
We began by employing an IHC approach to evaluate MMP-14 expression in 120 ESCC and 30 normal paracancerous tissue samples from human patients. This analysis revealed that MMP-14 expression was primarily restricted to the cytoplasm in ESCC cells, whereas it was largely undetectable in normal esophageal tissue samples (Fig 1). Representative tissue sections highlighting different levels of tissue MMP-14 staining are shown in Fig 2, with final sample IHC scores of 0–5 being considered to indicate low MMP-14 expression, while scores of 6–9 were considered to indicate high MMP-14 expression. We ultimately determined that 80/120 (66.67%) ESCC samples exhibited high MMP-14 expression, whereas the same was true for 0/30 (0%) paracancerous tissue samples (P < 0.05).
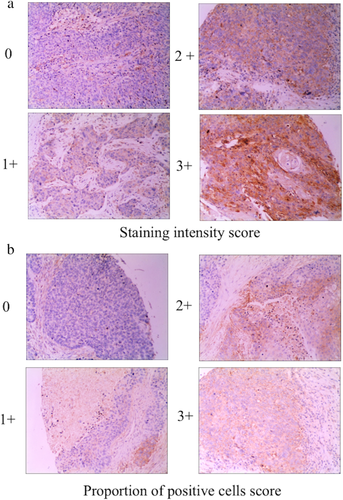
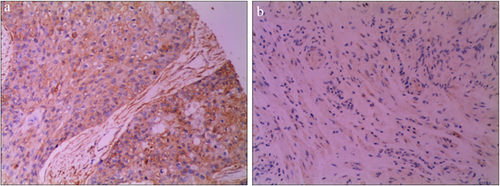
MMP-14 expression is associated with ESCC patient clinicopathological findings
To assess the clinical relevance of MMP-14 expression levels in ESCC tissues, we next assessed the association between MMP-14 expression and clinicopathological findings for these 120 ESCC patients. This analysis revealed high MMP-14 expression to be significantly related to tumor T classification, N classification, degree of differentiation, and clinical stage (P < 0.05, Table 2). In contrast, we detected no significant relationship between high MMP-14 expression and patient age or sex (P > 0.05, Table 2). These findings thus raise the possibility that elevated MMP-14 expression may enhance ESCC progression.
| MMP-14 expression (n) | |||||
|---|---|---|---|---|---|
| Clinicopathological features | No. of patients | High | Low | P-value | |
| Gender | Male | 84 | 26 | 58 | |
| Female | 36 | 14 | 22 | 0.398 | |
| Age | ≤60 | 79 | 31 | 48 | |
| >60 | 41 | 9 | 32 | 0.057 | |
| T classification | T1-2 | 31 | 4 | 27 | |
| T3 | 89 | 25 | 54 | 0.045 | |
| N classification | Negative | 72 | 19 | 53 | |
| Positive | 48 | 21 | 27 | 0.048 | |
| Differentiated degree | G3 | 37 | 7 | 30 | |
| G2 | 43 | 17 | 26 | 0.045† | |
| G1 | 40 | 16 | 24 | 0.043‡ | |
| Clinical stage | I–IIA | 72 | 19 | 53 | |
| IIB–III | 47 | 21 | 26 | 0.039 | |
- † Compared with well differentiated group;
- ‡ Compared with well differentiated group.
siRNA-mediated MMP-14 knockdown
We knocked down MMP-14 in ESCC cells via an siRNA approach and then evaluated mRNA and protein level expression at 48 and 72 hours post-siRNA transfection. Relative to mock-transfected cells, we observed clear reductions in MMP-14 expression at the RNA and protein levels in cells transfected with the MMP-14-specific siRNA (P < 0.05) (Fig 3a).
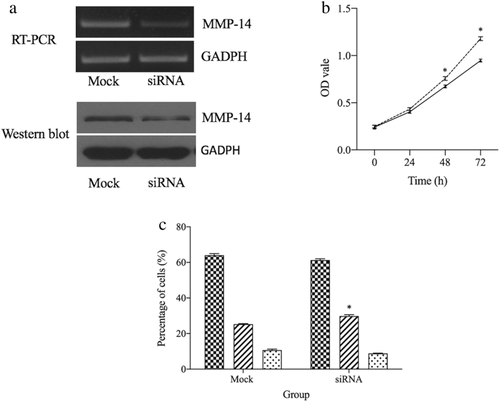
 ) Mock and (
) Mock and ( ) siRNA. (c) Downregulation of MMP-14 induced S phase arrest. (
) siRNA. (c) Downregulation of MMP-14 induced S phase arrest. ( ) GI, (
) GI, ( ) S and (
) S and ( ) G2-M. *P < 0.05 vs. Mock group.
) G2-M. *P < 0.05 vs. Mock group.MMP-14 knockdown suppresses ESCC cell proliferation
As MMP-14 expression has previously been shown to modulate cellular proliferation,11, 12 and we next examined the impact of MMP-14 knockdown on ECa-109 cell proliferative activity. Relative to mock-transfected cells, those in which MMP-14 had been knocked down markedly exhibited impaired proliferative activity (P < 0.05, Fig 3b). We additionally evaluated cell cycle progression in these cells using a flow cytometry-based approach, revealing that MMP-14 knockdown cells were more prone to cell cycle arrest than were ECa-109 cells that had been transfected with a control siRNA construct (Fig 3c).
MMP-14 knockdown inhibits ESCC cell migration
In order to further evaluate the functional importance of MMP-14 in ESCC cells, we utilized a wound healing assay to assess their migratory potential following MMP-14 knockdown or control siRNA transfection. This assay revealed that MMP-14 knockdown was associated with significantly impaired ECa-109 cell migration relative to control cells (P < 0.05) (Fig 4a).
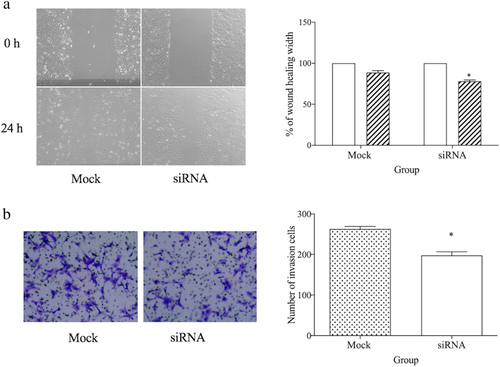
 ) 0 hour and (
) 0 hour and ( ) 24 hours. (b) Downregulation of MMP-14 inhibited cell invasion as compared to Mock group. *P < 0.05 vs. Mock group.
) 24 hours. (b) Downregulation of MMP-14 inhibited cell invasion as compared to Mock group. *P < 0.05 vs. Mock group.MMP-14 knockdown inhibits ESCC cell invasion
MMP-14 is well known to play important roles in regulating tumor invasive activity,13, 14 and as such we assessed the effects of MMP-14 knockdown on such activity. This analysis revealed that ESCC cells in which MMP-14 had been knocked down were less capable of penetrating the Matrigel membrane layer than were control cells, thus indicating that this protein serves as a key regulator of ESCC cell invasive activity (P < 0.05) (Fig 4b).
Discussion
MMP family proteases play essential roles in degrading and remodeling the extracellular matrix (ECM) in physiological and pathological contexts,15 with well documented roles for these proteins in the context of inflammation, oncogenesis, and angiogenesis.4, 15 MMP-14 is an MMP family member protein that is expressed on the cell surface and that is capable of degrading specific ECM components in order to facilitate cancer cell metastasis. The specific role of MMP-14 in ESCC, however, has not been clearly documented.
Herein, we found via IHC that MMP-14 expression was significantly increased in ESCC tumors relative to normal paracancerous esophageal tissue. When we assessed the relationship between MMP-14 expression and patient clinicopathological parameters, we found the upregulation of this protease was associated with tumor T classification, N classification, degree of differentiation and clinical stage in those with ESCC. These findings were consistent with those of a prior study which also found MMP-14 overexpression to be related to tumor depth, clinical stage, lymph node metastasis, and vascular invasion in ESCC patients.16 The upregulation of MMP-14 has also been linked to poor prognosis in patients with bladder, breast, colorectal, lung, and ovarian cancers and with melanoma, advanced neuroblastoma, and tongue squamous cell carcinoma.4, 17, 18 Together, these findings strongly suggest that MMP-14 is a key regulator of tumor progression.
In order to more fully explore the functional role of MMP-14 in ESCC, we utilized an siRNA-based approach to knock down the expression of this protein. We found that MMP-14 knockdown was associated with reduced proliferative, migratory, and invasive activity, and with increased S phase cell cycle arrest.
The accurate mechanism of MMP14 regulating tumor cell proliferation is still unclear. From previous studies, the possible mechanism may be that MMP14 contributes to cell proliferation through a proteolytic mechanism and/or proteolysis-independent mechanism. Another possible mechanism may be that MT1-MMP induces c-Src activation by modulating the extracellular microenvironment, which subsequently activates paxillin to scaffold signaling molecules for ERK pathway and thus leads to escape cells from growth-suppressive signals.
To metastasize, tumor cells need to acquire invasive and migratory capabilities that enable them to move away from the primary tumor site. The disruption of the basement membrane and the ECM are key steps in this metastatic process. Herein, we found that MMP-14 plays an important role in ESCC cell migration and invasion, consistent with prior reports indicating that EC cells require MMP-14 for their invasive activity.16 In line with these findings, MMP-14 has also been shown to be important for local migration and invasion in a range of tumor types,14, 19 emphasizing the broad importance of this protein as a regulator of tumor progression.
In summary, the results of this study indicate that MMP-14 upregulation commonly occurs in human ESCC and is correlated with tumor T classification, N classification, degree of differentiation, and clinical stage of ESCC patients. MMP-14 knockdown can additionally compromise the proliferative and invasive activity of these tumor cells, suggesting that MMP-14 may be a viable therapeutic target for the treatment of ESCC, although further research will be needed to validate these findings. Furthermore, downregulation of the MMP-14 expression level decreased cell proliferation and invasion ability. Given these findings, MMP-14 might be a potential therapeutic target in the treatment of ESCC, and future studies may be warranted to investigate these possibilities.
Disclosure
The authors have no conflicts of interest to declare.




