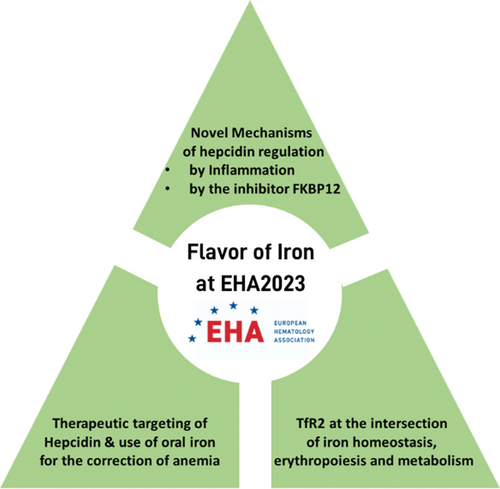
Flavor of Iron at EHA2023: Novel Regulatory Mechanisms and Therapeutic Options for the Correction of Anemia
At the Congress of the European Hematology Association (EHA) 2023 held in June in Frankfurt, Germany, 2 oral sessions were dedicated to iron metabolism and altered iron homeostasis in pathologic conditions. This HemaTopic will provide a taste of what we heard this year at the iron sessions.
Several talks were focused on the improvement of anemia in the context of inflammation and chronic diseases, and β-thalassemia. Furthermore, new mechanisms of hepcidin regulation and novel strategies for its therapeutic modulation have been presented.
Iron restriction is a host immune mechanism aimed at reducing systemic iron levels to diminish its availability for the growth of siderophilic pathogens.1,2 This mechanism is controlled by hepcidin, the key regulator of systemic iron homeostasis, that reduces iron absorption from duodenal enterocytes and release from iron-recycling macrophages by binding, blocking, and inducing the degradation of the sole iron exporter ferroportin. Hepcidin induction is triggered in hepatocytes in response to iron by the BMP (bone morphogenetic protein)-SMAD (suppressors of mothers against decapentaplegic) pathway, and in response to pathogens and inflammation, by the JAK (Janus kinase)-STAT3 (signal transducer and activator of transcription) pathway.1,2
During infection, innate immune cells detect pathogen-associated molecular patterns (PAMPs) and produce proinflammatory cytokines (eg, interleukin [IL]-6 and IL-1β), which directly promote hepcidin transcription in hepatocytes via the classical JAK2-STAT3 pathway.1,2 In this year’s EHA Congress, Dr Marques presented data showing the existence of novel mechanisms of hepcidin upregulation in inflammation, revealing a crucial role for toll-like receptors (TLRs).3 Dr Marques demonstrated that TLR2/6 and TLR5 promote a downstream inflammatory response in hepatocytes, which leads to the upregulation of hepcidin. Specifically, at an early time-point, hepcidin induction in response to stimulation with TLR ligands and heat-killed bacteria was shown to be independent from autocrine and paracrine regulation by key proinflammatory cytokines and dependent on the mitogen-activated protein kinase axis downstream TLR activation in hepatocytes. Ex vivo experiments revealed that PAMPs and pathogens directly pass through the sinusoidal barrier and reach hepatocytes to cause hepcidin upregulation. The fact that hepatocytes play immunological roles by detecting exogenous and potentially endogenous PAMPs places them as potential direct drivers of iron restriction in specific inflammatory conditions.
Although iron restriction is a protective mechanism during infection, it becomes detrimental in chronic inflammatory conditions, being the main cause of reduced iron availability for erythropoiesis and resulting in anemia. Targeting hepcidin is therefore a potential therapeutic approach for the treatment of anemia of inflammation or anemia associated with chronic diseases.
A crosstalk between the BMP-SMAD and the JAK-STAT3 pathways is essential for mounting a proper hepcidin upregulation in inflammation. Thus, reduced hepcidin synthesis can be achieved by targeting one of these pathways. In this context, Dr Asperti and Dr Poli presented a study on the application of heparin-like molecules to reduce hepcidin production.4,5 In collaboration with Modus Therapeutics, Dr Asperti tested the anti-hepcidin effect of sevuparin, a novel clinical stage, low-molecular weight heparinoid compound with reduced anticoagulant activity.6 Sevuparin strongly suppressed basal and BMP6-induced hepcidin expression in a hepatocyte cell line through the inhibition of the BMP-SMAD signaling pathway, in a dose- and time-dependent manner, achieving 90% hepcidin reduction after 6 hours of exposure. This anti-hepcidin effect was confirmed in mice in vivo, achieving a 70% reduction of hepatic hepcidin mRNA levels 6 hours after a single administration. Importantly, in healthy volunteers, a single dose of sevuparin administered subcutaneously caused a 50%–70% reduction of plasma hepcidin with a maximal suppression between 6 and 24 hours. Altogether, the results demonstrate the anti-hepcidin properties of sevuparin at safe and tolerable doses, suggesting therapeutic utility in patients with diseases hallmarked by high hepcidin levels and iron-restricted anemia.
Other mechanisms to potentially target hepcidin for therapeutic purposes were hypothesized by Dr Silvestri, who presented a study on the mechanism of action of the immunophilin FKBP12, recently described as a novel hepcidin inhibitor.7,8 The inhibitor activity of FKBP12 is mediated by its ability to block the BMP type I receptor ALK2, which activates the BMP-SMAD signaling pathway. Both the physiological ALK2 ligand BMP6 and the immunosuppressive drug Tacrolimus (TAC) displace FKBP12 from ALK2 and activate signal transduction. Dr Silvestri recently investigated the molecular mechanism underlying FKBP12 regulation of BMP-SMAD pathway activation and hepcidin expression.9 Through pharmacologic and genetic studies, FKBP12 was shown to modulate BMP-receptor interactions and ligand sensitivity. Mechanistically, BMP6 and TAC increase ALK2 homo- and ALK2-ALK3 hetero-oligomerization and the interaction between ALK2 and the type II receptors. As proven by both in vitro and in vivo experiments, these ligands cooperate in activating the BMP pathway and hepcidin expression by acting on the same receptors. Overall, by elucidating the mechanism through which FKBP12 regulates BMP-SMAD signaling and hepcidin expression in hepatocytes, these findings indicate that diseases characterized by high and low hepcidin levels can benefit from the therapeutic administration of FKBP12-like molecules and from the pharmacological disruption of FKBP12-ALK2 interaction, respectively.
Another approach to improve anemia associated with inflammation and chronic diseases is the administration of oral or intravenous iron preparations. The use of iron formulations that minimally induce inflammation should be preferred to avoid further hepcidin induction and aggravate the underlying inflammation. Macrophages are well known targets of iron formulations and free iron (nontransferrin-bound iron [NTBI]) and are implicated in iron recycling to support erythropoiesis.10,11 Dr Vinchi explored whether oral iron formulations administered to correct anemia alter macrophage iron status and induce cell inflammatory skewing, comparing the effect of the commonly used oral iron salt, iron sulfate (FeSO4), with that of an innovative formulation, sucrosomial iron (SI), in which ferric pyrophosphate is protected by a phospholipid/sucrester matrix.12 Because of a different mechanism of intestinal absorption, FeSO4 generated higher NTBI than SI, leaving macrophages exposed to free iron in anemic mice. This translated into a faster correction of macrophage iron deficiency by FeSO4 than SI, as indicated by a more pronounced suppression of transferrin receptor 1 (Tfr1) and bigger cell labile iron pool in hepatic and splenic reticulo-endothelial macrophages. Importantly, this was associated with more elevated production of reactive oxygen species (ROS) and release of tumor necrosis factor-α, IL-1β, and IL-6, together with increased cell apoptosis. In vivo and in vitro experiments showed that while FeSO4 exposure caused a massive increase in inflammatory cytokines, SI minimally affected cytokine production in macrophages, due to its ability to gradually alter cell iron status through progressive iron release from the sucrester matrix. In conclusion, the kinetic of macrophage iron deficiency correction by oral iron formulations determines ROS formation and cell proinflammatory activation. These studies show that SI is a superior oral iron formulation than FeSO4 in terms of reduced inflammatory action, with relevance for the treatment of anemia in individuals with pre-existing inflammatory conditions, including chronic kidney disease, inflammatory bowel disease, and cancer.
Erythropoiesis is strongly dependent on iron availability due to the crucial role of this metal for hemoglobin synthesis and red blood cell production. Novel data have been presented on TFR2, a receptor expressed in the hepatocytes and erythroid cells that modulates liver hepcidin expression on the one hand and erythropoietin (EPO) sensitivity on the other hand to adjust erythropoiesis to iron availability. The exploitation of TFR2 for the modulation of erythropoiesis in conditions of anemia is also of great therapeutic potential. To better understand the mechanism of action of TFR2, Dr Nai investigated the potential crosstalk between erythropoiesis and systemic glucose metabolism. Previous results from her laboratory demonstrated that in β-thalassemia mouse models and in conditions of anemia of inflammation, the inactivation of the iron sensor TFR2 ameliorates anemia and stimulates erythropoiesis through the modulation of EPO-EPOR signaling.13,14 Increased oxidative phosphorylation and mitochondrial activity of erythroid cells, as observed in mice with expanded and effective erythropoiesis, promotes systemic glucose consumption and systemic hypoglycemia. Similar alterations were detected in erythroid tissues of thalassemic mice lacking Tfr2 in hematopoietic cells.13 Thus, the authors postulated that the enhanced erythropoiesis induced by Tfr2 deletion, by stimulating the metabolic activity of erythroid cells, might prevent hyperglycemia and related complications in β-thalassemia mice. In line with this hypothesis, Dr Nai and colleagues demonstrated that mice lacking Tfr2 in the hematopoietic compartment showed reduced blood glycemia and improved glucose sensitivity both in a wild-type and in a β-thalassemic context, resulting also in a complete rescue of muscle functionality in β-thalassemic animals.15 Blood glycemia negatively correlated with both red blood cells count and hemoglobin levels, supporting the concept that enhanced erythropoiesis drives increased glucose consumption. Overall, the presented data suggest that early metabolic alterations of β-thalassemic mice depend on the degree of ineffective erythropoiesis and are corrected by hematopoietic Tfr2 deletion that rewires systemic metabolism to sustain augmented erythropoiesis. Enhancing erythropoiesis through hematopoietic TFR2 targeting may become a novel strategy for the control of blood glucose levels.
AUTHOR CONTRIBUTIONS
FV, MA, OM, AN, and LS conceptualized, researched, and wrote the article. In addition, FV edited and finalized the article.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
These works were supported by research funding from PharmaNutra to F.V.; Fondazione Cariplo (Project code: 2021-1558) and Modus Therapeutics AB to M.A.; EHA Junior Research Grant (RG66) and Olympia Morata Fellowship to O.M.; EHA Advanced grant 2021 and the Italian Ministry of Health (“Ricerca Finalizzata” Young Investigators grant number GR-2019-12369583) to A.N.; Cariplo Telethon Alliance GJC2021 (GJC21117) to L.S.