MicroRNAs in type 2 diabetes mellitus: Important for the pathogenesis but uncertain as biomarkers
2型糖尿病中的miRNA:病理发生过程中的重要参与者, 但尚未明确能否作为生物标记物
MicroRNAs (miRNAs) are single-stranded non-coding RNAs (19–22 nucleotides) generated from a well-organized RNA processing event. Drosha and Dicer, two key endoribonucleases acting in nuclei and the cytoplasm, respectively, are essential for miRNA biogenesis. MicroRNAs usually function as post-transcriptional regulators by pairing with the 3′-untranslated region (UTR) of target mRNA(s) to regulate mRNA degradation or translation.1 Thus far, approximately 2000 miRNAs across the human genome have been identified (http://www.mirbase.org/cgi-bin/browse.pl, accessed 1 May 2018) and play an important role in physiology and pathophysiology, because deletion of either Drosha or Dicer in mice leads to a loss of most miRNAs, causing lethality or severe pathology.2
Type 2 diabetes mellitus (T2DM) is a systemic metabolic disease involving various cell types and multiple tissues and complex mechanisms. A role for miRNAs in the pathogenesis of T2DM has been extensively studied and is now well appreciated. Many miRNAs have been found to be associated with the risk factors important for T2DM pathogenesis, including obesity, insulin resistance, and β-cell death.3 Targeted deletion of Dicer in β-cells results in decreased β-cell mass, less insulin secretion, and diabetes.4 Specifically, miR-103, miR-107, and miR-143 were found to regulate adipocyte differentiation and adipogenesis, whereas miR-9, miR-375, and miR-124a were reported to regulate insulin secretion.3 In addition, miR-155 has been shown to decrease insulin sensitivity via the inhibition of peroxisome proliferator-activated receptor (PPAR) γ,5 and miR-200 has been shown to promote β-cell apoptosis by decreasing anti-apoptotic DnaJ homolog subfamily C member 3 (Dnajc3) and X-linked inhibitor of apoptosis protein (Xiap) and increasing pro-apoptotic Trp53.6 The list of miRNAs that may contribute to the pathogenesis of T2DM is still expanding. The most important question remaining is whether changes in these miRNAs are specific for T2DM, because specificity is a prerequisite for a miRNA to be a therapeutic target or a clinical biomarker.
There are a significant number of circulating miRNAs derived from cells and tissues. Circulating miRNAs are relatively stable with a significantly longer half-life than those inside cells.7 MicroRNAs are also stable in vitro under various storage and transport conditions and withstand multiple freeze–thaw cycles.7 Therefore, miRNAs are very feasible as biomarkers for certain diseases. Importantly, there are tissue-specific miRNAs, although overall 87.3% of miRNAs show a broad expression pattern across different tissues. For example, the miR-378 family is highly expressed in the muscle and myocardium, whereas 18 members of the miR-506 family are abundant in the testis.5 In addition, miR-449c-3p and miR-449b-3p are expressed specifically in the spleen, and miR-449c-5p and miR-449b-5p are relatively specific for the kidney and small intestine.5 Therefore, different diseases may have distinct circulating miRNA profiles.7 To date, considerable efforts have been made to identify circulating biomarkers for many diseases, including T2DM.8 Although these studies provide many novel insights into the pathogenesis of T2DM, there are large discrepancies among studies, likely due to different patient cohorts, limited sample size, and variations in methodology.
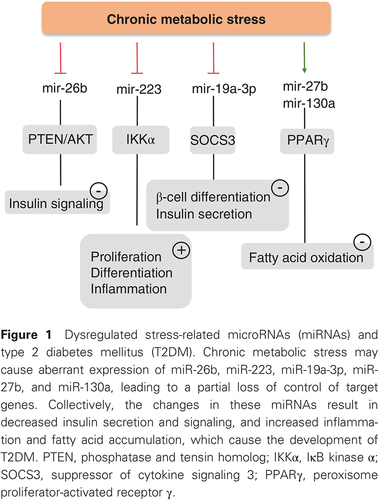
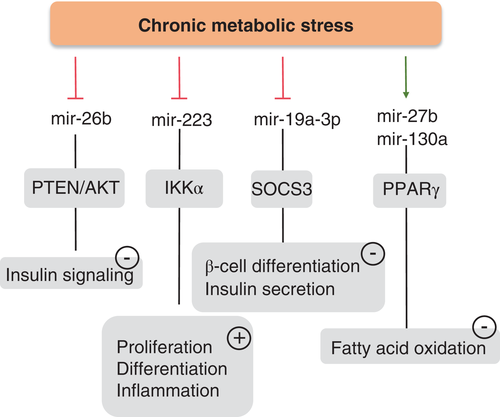
In this issue of the Journal, Liang et al.9 attempted to overcome some of these pitfalls by selecting eligible results from 23 human studies. They also included 18 studies from animals and collected reports on diabetic tissues. Because metabolic stress has been shown to be critically involved in T2DM and to be associated with an altered miRNA profile, Liang et al.9 specifically identified studies on stress-related miRNAs in T2DM. They found 16 miRNAs to be dysregulated in T2DM and suggested a few miRNAs, including mir148b, mir223, mir-130a, mir-19a, mir-26b, and mir-27b, as potential circulating biomarkers, with miR-146a and miR-21as potential tissue biomarkers. In their report, circulating miR-19a, miR-26b, miR-27b, miR-130a, miR-146a, miR-148b, and miR-223 were consistently downregulated in T2DM. Of these, miR-223 may be important for T2DM because miR-223-deficient mice develop severe inflammation and insulin resistance after feeding of a high-fat diet.10 Moreover, decreased miR-223 has been reported to predict the onset of T2DM in a 10-year follow-up.11 It has been shown that mir-130a-3p increases insulin signaling in both human and mouse hepatocytes, whereas silencing of miR-130a-3p has the opposite effect.12 Conversely, miR-19a-3p regulates the expression of 5-lipoxygenase (5-LO),13 and has been recently identified as inhibiting the apoptosis of pancreatic β-cells via suppression of suppressor of cytokine signaling (SOCS) 3.14 The miR-148/152 family includes miR-148a, miR-148b, and miR-152, the aberrant expression of which has been observed in many diseases, such as diabetes, atherosclerosis, IgA nephropathy, and cancers.15 In previous studies, miR-148b and miR-152 have been found to participate in regulating insulin receptor substrate-1, insulin-like growth factor-1 receptor, AMP-activated protein kinase, phosphatase and tensin homolog, phosphatidylinositol 3-kinase, and Ca2+/calmodulin-dependent protein kinase II.15 Collectively, all these stress-related miRNAs identified by Liang et al.9 seem to be involved in T2DM. In Fig. 1, we have summarized the possible roles and potential mechanisms by which decreased expression of miR-19a, miR-26b, miR-27b, miR-130a, miR-146a, miR-148b, and miR-223 contributes to the pathogenesis of T2DM. To date, the exact cause(s) of the changes in these miRNAs remain unknown.
The findings by Liang et al.9 are clinically relevant and may advance our understanding of the role of miRNAs in the pathogenesis of T2DM. However, the study also has many limitations, primarily because of the small numbers of patients, the nature of meta-analyses, and the lack of validation. In 2015, Zhu and Leung16 reported the first meta-analysis on the miRNA expression profile in T2DM from 38 studies including both human and animals. In that study, eight miRNAs, namely miR-103, miR-107, miR-132, miR-144, miR-29a, miR-34a, miR-142-3p, and miR-375, were suggested as circulating biomarkers, whereas miR-199a-3p and miR-223 were suggested as potential tissue biomarkers for T2DM.16 Surprisingly, these findings were not reproduced by Liang et al.9 There may be many reasons for this discrepancy. For example, the selection criteria for published data were different between the two meta-analyses. Liang et al.9 narrowed the miRNAs down to stress-related miRNAs instead of collecting all available data. Perhaps the most notable cause of the controversy comes from inappropriate combination of studies from humans and animals. Although both patients and animals had T2DM and hyperglycemia, the etiologies of the two entities are quite different. Animal models for T2DM were generated by either genetic mutation of leptin or the leptin receptor or streptozotocin injection. In contrast, most cases of T2DM in humans are caused by multiple unidentified genes. In addition, because rodents and humans exhibit different sensitivities to diabetic complications, especially nephropathy,17 it is likely that there may be a significant difference in the miRNA profile between human and animal T2DM.
Diabetic complications are a major cause of morbidity and mortality for diabetes. Early detection and treatment are essential to reduce progression and avoid worse outcomes. MicroRNAs in the circulation and biofluids, including urine, are candidates for non-invasive early diagnosis. Accordingly, Zampetaki et al.11, 18 reported that miR-27b and miR-320a, two miRNAs that target thrombospondin 1 and are involved in angiogenesis, are highly correlated with the incidence and progression of diabetic retinopathy. However, that study is a retrospective study from two type 1 diabetes cohorts and there is also a concern over the choice of one standard deviation as a cut-off. In addition, it seems that some other diseases, such as hypertension, hypertensive cardiomyocyte hypertrophy, and metabolic syndrome are associated with elevated circulating miR-27b or miR320a.19 MicroRNAs have also been reported to play a significant role in diabetic nephropathy,19, 20 and miR-192 has been previously identified to contribute to diabetic renal fibrosis via regulating transforming growth factor β and Type I collagen.21 Moreover, circulating miR-377, miR-21, miR-192, miR-216a, miR-217, miR-375, and miR-144 are dysregulated, whereas urinary miR-638 is elevated in diabetic nephropathy.22 The specificity and clinical value of these miRNAs in the diagnosis of diabetic complications remain to be determined.
In summary, dysregulated miRNAs are likely involved in every facet of T2DM. The next step is to identify key miRNAs that contribute to the pathogenesis of T2DM and its complications. As for the application of recently identified miRNAs as biomarkers for disease diagnosis, one would have to first put them to the test in a large sample cohort for specificity and feasibility.
Acknowledgements
The authors’ work described herein was supported by the National Natural Science Foundation of China (81722010, 81570636, 81500538, 81670668/91639201) and the Dalian High-level Talent Innovation Support Program (2016RD13).
Disclosure
None declared.
microRNA(miRNA)是一类单链非编码RNA, 其长度为19-22个碱基, 在其编码基因转录为前体RNA之后, 由Drosha与Dicer这两个核酸内切酶在细胞核内切割, 然后转运到细胞浆发挥其生物学功能。在哺乳动物体内, miRNA主要通过序列部分互补的方式结合靶标mRNA的3`UTR区域, 形成RNA介导的沉默复合物引起mRNA降解, 发挥转录后调控的作用。迄今为止, 从人类基因组中已发掘出2000余个miRNA (http://www.mirbase.org/cgi-bin/browse.pl, accessed 1 May 2018), 有众多证据显示miRNA在生理和病理生理过程中发挥了重要作用, 如果通过敲除Drosa或Dicer来造成miRNA的缺失, 会导致多种疾病的发生。
2型糖尿病(T2DM)是一种系统性的代谢疾病, 其发生发展涉及了多种细胞与组织, 机制复杂, 而miRNA在其中所发挥的作用也受到了广泛研究并得到了一些结果。例如, 一些miRNA与糖尿病发病的诸多风险因素有关, 包括肥胖, 胰岛素抵抗及胰岛β细胞死亡等。如果将胰岛β细胞的Dicer基因敲除, 整体miRNA的缺失会导致β细胞体积减小, 胰岛素分泌量下降并由此诱发糖尿病。目前miR-9, miR-375和miR-124a已被证实可以参与调控胰岛素的分泌, 同时miR-103, miR-107和miR-143等参与脂肪细胞分化和脂质生成的调控;此外, miR-155可以通过靶向抑制过氧化物酶体增殖物激活受体γ(PPARγ), 降低胰岛素敏感性, miR-200可以下调Dnajc3, Xiap等抗凋亡蛋白, 并上调促凋亡因子Trp53, 最终诱导β细胞凋亡。另外也发现了一些参与T2DM发生, 发展的miRNA, 其作用的分子靶标也得到阐释。但是这些miRNA的变化是否为T2DM所特有?这个重要的问题决定了这些miRNA是否可以作为T2DM治疗与诊断的靶标。
外周循环中有着多种分泌自不同细胞与组织的miRNA, 被称之为循环miRNA(circulating miRNA), 这些循环miRNA比较稳定, 半衰期要远长于胞内的miRNA, 并且可以耐受多次冻融, 在各种运输和储存环境中保持稳定, 因此miRNA被认为是一种极具潜力的生物标志物, 可用于疾病诊断。更为重要的是, 虽然87.3%的miRNA广泛表达于不同的组织中, 但仍然有许多miRNA具有组织表达的特异性:miR-378家族成员在肌肉与心肌中高度表达, miR-506家族的18个成员则在睾丸中含量丰富;即使来源于同一个pre-miRNA前体序列, miRNA的分布也有很大差别, 如miR-449c-3p和miR-449b-3p虽在胰腺中大量存在, 其同源序列miR-449c-5p和miR-449b-5p却富含于肾脏和小肠。因此研究人员推测, 是否不同的疾病拥有不同的循环miRNA表达谱, 并可用于提示疾病的发生与进展。时至今日, 多项研究致力于挖掘具有疾病生物标记物潜质的miRNA, 其中就有针对T2DM的工作。尽管这些工作为人们了解2型糖尿病的病理和发病机制提供了一些帮助, 但是不同的研究所得的结果存在着较大的差异, 这些差异可能由于不同的患者队列, 有限的样本量以及方法上的差异所致。
在本期杂志中, Liang和他的同事试图总结23项糖尿病的miRNA研究结果, 同时参考18项动物实验的结果, 希望从中寻找合适的miRNA作为疾病发生和发展的生物标记物。考虑到T2DM常常会造成代谢应激, 而代谢应激与miRNA表达谱的变化密切相关, Liang首先关注T2DM中与应激反应密切相关的miRNA, 经过分析筛选, 他们找到16种miRNA在T2DM中表达失调。在此基础上, 他们提出包括mir148b, mir223, mir-130a, mir-19a, mir-26b和mir-27b在内的部分miRNA可作为疾病潜在的循环生物标志物, 而miR-146a和miR-21as则可能作为累及组织的生物学标志物。他们的研究还发现, 外周循环中的miR-19a, miR-26b, miR-27b, miR-130a, miR-146a, miR-148b和miR-223在T2DM中表达下调。其中, miR-223对T2DM的作用可能最为关键, 因为miR-223敲除小鼠在高脂饮食诱导后出现严重的炎症和胰岛素抵抗症状。不仅如此, 一项长达10年的临床随访研究报道了miR-223的降低可作为T2DM发生的预测指标。此外, 有研究发现在人和小鼠肝细胞中表达miR-130a-3p均可以增强胰岛素通路的信号传导, 而沉默miR-130a-3p具有相反的作用。另一方面, miR-19a-3p可以调节5-脂氧合酶(5-LO)的表达, 通过影响细胞因子信号传导的(SOCS)3来抑制胰腺β细胞的凋亡。miR-148/152家族包括miR-148a, miR-148b和miR-152, 它们的异常表达已在许多疾病中观察到, 例如糖尿病, 动脉粥样硬化, IgA肾病和癌症等。之前的研究发现该家族成员miR-148b和miR-152可以靶向调控胰岛素信号通路相关的诸多基因, 如调节胰岛素受体底物-1(IRS1), 胰岛素样生长因子1受体(IGF1R), 腺苷酸活化蛋白激酶(AMPK), 同源性磷酸酶(PTEN), 磷脂酰肌醇3激酶(PI3K)和Ca2+/钙调蛋白依赖性蛋白激酶II(CaMKII)等。由Liang等所筛选出的所有这些与代谢应激相关的miRNA似乎都参与了T2DM。 miR-19a, miR-26b, miR-27b, miR-130a, miR-19a-3p, miR-223等的表达下降可能都参与T2DM发生的机制。迄今为止, 这些miRNA表达变化的确切原因仍然未知。
Liang等人的发现与临床应用密切相关, 并会增进我们对miRNA在T2DM发病机理中的作用的了解。但是该研究存在许多局限性。首先用于分析的患者人数较少, 其次meta分析的可信度存疑, 而且研究者并没有去实际验证分析得到的结果。在2015年, Zhu和Leung就率先报告了来自38项包括临床和动物试验在内的T2DM相关miRNA表达谱的meta分析, 在该研究中, 作者建议将8个miRNA, 即miR-103, miR-107, miR-132, miR-144, miR-29a, miR-34a, miR-142-3p和miR-375用作疾病的循环生物标志物, 同时miR-199a-3p和miR-223也被推荐作为T2DM的潜在组织生物学标志物。然而, Liang等人并没有同样的发现, 有许多原因可能导致了这种差异。首先, 两次meta分析之间发布数据的选择标准不同。 Liang等将分析范围缩小为与代谢应激相关的miRNA, 而不是收集所有可用数据。此外, 引起争议的最明显原因可能是来自于对人类和动物研究的不适当组合, 尽管患者和动物模型都患有T2DM和高血糖症, 但研究T2DM的动物模型与人类疾病实际发生的病因却大不相同。糖尿病动物模型通常是应用瘦素或瘦素受体基因突变或链脲佐菌素注射来制作, 与之相反的是人类大多数T2DM病例是由多个未知基因引起。此外, 由于啮齿动物和人类对糖尿病并发症(尤其是肾病)的敏感性不同, 人类和动物T2DM之间的miRNA谱可能存在显着差异。
糖尿病并发症是糖尿病患者病情进展和死亡的主要原因, 早期发现和及时治疗对于减少疾病进展和避免不良后果至关重要。外周循环和包括尿液在内的体液中miRNA是无创早期诊断的候选靶标。因此, Zampetaki等人报道靶向血小板反应蛋白1并参与血管生成的两个miRNA: miR-27b和miR-320a与糖尿病性视网膜病变的发生和发展高度相关。但是该研究仅仅是对两个1型糖尿病队列的回顾性研究, 因此这两个miRNA应用到临床诊断时, 需考虑如何选择合适的阈值, 这还有待进一步研究。此外, 其他一些疾病如高血压, 高血压心肌细胞肥大和代谢综合征的发生也与循环中的miR-27b或miR-320a升高有关。miRNA在糖尿病肾病中也发挥着重要作用, 如miR-192先前已被发现可通过调节转化生长因子β和I型胶原, 促进糖尿病肾纤维化1。此外, 糖尿病肾病患者的循环miR-377, miR-21, miR-192, miR-216a, miR-217, miR-375和miR-144发生失调, 尿液miR-638升高。这些miRNA在糖尿病并发症诊断中的特异性和临床价值仍有待确定。
综上所述, 表达失调的miRNA可能与T2DM发生发展有关。后续研究需要确定导致T2DM发病及其并发症的关键miRNA。对于已明确调控功能的miRNA, 必须首先将它们在大样本队列中进行测试, 以确保特异性和可行性, 这样方可用作疾病诊断的生物标记物。