Glycemic control and risk factors for in-hospital mortality and vascular complications after coronary artery bypass grafting in patients with and without preexisting diabetes
血糖控制水平及其他危险因素对糖尿病与非糖尿病患者冠状动脉旁路移植术后院内死亡和血管并发症的影响
Yanyan Chen and Heng Zhang contributed equally to this study.
Funding information: Beijing Municipal Commission of Science and Technology, Grant/Award Number: D171100002917001; Chinese Academy of Medical Sciences Key Program of Discipline Development Grant, Grant/Award Number: 2011-YIRI; the major chronic noncommunicable disease prevention and control research during the 13th 5-year plan period, Grant/Award Number: 2016YFC1302001
Abstract
enBackground
The purpose of this study was to investigate risk factors of in-hospital mortality and vascular complications after coronary artery bypass grafting (CABG), particularly the effect of different glycemic control levels on outcomes in patients with and without previous evidence of diabetes.
Methods
A total of 8682 patients with and without previous diabetes undergoing CABG were categorized into strict, moderate, and liberal glucose control groups according to their mean blood glucose control level <7.8 mmol/L, 7.8 to 10.0 mmol/L, and ≥10.0 mmoL/L after in-hospital CABG.
Results
The patients with previous diabetes had higher rates of in-hospital mortality (1.3% vs 0.4%, P < .001) and major complications (7.0% vs 4.8%, P < .001) than those without diabetes. Current diabetes was significantly associated with a higher risk of in-hospital mortality (odds ratio [OR] = 3.14, 95% confidence interval [CI] 1.87-5.27) and major complications (OR = 1.49, 95% CI 1.24-1.80), and smoking and higher low-density lipoprotein cholesterol (LDL-C) levels showed similar results. Among patients with previous diabetes, strict glucose control was significantly associated with an increased risk of in-hospital mortality (OR = 8.32, 95% CI 3.95-17.51) compared with moderate glucose control. Nevertheless, among non-previous diabetic patients with stress hyperglycemia, strict glucose control led to a lower risk of major complications (OR = 0.71, 95% CI 0.52-0.98).
Conclusions
Diabetes status, smoking, and LDL-C levels were modifiable risk factors of both in-hospital mortality and major complications after CABG. Strict glucose control was associated with an increased risk of in-hospital mortality among patients with diabetes, whereas it reduced the risk of major complications among non-previous diabetic patients.
摘要
zh目的
探讨冠状动脉旁路移植术患者院内死亡和血管并发症的影响因素, 重点分析不同血糖控制水平对有, 无糖尿病史患者术后并发症的影响。
方法
8682名接受冠状动脉旁路移植术治疗患者依术后平均血糖控制水平分为严格, 中度和宽松血糖控制三个组。各组平均血糖值依次为<7.8mmol/L, 7.8-10.0mmol/L和≥10.0mmoL/L。
结果
有糖尿病史的患者院内死亡率及术后主要并发症明显高于无糖尿病史患者(院内死亡率:1.3% vs 0.4%, P <0 .001; 主要并发症:7.0% vs 4.8%, P <0 .001)。糖尿病状态显著增加院内死亡风险(OR:3.14, 95%CI:1.87-5.27)和主要并发症风险(OR = 1.49, 95%CI 1.24-1.80); 吸烟及低密度脂蛋白胆固醇升高亦增加死亡和主要并发症风险。在有糖尿病史患者中, 与中度血糖控制组相比, 严格血糖控制组院内死亡风险明显增加(OR:8.32, 95% CI:3.95-17.51)。与之相反, 在无糖尿病史患者中, 严格血糖控制组的主要并发症风险却明显减少(OR:0.71,95% CI:0.52-0.98)。
结论
糖尿病, 吸烟和低密度脂蛋白胆固醇升高是冠状动脉旁路移植术后院内死亡和主要并发症的三个可变危险因素。严格血糖控制增加糖尿病手术患者院内死亡风险, 但却可以减少非糖尿病患者术后主要并发症风险。
1 INTRODUCTION
Coronary artery bypass grafting (CABG) is widely used worldwide to treat patients with severe coronary artery disease in both the diabetic and nondiabetic population. In patients with diabetes, surgical outcomes are poor and unpredictable.1-3 Age and several other nonmodifiable risk factors related to the severity of cardiovascular disease have been identified. Efforts have been made to ensure the perioperative safety of patients in terms of patient selection, evaluation of preoperative myocardial viability, completion of myocardial revascularization with arterial grafts, and other factors.4-8 Smoking has been found to be associated with poor clinical outcomes after revascularization in patients with a complex of coronary artery disease.9 Poor glycemic control, defined as a serum glucose level >11.1 mmol/L before surgery, has also been recognized as a contributor to negative cardiovascular outcomes after CABG, but it remains controversial as to whether stricter perioperative glucose control can further reduce rates of adverse outcomes.10-12 The current guidelines recommend that patients with hyperglycemia undergoing surgical procedures should maintain plasma glucose levels <10.0 mmol/L or between 7.8 and 10.0 mmol/L.13, 14 However, the evidence is limited on the appropriateness of using the same glucose target for hyperglycemic patients with and without a history of diabetes. We investigated modifiable risk factors of in-hospital mortality and vascular complications after CABG, particularly the role of the perioperative period of glycemic control in patients with vs without preexisting diabetes.
2 METHODS
2.1 Study design and endpoints
The present study retrospectively investigated the risk factors of in-hospital mortality and vascular complications after CABG and particularly analyzed the effect of glycemic control levels in patients with and without previous evidence of diabetes. The stratification of the participantsʼ three glucose control level groups was prespecified before the data collection and patient recruitment based on the guidelinesʼ recommendations. The primary endpoint was all-cause in-hospital mortality, and the secondary endpoint was major complications, which include postoperative acute myocardial infarction, stroke, or acute renal failure. All of the outcome components were determined according to the definitions provided by the American Heart Association and Society of Thoracic Surgeons.15, 16 Acute myocardial infarction was defined as cardiac biomarker levels >10 times the hospitalʼs upper reference limit within 48 hours of CABG and at least one of the following: new pathological Q wave, new left bundle branch block, angiographic evidence of new graft or native coronary occlusion, or evidence of new loss of myocardium or regional wall motion abnormalities. Stroke was defined as a sudden onset of neurologic deficits resulting from vascular brain lesions and persisting for >24 hours. Acute renal failure was defined as serum creatinine ≥353.6 μmol/L or >3 times the preoperative level. Diabetes was defined as preoperative glycosylated hemoglobin (HbA1c) ≥47.5 mmol/mol (6.5%), fasting blood glucose ≥7.0 mmol/L, or documented history of diabetes.17 According to American Diabetes Association criteria, hypoglycemia was defined as a glucose concentration <3.9 mmol/L (70 mg/dL) at any one time measurement after CABG during hospitalization.18 The Fuwai Hospital Institutional Review Board approved this study and waived the informed consent requirement.
2.2 Participants
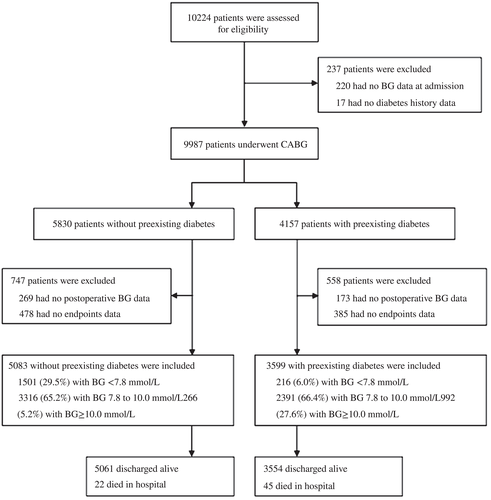
Medical records of 10 224 patients who underwent CABG at Fuwai Hospital from January 2011 to December 2014 were collected initially in the present study, and 1542 patients were excluded due to missing plasma glucose and/or endpoint data during the admission period (Figure 1). Ultimately, 8682 patients were recruited in the present analysis. Among them, 3599 (41.5%) had diabetes and 5083 (58.5%) did not have diabetes. Data on the patientsʼ baseline characteristics, medications, surgical procedures, and clinical outcomes after CABG were collected via chart review by trained physicians and evaluated for completeness and accuracy by at least two researchers. All of the patients with hyperglycemia in different groups had received antidiabetes treatment, continuous intravenous insulin infusion, or subcutaneous multiple insulin injection following the current diabetes management guidelines with target blood sugar levels of 7.8 to 10 mmoL/L during admission.13, 14 Blood glucose measurements were obtained via venous or capillary blood four to nine times per day after in-hospital CABG surgery. Mean blood glucose (MBG) was calculated as the mean of the daily average blood glucose levels after CABG during hospital admission. The admission time after CABG was 5.8 ± 3.7 days. To evaluate the different risks of outcomes induced by glucose control levels, the participantsʼ glycemic control status was categorized into the following three subgroups according to MBG during admission: strict group, MBG <7.8 mmol/L; moderate group, MBG 7.8 to 10.0 mmol/L; and liberal group, MBG ≥10.0 mmol/L. In addition to glycemic control therapy, other pharmacological treatments including angiotensin-converting enzyme inhibitors, beta-blockers, and statins were recommended based on current practice.
2.3 Statistical analysis
Baseline patient characteristics and in-hospital outcomes were described as mean ± standard deviation or median (interquartile range) for continuous variables and percentages for categorical variables. Comparisons by diabetes status and glycemic control levels were made using analysis of variance or Kruskal-Wallis tests for continuous variables and chi-square tests for categorical variables. Stepwise regression was used to explore the modifiable and nonmodifiable risk factors for in-hospital mortality and major complications. In these models, variables including age, sex, smoking, systolic blood pressure (SBP), low-density lipoprotein cholesterol (LDL-C), left ventricular heart failure, previous myocardial infarction, previous stroke, peripheral vascular disease, CABG combined with valve surgery, extracorporeal circulation, and diabetes history were included as independent variables. Logistic regression analyses were used to estimate the effect of glycemic levels and other variables on outcomes, and propensity score-matching methods were used to reduce confounding by measuring covariates among the patients in the three glycemic control categories. We first estimated propensity scores separately for each glucose stratum using logistic regression models that included age, sex, smoking, SBP, LDL-C, left ventricular heart failure, previous myocardial infarction, previous stroke, peripheral vascular disease, CABG combined with valve surgery, and extracorporeal circulation. Then propensity score-weighted multivariable logistic regression was used to determine the impact of differing glycemic levels on outcomes after adjusting for perioperative risk factors.19 Propensity score matching (1:1) was performed as a sensitivity analysis. Statistical analysis was conducted using SAS v9.4 (SAS Institute, Cary, North Carolina) with a two-sided α of .05.
3 RESULTS
3.1 Baseline characteristics and outcomes of patients with different diabetes status
Of the participants, 77.6% were male. Their median age was 61 years (interquartile range, 55-67 years). A total of 43% were smokers, 61% had hypertension, 55.8% had hyperlipidemia, 26.7% had previous myocardial infarction, and 10.3% had previous stroke. There were 5083 (58.5%) patients without diabetes and 3599 (41.5%) patients with diabetes. Compared with the patients without diabetes, those with diabetes had higher BMI, SBP, fasting blood glucose, and HbA1c (all P < .001) and more frequently had a history of hypertension (66% vs 58.5%, P < .001), dyslipidemia (62.5% vs 51.1%, P = .01), previous myocardial infarction (28.5% vs 25.5%, P = .01), stroke (11.9% vs 9.1%, P < .001), and peripheral vascular disease (16.6% vs 12.5%, P < .001) (Table 1).
| Characteristica | All patients (N = 8682) | Patients without preexisting diabetes (n = 5083) | Patients with preexisting diabetes (n = 3599) | P value |
|---|---|---|---|---|
| Age, years | 61.0 (55.0-67.0) | 60.0 (55.0-67.0) | 61.0 (56.0-67.0) | <.001 |
| Male | 6737 (77.6) | 4077 (80.2) | 2660 (73.9) | <.001 |
| Body mass index, kg/m2 | 25.5 (23.5-27.6) | 25.3 (23.3-27.3) | 25.9 (23.9-27.9) | <.001 |
| Systolic blood pressure, mm Hg | 128.0 (119.0-140.0) | 126.0 (118.0-140.0) | 130.0 (120.0-140.0) | <.001 |
| Diastolic blood pressure, mm Hg | 76.0 (70.0-80.0) | 76.0 (70.0-80.0) | 76.0 (70.0-80.0) | .38 |
| Fasting blood glucose, mmol/L | 5.5 (4.9-6.6) | 5.1 (4.7-5.5) | 6.9 (5.8-8.4) | <.001 |
| Glycosylated hemoglobin, % | 6.2 (5.8-7.1) | 5.9 (5.6-6.1) | 7.3 (6.6-8.3) | <.001 |
| Triglycerides, mmol/L | 1.5 (1.1-2.0) | 1.5 (1.1-2.0) | 1.6 (1.2-2.1) | <.001 |
| Total cholesterol, mmol/L | 4.1 (3.5-4.8) | 4.1 (3.5-4.9) | 4.0 (3.4-4.8) | <.001 |
| High-density lipoprotein cholesterol, mmol/L | 1.0 (0.9-1.2) | 1.1 (0.9-1.2) | 1.0 (0.8-1.2) | <.001 |
| Low-density lipoprotein cholesterol, mmol/L | 2.4 (1.9-3.1) | 2.5 (2.0-3.1) | 2.4 (1.8-3.0) | <.001 |
| Smoker | 3728 (43.0) | 2283 (44.9) | 1445 (40.2) | .001 |
| Hypertension | 5346 (61.6) | 2971 (58.5) | 2375 (66.0) | <.001 |
| Dyslipidemia | 4846 (55.8) | 2596 (51.1) | 2250 (62.5) | .01 |
| Chronic renal failure | 13 (0.12) | 7 (0.1) | 6 (0.2) | .73 |
| Congestive heart failure | 108 (1.2) | 59 (1.2) | 49 (1.4) | .41 |
| Previous myocardial infarction | 2320 (26.7) | 1294 (25.5) | 1026 (28.5) | .01 |
| Previous stroke | 889 (10.3) | 461 (9.1) | 428 (11.9) | <.001 |
| Peripheral vascular disease | 1233 (14.2) | 637 (12.5) | 596 (16.6) | <.001 |
| Off-pump CABG | 4333 (49.9) | 2520 (49.6) | 1813 (50.4) | .46 |
- Abbreviation: CABG, coronary artery bypass grafting.
- a Values are medians (interquartile ranges) or n (%).
Overall, 22 of the 5083 patients without preexisting diabetes and 45 of the 3599 with diabetes died, and in 244 of the 5083 and 253 of the 3599, major complications occurred in these two groups, respectively, after CABG during the admission period. Compared with the patients without preexisting diabetes, those with diabetes had significantly higher rates of in-hospital mortality (1.3% vs 0.4%, P < .001) and major complications (7.0% vs 4.8%, P < .001) (Table 2).
| Outcomea | All patients (N = 8682) | Patients without preexisting diabetes (n = 5083) | Patients with preexisting diabetes (n = 3599) | P value |
|---|---|---|---|---|
| In-hospital mortality | 67/8671 (0.8) | 22/5083 (0.4) | 45/3599 (1.3) | <.001 |
| Major complicationsb | 497/8682 (5.7) | 244/5083 (4.8) | 253/3599 (7.0) | <.001 |
| Acute myocardial infarction | 292/8682 (3.4) | 168/5083 (3.3) | 124/3599 (3.4) | .98 |
| Stroke | 73/8682 (0.8) | 25/5083 (0.5) | 48/3599 (1.3) | <.001 |
| Acute renal failure | 186/8682 (2.1) | 74/5083 (1.5) | 112/3599 (3.1) | <.001 |
- Abbreviation: CABG, coronary artery bypass grafting.
- a Values are the number of patients with outcome events/total number of patients (%).
- b Major complications included acute myocardial infarction, acute stroke, and acute renal failure.
3.2 Blood glucose levels and hypoglycemic therapy after CABG
During the perioperative period, all of the patientsʼ blood glucose levels were significantly elevated on the first postoperative day (9.2 mmol/L) and gradually decreased during the subsequent 2 days in the intensive care unit (Table S1). The same change trend was found in the patients without diabetes and those with diabetes, but the MBG levels in the first 3 days were all significantly higher in the patients with diabetes than in those without diabetes (P < .001). On the first day after CABG, 83% of the patients with diabetes were treated with insulin, and the average insulin dosage (50.9 IU) was considerably higher than that of the patients without diabetes (50.7% received insulin therapy, and the average dosage was 16.3 IU). Hypoglycemia (MBG <3.90 mmol/L) occurred in 33 (0.40%) patients on the first postoperative day, with a similar frequency in the nondiabetic and diabetic patients. However, the hypoglycemia rate was higher on the second and third days in the patients with diabetes compared to those without diabetes.
3.3 Risk factors of in-hospital mortality and major complications
Among all of the patients, older age, smoking, LDL-C, CABG combined with valve surgery, extracorporeal circulation, and diabetes were significantly associated with increased risk of in-hospital mortality (P < .05) after CABG, and older age, smoking, LDL-C, previous stroke, previous vascular disease, CABG combined with valve surgery, and diabetes were associated with major complications (P < .05). Diabetes status had a stronger impact on the increased risk of in-hospital mortality, and the odds ratio (OR) of in-hospital mortality for diabetes (OR = 3.14, 95% confidence interval [CI] 1.87-5.27) was close to that for CABG combined with valve surgery (OR = 3.05, 95% CI 1.82-5.09). An increased risk of major complications was also significantly associated with diabetes (OR = 1.49, 95% CI 1.24-1.80) and smoking status (OR = 1.24, 95% CI 1.03-1.49) (Table 3).
| Variable | Odds ratio | 95% confidence interval | P value | Standardized estimate |
|---|---|---|---|---|
| In-hospital mortality | ||||
| Age ≥60 years | 3.21 | 1.76-5.84 | .0001 | 0.3051 |
| Smoking | 2.38 | 1.45-3.91 | .001 | 0.2537 |
| LDL-C | 1.34 | 1.06-1.69 | .016 | 0.1197 |
| CABG combined with valve surgery | 3.05 | 1.82-5.09 | <.001 | 0.2618 |
| Extracorporeal circulation | 2.86 | 1.62-5.06 | .0003 | 0.2906 |
| Diabetes history | 3.14 | 1.87-5.27 | <.001 | 0.2637 |
| Major complications | ||||
| Age ≥60 years | 1.61 | 1.32-1.96 | <.001 | 0.1007 |
| Smoking | 1.24 | 1.03-1.49 | .02 | 0.0945 |
| LDL-C | 1.17 | 1.06-1.28 | .001 | 0.0469 |
| Previous stroke | 1.61 | 1.25-2.08 | .0002 | 0.1295 |
| Peripheral vascular disease | 1.65 | 1.32-2.07 | <.001 | 0.0959 |
| CABG combined with valve surgery | 1.83 | 1.46-2.29 | <.001 | 0.1143 |
| Extracorporeal circulation | 1.21 | 1.00-1.45 | .04 | 0.0943 |
| Diabetes history | 1.49 | 1.24-1.80 | <.001 | 0.0939 |
- Abbreviation: CABG, coronary artery bypass grafting; LDL-C, low-density lipoprotein cholesterol.
3.4 Impact of glycemic control on outcomes
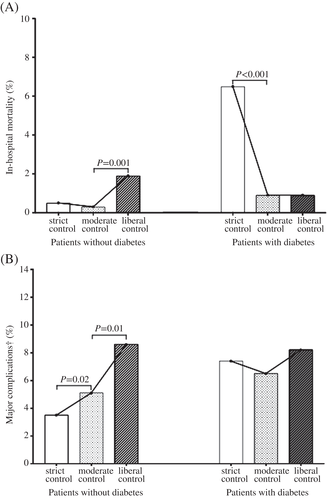
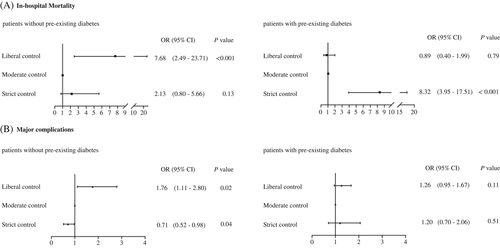
In all of the patients, compared with the moderate control group, the rates of in-hospital mortality were significantly higher in both the liberal control group (liberal vs moderate: 1.3% vs 0.5%, P = .002) and strict control group (strict vs moderate: 1.1% vs 0.5%, P = .03). However, the rate of major complications showed a different trend: The strict control group had the lowest risk, the liberal control group had the highest risk, and the moderate control group was in the middle (Table S2). Because of the significant interaction between glycemic control and diabetes for both in-hospital mortality (liberal control vs moderate control, P ≤ .001; strict control vs moderate control, P ≤ .001) and major complications (liberal control vs moderate control, P = .037; strict control vs moderate control, P = .045), the analyses were stratified by diabetes status. In the patients without previous diabetes with stress hyperglycemia, the liberal control group had higher rates of in-hospital mortality (1.9% vs 0.3%, P = .001) and major complications (8.6% vs 5.1%, P = .01) compared with the moderate control group, but the strict control group had lower rates of major complications (3.5% vs 5.1%, P = .02) (Figure 2). Propensity score-weighted multivariable logistic regression showed that liberal glucose control increased both in-hospital mortality (OR = 7.68, 95% CI 2.49-23.71) and major complications (OR = 1.76, 95% CI 1.11-2.80) compared with moderate control after adjusting for the previously mentioned confounders. However, the strict glucose control group had a lower rate of major complications (OR = 0.71, 95% CI 0.52-0.98) than the moderate glucose control group (Figure 3, Table S3). Among patients with preexisting diabetes, those with strict vs moderate control had higher rates of in-hospital mortality (6.5% vs 0.9%, P < .001) and similar rates of major complications (7.4% vs 6.5%, P = .62) (Figure 2). In addition to the previously mentioned confounders, strict glucose control was significantly associated with increased risk of in-hospital mortality (OR = 8.32, 95% CI 3.95-17.51) compared with moderate control but did not increase the rate of major complications (OR = 1.20, 95% CI 0.70-2.06) (Figure 3, Table S3).
3.5 Sensitivity analysis
There were 798 patients without diabetes (266 per glycemic control group) and 648 with diabetes (216 per glycemic control group) in the propensity score-matching analysis (Supplementary Table S4). Analyses of these cohorts yielded largely similar results (Figure S1).
4 DISCUSSION
Diabetes mellitus is an established risk factor for adverse cardiovascular and infective complications after CABG.20-22 The present study found that in addition to old age, male sex, smoking, LDL-C, CABG combined with valve surgery, and extracorporeal circulation, diabetes was an important risk factor for both in-hospital mortality and major complications after CABG among all of the study participants. Except for old age, CABG combined with valve surgery and extracorporeal circulation were dependent mainly on the preoperative severity of coronary disease; hence, they were likely not to be modified. Moreover, smoking, LDL-C, and diabetes status were three modifiable risk factors. Of note, in terms of the OR of in-hospital mortality, diabetes was very close to extracorporeal circulation and CABG combined with valve surgery, both of which are generally accepted as strong nonmodifiable risk factors. This finding highlights the important role of diabetes management, especially diabetes prevention, in the reduction of negative outcomes after CABG. China has the worldʼs largest population of persons with diabetes. The most recent reported prevalence is 11.6%, indicating that the numbers of adult diabetic patients are 112 million.23 Smoking was another modifiable risk factor. The prevalence of smoking in Chinese people older than 15 years of age is 62%, which means that 340 million men are smokers.24 More than 40% of the patients in our study were smokers. In terms of lipid control, it was reported that treating the LDL-C target (<2.59 mmol/L) in 4778 patients with coronary heart disease from 52 centers in six provinces in China was only 36.2%.25 Thus, diabetes prevention, smoking cessation, and treating lipids to target levels is an urgent task for Chinese medical professionals, not only to prevent cardiovascular events but also to reduce the risks of poor clinical outcomes after CABG.
It is commonly accepted that uncontrolled hyperglycemia in patients undergoing cardiac surgery increases the risk of postoperative mortality and morbidity.16, 17 Most cardiac centers aim for a perioperative serum glucose concentration <10 mmol/L, but there is evidence from several studies suggesting tighter glucose control could reduce adverse outcome rates. Will further decreasing the plasma glucose level to less than 7.8 mmol/L favor a further decrease in the rate of adverse outcomes? Possibly. Several studies have demonstrated encouraging results. In the Portland Diabetes Project, an upper serum glucose concentration of 6.1 mmol/L was associated with reductions in operative and cardiac-related death.26 Similarly, a retrospective analysis of more than 4300 cardiac surgery patients at the Cleveland Clinic found that a glucose concentration ≤7.8 mmol/L during ICU stay was associated with improved outcomes.27 Should the glycemic target be adjusted according to diabetes status? There has been limited research on this topic. In the present study, after adjusting for significant confounders, moderate glucose control was associated with a lower risk of in-hospital mortality compared with strict glucose control in patients with preexisting diabetes. In contrast, among previously nondiabetic patients with stress hyperglycemia after CABG, patients with liberal glucose control (≥10.0 mmol/L) had the highest risks of both in-hospital mortality and major complications compared to patients with strict and moderate control. However, strict glucose control (<7.8 mmol/L) further decreased the rate of major complications but did not increase the risk of in-hospital mortality compared with moderate control. This finding suggests that the glucose control target level should be adjusted based on diabetes status to achieve more favorable outcomes. Further studies are required to clarify whether near-normal glucose control will lead to fewer cardiovascular events and lower mortality among patients with stress hyperglycemia.
Studies have noted the impact of stress hyperglycemia on mortality and found that the development of hyperglycemia in patients without a history of diabetes was associated with higher mortality and complication rates than in patients with diabetes.28 Similarly, a study of 5050 CABG patients showed that postoperative hyperglycemia with a maximum blood glucose level >13.9 mmol/L increased in-hospital mortality among patients without diabetes but not among patients with diabetes.29 Another study of 8727 hospitalized adults without diabetes after cardiac surgery reported a stepwise reduced rate of death in patients with better glucose control. The mortality rate was 1.8% in those with good glucose control (<11.1 mmol/L), 4.2% in those with moderate control (11.1-13.9 mmol/L), and 9.6% in those with poor control (>13.9 mmol/L).30 There are some possible explanations for the differential impact of strict blood glucose control on patients with and without diabetes. First, the response to hypoglycemia could be more severe in patients with diabetes because of preexisting endothelial dysfunction and a tendency for ischemic events than in those without diabetes, especially in patients with diabetes and cardiovascular disease. Second, patients with diabetic autonomic dysfunction might be predisposed to arrhythmias as a result of hypoglycemia.31 Third, rapid glucose swings were more likely to be observed in relatively low glycemic control in patients with diabetes. They usually need more insulin injection therapy, and acute glucose fluctuations might stimulate oxidative stress and cause adverse outcomes. Thus, glycemic variability resulting from intensive glycemic control might alter the stability of regulatory mechanisms.32 Accordingly, an integrated strategy focusing on safety, multifactorial approaches, and reduction of risk in therapy for diabetes is essential in the cardiovascular surgery setting.33
Although the strict glucose control group in the patients with diabetes showed a higher mortality rate than the other two groups, we posit that the high mortality rate in this group might not be attributed to the occurrence of hypoglycemia in this study since hypoglycemia seldom occurred in those who died. Why did the strict glucose control group demonstrate a higher mortality rate than the moderate glucose control group? There might be several possible explanations. First, diabetic patients have increased blood glucose levels with postoperative stress, and the near normal MBG concentration is likely to represent a sharp fluctuation, which may also induce oxidative stress and lead to adverse outcomes. Second, it was suspected that once the blood vessel is rebuilt and blood flow is restored after CABG, the original “hibernating” myocardial cells may require a higher glucose level to provide enough energy, so a relatively higher glucose level may favor good outcomes.
This study has some limitations. First, as it was a retrospective observational study, glycemic control targets could not be randomly assigned. However, we used propensity score methods to reduce the impact of confounding after applying propensity score weights. Moreover, we further controlled for potentially influential covariates in our regression models, and a sensitivity analysis using propensity score matching confirmed our findings. Second, outcomes were assessed in hospital, and subsequent follow-up information was not collected. The long-term impact of diabetes status and glycemic control on outcomes should be explored in future analyses. Third, the present study mainly focused on the impact of postoperative blood glucose control levels on mortality and complications, and the differentiated impact of preoperative blood glucose needs to be further assessed in future research. Finally, our data were drawn from a single hospital and might not be generalizable to other institutions, although these real-world data were collected from a large sample of CABG patients.
In conclusion, smoking, LDL-C, and diabetes status are the three major modifiable risk factors of both in-hospital mortality and major complications after CABG. Compared to patients with moderate glucose control, strict glucose control significantly increased the risk of in-hospital mortality among those with preexisting diabetes. In contrast, strict glucose control reduced the risk of major complications but did not increase in-hospital mortality among patients without preexisting diabetes. This highlights that smoking cessation, treating LDL-C to target levels, preventing diabetes, and highly individualized glucose control are crucial to minimize the risk of subsequent in-hospital death and major complications after CABG. In addition, this study suggests that optimal individualized perioperative glycemic control targets before and after CABG may differ based on patient diabetes status.
ACKNOWLEDGEMENTS
The authors thank the database staff, including Yan Zhao, Ying Zhang, and Xianglong Jiao. We are indebted to Yun Wang, PhD, at Harvard T.H. Chan School of Public Health for his assistance in preparing and revising the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.




