COVID-19 and comorbidities: A role for dipeptidyl peptidase 4 (DPP4) in disease severity?
新冠肺炎与合并症:二肽基肽酶4在疾病严重程度中的作用?
Funding information: National Medical Research Council; Northumbria University; Action Medical Research
Abstract
enThe coronavirus disease 2019 (COVID-19) pandemic is caused by a novel betacoronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), similar to SARS-CoV and Middle East respiratory syndrome (MERS-CoV), which cause acute respiratory distress syndrome and case fatalities. COVID-19 disease severity is worse in older obese patients with comorbidities such as diabetes, hypertension, cardiovascular disease, and chronic lung disease. Cell binding and entry of betacoronaviruses is via their surface spike glycoprotein; SARS-CoV binds to the metalloprotease angiotensin-converting enzyme 2 (ACE2), MERS-CoV utilizes dipeptidyl peptidase 4 (DPP4), and recent modeling of the structure of SARS-CoV-2 spike glycoprotein predicts that it can interact with human DPP4 in addition to ACE2. DPP4 is a ubiquitous membrane-bound aminopeptidase that circulates in plasma; it is multifunctional with roles in nutrition, metabolism, and immune and endocrine systems. DPP4 activity differentially regulates glucose homeostasis and inflammation via its enzymatic activity and nonenzymatic immunomodulatory effects. The importance of DPP4 for the medical community has been highlighted by the approval of DPP4 inhibitors, or gliptins, for the treatment of type 2 diabetes mellitus. This review discusses the dysregulation of DPP4 in COVID-19 comorbid conditions; DPP4 activity is higher in older individuals and increased plasma DPP4 is a predictor of the onset of metabolic syndrome. DPP4 upregulation may be a determinant of COVID-19 disease severity, which creates interest regarding the use of gliptins in management of COVID-19. Also, knowledge of the chemistry and biology of DPP4 could be utilized to develop novel therapies to block viral entry of some betacoronaviruses, potentially including SARS-CoV-2.
摘要
zh摘要
2019年新冠肺炎冠状病毒大流行是由一种新型β新冠状病毒-严重急性呼吸综合征冠状病毒2型(SARS-CoV-2)引起的, 类似于SARS-CoV和中东呼吸综合征(MERS-CoV), 可引起急性呼吸窘迫综合征和死亡。新冠肺炎病的严重程度在患有糖尿病, 高血压, 心血管疾病和慢性肺部疾病的老年肥胖患者中更严重。β冠状病毒结合并进入细胞是通过它们的表面刺状糖蛋白;SARS-CoV是与金属蛋白酶血管紧张素转换酶2(ACE2)结合, 而MERS-CoV利用二肽肽酶4(DPP4)进入细胞。最近对SARS-CoV-2刺激性糖蛋白结构的模拟, 预测除了ACE2之外, 它还可以与人DPP4相互作用。DPP4是一种无处不在的膜结合型氨基肽酶, 在血浆中循环, 具有多种功能, 在营养, 代谢, 免疫和内分泌系统中发挥作用。DPP4通过其酶活性和非酶免疫调节作用, 调节葡萄糖稳态和炎症。DPP4抑制剂或格列普汀被批准用于治疗2型糖尿病, 这突显了DPP4对医学界的重要性。本文就新冠肺炎合并疾病时DPP4的异常调节进行综述。老年人DPP4活性较高, 血浆DPP4升高是代谢综合征发病的预测因子。DPP4上调可能是新冠肺炎疾病严重程度的决定因素, 这引起了人们对格列普汀在新冠肺炎治疗中使用的兴趣。此外, DPP4的化学和生物学知识可以用来开发新的疗法, 阻止一些β冠状病毒如SARS-CoV-2等入侵体内。
1 INTRODUCTION
The rampant new clinical infectious disease that emerged in late 2019 and rapidly became a world pandemic is caused by a novel betacoronavirus; the disease is now called COVID-19 (Coronavirus Disease 2019). The natural reservoir of these enveloped, single-stranded positive-sense RNA viruses is thought to be bats. The zoonotic potential of betacoronaviruses is well recognized, with species such as pangolins, the most illegally trafficked mammal,1 civets, and dromedary camels serving as intermediate hosts. The sudden emergence and spread of COVID-19 is similar to severe acute respiratory syndrome (SARS)—a related disease that appeared in 2002 in Asia.2 Nearly a decade later in 2012 another respiratory syndrome erupted in the Middle East, Middle East respiratory syndrome (MERS), which was also caused by zoonotic transmission of a coronavirus.3 The coronaviruses that cause these 21st century clinical respiratory syndromes are now classified as SARS coronavirus (SARS-CoV), MERS coronavirus (MERS-CoV), and SARS-CoV-2.
In SARS and MERS cases a number of risk factors were noted to be associated with progression to acute respiratory distress syndrome (ARDS), especially advanced age and male sex. For MERS, additional risk factors included chronic conditions such as diabetes mellitus, hypertension, cardiac diseases, and obesity.4 A similar picture is emerging with COVID-19. An analysis of 46 248 SARS-CoV-2 infected patients found that the most prevalent comorbidities were hypertension and diabetes, followed by cardiovascular diseases and respiratory system disease. Compared with the nonsevere patient, the pooled odds ratios (OR) of hypertension/diabetes, respiratory system disease and cardiovascular disease in severe patients were OR 2.36, OR 2.46, and OR 3.42 respectively.5 Another meta-analysis of six Chinese studies showed the highest odds ratio (5.97) for COVID-19 in patients with preexisting chronic obstructive pulmonary disease (COPD).6, 7 Similarly, a report of 72 314 confirmed and suspected cases of COVID-19 published by the Chinese Centre for Disease Control and Prevention showed increased mortality in people with diabetes (2.3%, overall in 44 672 confirmed cases and 7.3% for patients with diabetes).8 Italian COVID-19 patients show similar comorbidities; more than two-thirds of the COVID-19 case fatalities had diabetes, cardiovascular diseases, cancer, or were former smokers.9 Older age and male sex have also been confirmed as important factors in disease severity in a recent meta-analysis.10 Clinical features of severe COVID-19 include not only acute lung injury but also acute cardiac injury and heart failure, irrespective of whether the patient has a history of hypertension.11 Acute kidney injury was also associated with mortality in 25% of these 113 deceased COVID-19 patients. The disease can rampage throughout the body, with gastrointestinal symptoms such as diarrhea and seizures in some severe patients.
The course of any acute viral infection is determined by host factors including the immune response and viral strategies to evade that response to establish primary and rapid replication (Figure 1).12, 13 In both SARS and MERS the viral load in those with severe illness was higher than in those with mild disease, and viral shedding in respiratory secretions was more prolonged.14 This implies that the balance was tipped toward the virus in those with comorbidities. Initial interactions between a virus and host cell are required for productive viral infection and initiation of the viral life cycle. Thus, the host factors required for SARS-CoV-2 - host interactions during attachment, binding, and entry are important not only in vaccine development but also in disease pathogenesis and treatment.
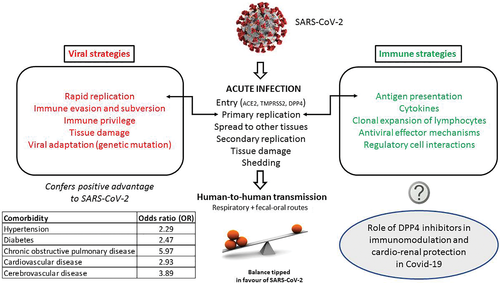
1.1 Coronavirus (CoV) entry via the spike glycoprotein
Coronavirus entry is via the spike (S) protein, a type I transmembrane glycoprotein, which contains an N-terminal S1 subunit receptor binding domain (RBD) and a C-terminal S2 subunit domain that enables membrane fusion. Binding by the S-protein to a receptor causes a conformational change that is necessary for activation by host cell proteases, and both steps are essential for coronavirus infection of a cell.15
At the cell surface, a type II transmembrane serine protease, TMPRSS2, activates the SARS coronavirus S protein such that it can then bind to the metalloprotease angiotensin-converting enzyme 2 (ACE2)16, 17; these proteases are co-expressed by type II pneumocytes. TMPRSS2 dependency occurs in both SARS-CoV and SARS-CoV-2. This is important as TMPRSS2 inhibitors, for example, Camostat, that are already licensed for other indications could be repurposed as antiviral therapy.17, 18 Camostat is being evaluated in Phase I and II clinical trials (ClinicalTrials.gov Identifier: NCT04321096), with an upper age limit of 110 years to encompass older adults at risk of severe disease. Trials with Nafamostat mesylate (Fusan), licensed in Japan for treatment of acute pancreatitis, are due to start at the University of Tokyo.
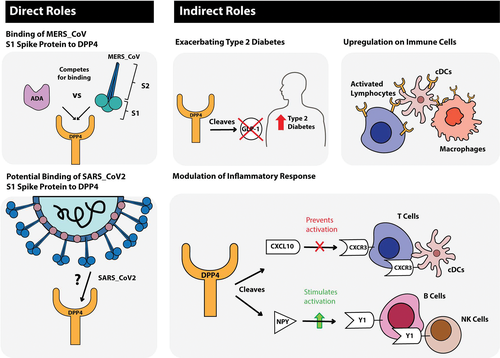
MERS-CoV on the other hand utilizes dipeptidyl-peptidase 4 (DPP4), also termed cluster of differentiation 26 (CD26), as its main entry receptor.19, 20 The tetraspanin CD9 facilitates MERS-CoV entry by scaffolding host cell receptors including DPP4.21 MERS-CoV infection is mediated by the binding of its S1 protein to the β-propeller domain of DPP4; there are 11 critical residues within the β-propeller domain that directly interact with the S1 protein of the virus (reviewed in22). Species permissivity to MERS-CoV is governed by both protein sequence and the variable glycosylation of DPP4.23 Adaptive evolution of the MERS-CoV spike protein has been demonstrated to occur by multiple paths in vitro all of which alter the surface charge of the spike.24 Human adenosine deaminase (ADA), which binds to extracellular DPP4 of all mammals except rodents,25, 26 has been shown to compete for MERS-CoV binding, acting as a natural antagonist for MERS-CoV infection.27
The interaction of MERS-CoV spike protein with DPP4 involves not only the RBD in domain B of the S1 subunit but also interactions of domain A with sialylated receptors that facilitate viral entry into airway epithelial cells. It is suggested that this additional attachment factor may also be a determinant of tissue and host tropism.20 The number of sialylated DPP4 isoforms isolated from human peripheral blood mononuclear cells increases significantly with age greater than 40 years.28 That study also found that hypersialylation of DPP4 modifies its surface charge, promoting binding of human immunodeficiency virus (HIV) peptides such that certain HIV moieties are likely to engage this phenomenon as an auxiliary adhesion mechanism to fuse with cells.
Recent modeling of the homotrimer structure of SARS-CoV-2 spike protein predicts that the S1 domain binds to human DPP4.29 The model demonstrated a large binding interface between the SARS-CoV-2 S1 glycoprotein and DPP4 suggesting a tight interaction. It might be that this binding is influenced by the sialylation of DPP4, discussed previously. Other studies have not shown that SARS-CoV-2 binds to DPP4 but have shown that betacoronaviruses are capable of entering human cells through an unknown receptor.30 There are thus important commonalities between coronaviruses in relation to the first step in the viral life cycle, host cell binding and entry. This review focuses on DPP4 as its expression may be a factor in determining the balance between virus and host in COVID-19 and can potentially be linked to some of the known comorbidities.
2 DIPEPTIDYL-PEPTIDASE 4
The abundant ubiquitous DPP4 protein was first isolated in the 1970s and is now well characterized. It is a 110 kDa glycoprotein, a membrane-bound aminopeptidase that preferentially cleaves peptides containing a proline or alanine residue in the penultimate amino-terminal position. The extracellular domain of homodimeric DPP4 is often cleaved from cell membranes and circulates in plasma, where it remains enzymatically active. DPP4 is multifunctional with roles in nutrition, metabolism, the immune and endocrine systems, bone marrow mobilization, cancer growth, and cell adhesion. The importance of DPP4 for the medical community has been highlighted by the approval of DPP4 inhibitors for the treatment of type 2 diabetes mellitus (T2DM) (reviewed in31).
2.1 Tissue distribution of DPP4 and dysregulation in disease(s)
The clinical features of COVID-19 reflect the ability of SARS-Cov-2 to infect different tissues and their constituent cells, which in turn echoes the expression of viral attachment/binding receptors. Although ACE2 is expressed on pneumocytes and enterocytes of the small intestine,32 a recent study has shown that the expression on alveolar type 2 cells is rather low compared to other cells expressing ACE2, raising the possibility that SARS-CoV-2 utilizes coreceptors.33 That study demonstrates ACE2 expression is high in kidney and intestine (esophagus, stomach, ileum, colon, and rectum) and is correlated with expression of other peptidases that are used as entry receptors by coronaviruses. DPP4 was found to be the first gene clustered with ACE2 and, as modeling suggests a tight interaction between SARS-CoV-2 S1 and DPP4,29 the tissue distribution of DPP4 is relevant.
In the natural reservoir of betacoronaviruses, bats, DPP4 is in epithelial cells of both the respiratory and the intestinal tract of frugivorous bats, similar to humans, but in insectivorous bats it is preferentially expressed in the intestinal tract.34 This suggests that in some bat species the mode of transmission is via the fecal-oral route. Hence differences in the distribution of DPP4 expression among MERS-CoV susceptible species might influence virus tropism, pathogenesis, and transmission route.
In humans the most intense expression of DPP4 is in salivary gland, kidney, seminal fluid, and liver (Figure 2). DPP4 is also high in enterocytes, particularly colonic enterocytes, as well as blood vessel capillaries, lung epithelium, and immune cells (activated T, B, and natural killer cells and myeloid cells). The level of expression depends on the cell type, differentiation state, and/or the activation state.35, 36 DPP4 activity is in saliva of humans and ferrets37, 38 and mucosal-associated invariant T cells (MAITs) express high levels of CD26/DPP4.39 SARS-CoV-2 is probably spread by the fecal-oral route in humans and ferrets.38, 40
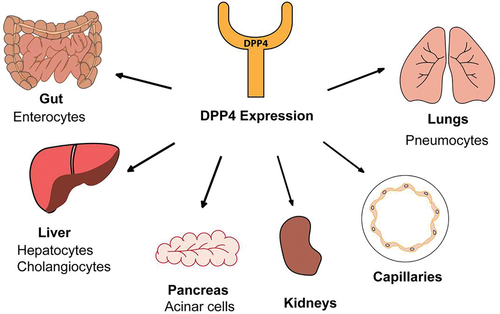
DPP4 activity is higher in older individuals compared to younger ones and, in vitro, is selectively expressed on the surface of senescent, but not proliferating, human diploid fibroblasts.41 DPP4 is present in the lower respiratory tract epithelium, mainly on alveolar type 2 cells,33 but patients with COPD and cystic fibrosis exhibit increased DPP4 not only in these cells but also alveolar macrophages.42, 43 DPP4 upregulation in type I pneumocytes may be a crucial determinant for severe MERS-CoV infection.22
The widespread expression of DPP4 on blood capillaries, myocardium, and myeloid cells and the nonenzymatic function of DPP4 as a signaling and binding protein, across a wide range of species, imply a role in angiogenesis, cardiovascular regulation, and inflammation.44, 45 DPP4 inhibition is beneficial in animal models of acute and chronic kidney injury (reviewed in46). In patients with different stages of chronic kidney disease the activities of both DPP4 and ACE2 correlate with estimated glomerular filtration rates (eGFR).47 This study of 268 participants found that those with the lowest eGFR exhibited the highest DPP4 and ACE2 activities and that DPP4 also correlated with age.
The liver plays a crucial role by contributing to systemic DPP4 levels. DPP4 is highly expressed in the healthy liver and this expression is increased in liver disease and cirrhosis.48 Elevated expression of DPP4 in the liver promotes non-alcoholic fatty liver disease (NAFLD) and insulin resistance.49-51 Glucose-induced expression of DPP4 in the liver is facilitated by demethylation of the DPP4 gene early in life52 and this epigenetic regulation is responsive to dietary protein restriction.53 There is important crosstalk between the liver and visceral adipose tissue in obesity-induced metabolic syndrome (MS).54 In this mouse model obesity stimulates hepatocytes to synthesize and secrete DPP4, which acts with plasma factor Xa to increase inflammation in adipose tissue. Adipose tissue is also a relevant source of DPP4 in diet-induced obesity55 and is involved in linking obesity to MS.56, 57 Obese individuals have higher circulating levels of DPP457 and the level is correlated with body mass index (BMI) in young healthy people.58
2.2 DPP4, immunomodulation, and infection
DPP4 also possesses noncatalytic functions through its interaction with ligands, primarily ADA.25 DPP4 is a co-stimulator for T cell activation by binding to ADA and additionally enhances lymphocyte proliferation independent of ADA binding.59 As adenosine is a potent suppressor of T cell proliferation, inducing its degradation through increased DPP4 activity can increase T cell proliferation. Obese humans demonstrate increased levels of DPP4 expression in dendritic cell/macrophage cell populations from visceral adipose tissue, potentiating inflammation in obesity by interacting with ADA.60 Hence increased DPP4 in conditions such as obesity-induced MS results in failure to resolve inflammation and chronic subclinical activation of the immune system. This metabolic/immune dysregulation may provide the “ideal home” for some coronavirus infections.
The adenosine nucleoside analogue, galidesivir (BCX4430), which has broad-spectrum activity against a wide range of RNA viruses, including flaviviruses such as Zika,61 is now being evaluated in COVID-19 (ClinicalTrials.gov Identifier: NCT03891420). The rationale is that it acts to block viral RNA-dependent RNA polymerase, which plays a crucial role in the viral replication process. It is unknown how such a therapy could interact with the immunoregulatory, mostly anti-inflammatory, effects of adenosine in different immune cell types62 and how increased DPP4 expression and chronic subclinical inflammation in obesity-induced MS will affect the clinical response.
DPP4 has identified roles in other infections. In chronic hepatitis C virus (HCV), DPP4 generates an antagonist form of the chemokine CXCL10 (also known as IP-10) by amino-terminal truncation of the protein,63 such that the elevated plasma CXCL10 found in patients with chronic HCV can modulate immune responses by chemokine receptor antagonism.64 CXCR3 antagonism via truncated CXCL10 may also be an important regulatory mechanism occurring in tumours65 and in sites of tuberculosis pathology.64
3 COVID-19 COMORBIDITIES, DISEASE MANIFESTATIONS, AND SEVERITY
Older age and comorbidities play important roles in influencing the severity of COVID-19. In one study the incidences of hypertension, cardio-cerebrovascular diseases, and diabetes mellitus (DM) were 2-3-fold higher in intensive care unit (ICU)/severe cases than in their non-ICU/severe counterparts.66 In individuals with DM but without other comorbidities, serum levels of inflammation-related biomarkers such as interleukin (IL)-6, C-reactive protein, serum ferritin and coagulation index, and D-dimer, were significantly higher (P < .01) compared with those without DM, suggesting that patients with diabetes are more susceptible to an inflammatory storm eventually leading to rapid deterioration and increased mortality.8, 67 As approximately 425 million adults (1 in 11) are living with diabetes, therapeutic options in diabetes become important.
In a French study of 124 consecutive hospitalized COVID-19 patients, obesity (BMI >30 kg/m2) and severe obesity (BMI >35 kg/m2) were present in 47.6% and 28.2% of cases, respectively.68 The need for invasive mechanical ventilation was significantly associated with male sex (P < .05) and BMI (P < .05), independent of age, diabetes, and hypertension. As noted previously, increased DPP4 expression and activity are associated with obesity and MS.54, 57, 58, 69 Hence extensive emerging data is pointing to an important direct metabolic and endocrine mechanistic link to the viral disease process (Figure 3) leading to the suggestion that more attention should be paid to the treatment of comorbidities in COVID-19 as these patients often die.6
4 DPP4 INHIBITION
DPP4 inhibitors, also known as gliptins, are now a firmly established class of oral antidiabetic agents for the treatment of T2DM (reviewed in70).The gliptins are not a homogenous class of compounds but show different interactions with the active site of the enzyme molecule.31, 70 The gliptins are classified into three classes depending on their mode of binding.71 Eight DPP4 inhibitors are currently approved for T2DM, some only in Japan and Korea. For example teneligliptin, which was developed in Japan, has a unique structure characterized by a J-shape and an anchor-lock domain and not only has strong DPP4 inhibitory function but also is safe for patients with end-stage renal disease.72
As modeling suggests that DPP4 is a co-receptor for SARS-CoV-2 viral entry29 and disease severity in COVID-19 is associated with conditions in which there is dysregulation of DPP4, the role of DPP4 inhibition in the COVID-19 disease process needs to be explored.
Although the gliptins do not bind to the putative receptor binding site of SARS-CoV-2, research over the past two decades toward drug discovery of the gliptins has yielded vast knowledge of the chemistry and biology of DPP4 and its binding interactions26, 31, 71; this could be utilized in developing new anticoronavirus drugs. It is feasible that a therapy could be designed to shield the human viral entry protein(s) from the virus, using an antibody or peptide that binds to the human protein. A therapeutic vaccine to human DPP4 has already been developed as a novel potential therapy for T2DM.73 Pang et al selected three suitable regions in DPP4 as candidate targets for a vaccine and conjugated these peptides to the adjuvant, keyhole limpet hemocyanin, which presents a variety of T cell epitopes to induce helper T cell responses and demonstrated its efficacy in improving the diabetic phenotype in animal models.
Altered DPP4 expression in comorbid conditions of COVID-19 may favor the virus in ways that are not currently understood. There is a possibility not only that gliptins may modulate the severity of COVID-19 but also each DPP4 inhibitor/class may have differential effect(s). It would be of interest to compare the diabetic (and other) therapy in those with severe vs nonsevere disease.8 For example, in Japan a DPP4 inhibitor was chosen as initial monotherapy in the majority of T2DM patients (1410/2666 patients), a decision influenced by older age.74 Does such therapy influence severity of COVID-19 compared to treatment with a sulfonylurea or a biguanide? Would the addition of a DPP4 inhibitor improve the hyperglycemia and inflammatory activation in older patients with COVID-19? Are any potential beneficial effects of gliptins outweighed by treatment with ACE inhibitors, which increase ACE2 expression in diabetics?75 It is unclear whether the use of renin-angiotensin-aldosterone system inhibitors alone have potential for more benefit than harm in patients with COVID-19.76 Data collected during this pandemic need to be interrogated for medicinal drug usage and interactions in all groups with comorbidities.
In rodent models, different gliptins have been shown to have a variety of effects that could be beneficial in COVID-19 pathology. Thus, vildagliptin and saxagliptin have significant anti-inflammatory activity comparable to that of aspirin in an acute model of inflammation.77 Saxagliptin-mediated DPP4 inhibition can attenuate DM-induced activation of the NLRP3 inflammasome and reduce the serum levels of CRP, tumor necrosis factor α, IL-1β, IL-18, and IL-6.78 There is also evidence of crosstalk between DPP4 and the renin-angiotensin system in the pathophysiology of cardiorenal syndromes and that gliptins are cardioprotective and may delay the onset of cardiovascular impairment in chronic kidney disease.46, 79 Likewise, gliptins may have a role in treatment of the hepatic manifestation of obesity-induced metabolic syndrome, NAFLD.80, 81
In patients, gliptins exert a comprehensive and potent anti-inflammatory effect; it has been shown for example that sitagliptin treatment not only causes a significant fall of plasma concentrations of C-reactive protein (CRP) and IL-6 but also suppressed the mRNA expression of CD26/DPP4 in mononuclear cells by 16 ± 6% within 2 hours after a single dose.82 Mathematical modeling indicates that the spread of MERS-CoV infection could be controlled by decreasing the expression of DPP4.83 Hence, if DPP4 expression facilitates infection by SARS-CoV-2, perhaps the anti-inflammatory activity of clinically used DPP4 inhibitors84 could be repurposed to treat COVID-19 patients, especially those with elevated blood glucose, markers of virally driven hyperinflammation85, 86 or cardiorenal problems.46
Another question to ask is whether known genetic variation(s) in DPP4 87 affect viral entry and attenuate viral replication, as has been noted for MERS.88
5 CONCLUSIONS
At the time of writing SARS-CoV-2 has infected >3.75 million people in 187 countries, leading to at least 265 000 deaths and the numbers of COVID-19 cases continue to rise exponentially worldwide. Comorbid conditions that include obesity-induced metabolic syndrome and associated T2DM influence disease severity. T2DM has itself been called a pandemic with around 415 million adults living with the disease. As a higher proportion of these individuals can expect to become critically ill when infected with SARS-CoV-2 it is important not only to ask why but also to optimize treatment regimens.
In this review we focus on DPP4 as recent modeling of the structure of SARS-CoV-2 spike glycoprotein, which mediates viral-host-cell binding and entry, predicts that it interacts with human DPP4 in addition to ACE2. Furthermore, increased DPP4 expression and activity have been consistently associated with obesity, T2DM, and diabetes complications. DPP4 inhibitors, also known as gliptins, are now a firmly established class of oral antidiabetic agents for the treatment of T2DM. It is important to understand whether some or all gliptins modulate COVID-19 disease severity, possibly via their immunomodulatory and cardioprotective effects. Finally, although gliptins do not bind to the putative binding site of SARS-CoV-2, it may be that the vast knowledge of the biology and biochemistry of DPP4 and other proteases that has accumulated over past decades could be utilized in the development of novel therapies to block viral entry and in vaccine design.
ACKNOWLEDGEMENTS
MFB: no funding received. SHB: research funding from the UK charity, Action Medical Research, with additional funding from Northumbria University. GWM: Australian National Medical Research Council (NHMRC) project grant 1105238 and New South Wales Cancer Council grant 1183157. MDG: NHMRC project grants 1105238, 1099375 and 1148077. The authors wish to thank Dr R Dermot Neely, Trustee of HEART UK, for his critical review of the manuscript and Michelle Sui Wen Xiang for digital design of Figures 2 and 3.
DISCLOSURES
None declared.




