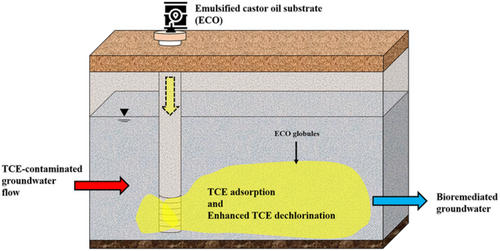
Bioremediation of trichloroethylene-polluted groundwater using emulsified castor oil for slow carbon release and acidification control
Wei-Ting Chen
Institute of Environmental Engineering, National Sun Yat-Sen University, Taiwan
Contribution: Investigation (equal), Methodology (equal)
Search for more papers by this authorKu-Fan Chen
Department of Civil Engineering, National Chi Nan University, Taiwan
Contribution: Conceptualization (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorRao Y. Surmpalli
Global Institute for Energy, Environment and Sustainability, Lenexa, Kansas, USA
WEF Members.Contribution: Conceptualization (equal), Data curation (equal), Visualization (equal)
Search for more papers by this authorTian C. Zhang
Department of Civil & Environmental Engineering, University of Nebraska-Lincoln, Omaha, Nebraska, USA
WEF Members.Contribution: Data curation (equal), Methodology (equal), Validation (equal)
Search for more papers by this authorJiun-Hau Ou
Institute of Environmental Engineering, National Sun Yat-Sen University, Taiwan
Contribution: Data curation (equal), Methodology (equal)
Search for more papers by this authorCorresponding Author
Chih-Ming Kao
Institute of Environmental Engineering, National Sun Yat-Sen University, Taiwan
WEF Members.Correspondence
Chih-Ming Kao, Institute of Environmental Engineering, National Sun Yat-Sen University, Kaohsiung City, Taiwan.
Email: [email protected]
Contribution: Funding acquisition (equal), Project administration (equal), Resources (equal), Supervision (equal)
Search for more papers by this authorWei-Ting Chen
Institute of Environmental Engineering, National Sun Yat-Sen University, Taiwan
Contribution: Investigation (equal), Methodology (equal)
Search for more papers by this authorKu-Fan Chen
Department of Civil Engineering, National Chi Nan University, Taiwan
Contribution: Conceptualization (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorRao Y. Surmpalli
Global Institute for Energy, Environment and Sustainability, Lenexa, Kansas, USA
WEF Members.Contribution: Conceptualization (equal), Data curation (equal), Visualization (equal)
Search for more papers by this authorTian C. Zhang
Department of Civil & Environmental Engineering, University of Nebraska-Lincoln, Omaha, Nebraska, USA
WEF Members.Contribution: Data curation (equal), Methodology (equal), Validation (equal)
Search for more papers by this authorJiun-Hau Ou
Institute of Environmental Engineering, National Sun Yat-Sen University, Taiwan
Contribution: Data curation (equal), Methodology (equal)
Search for more papers by this authorCorresponding Author
Chih-Ming Kao
Institute of Environmental Engineering, National Sun Yat-Sen University, Taiwan
WEF Members.Correspondence
Chih-Ming Kao, Institute of Environmental Engineering, National Sun Yat-Sen University, Kaohsiung City, Taiwan.
Email: [email protected]
Contribution: Funding acquisition (equal), Project administration (equal), Resources (equal), Supervision (equal)
Search for more papers by this authorAbstract
In this study, the emulsified castor oil (ECO) substrate was developed for a long-term supplement of biodegradable carbon with pH buffering capacity to anaerobically bioremediate trichloroethylene (TCE)-polluted groundwater. The ECO was produced by mixing castor oil, surfactants (sapindales and soya lecithin [SL]), vitamin complex, and a citrate/sodium phosphate dibasic buffer system together for slow carbon release. Results of the emulsification experiments and microcosm tests indicate that ECO emulsion had uniform small droplets (diameter = 539 nm) with stable oil-in-water characteristics. ECO had a long-lasting, dispersive, negative zeta potential (−13 mv), and biodegradable properties (viscosity = 357 cp). Approximately 97% of TCE could be removed with ECO supplement after a 95-day operational period without the accumulation of TCE dechlorination byproducts (dichloroethylene and vinyl chloride). The buffer system could neutralize acidified groundwater, and citrate could be served as a primary substrate. ECO addition caused an abrupt TCE adsorption at the initial stage and the subsequent removal of adsorbed TCE. Results from the next generation sequences and real-time polymerase chain reaction (PCR) indicate that the increased microbial communities and TCE-degrading bacterial consortia were observed after ECO addition. ECO could be used as a pH-control and carbon substrate to enhance anaerobic TCE biodegradation effectively.
Practitioner Points
- Emulsified castor oil (ECO) contains castor oil, surfactants, and buffer for a slow carbon release and pH control.
- ECO can be a long-term carbon source for trichloroethylene (TCE) dechlorination without causing acidification.
- TCE removal after ECO addition is due to adsorption and reductive dechlorination mechanisms.
Graphical Abstract
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| wer1673-sup-0001-Supplementary Materials.docxWord 2007 document , 185.2 KB |
Table S1. Properties of the soils and groundwater used in this study. Table S2. Components of five groups of microcosms. Table S3. Similarity between the nucleotide sequences of 16S rDNA of 31 specific bacteria in microcosm samples collected on Day 95 and the GenBank database. Table S4. Functions of the identified bacteria in microcosm samples. Figure S1. DGGE patterns of the PCR-amplified 16S rDNA for soils collected from LC, ES, ECON, and ECO groups of microcosms on Days 5 and 95. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- Aziz, F. A., Suzuki, K., Amano, K., Moriuchi, R., Dohra, H., Tashiro, Y., & Futamata, H. (2020). Draft genome sequence of phenol-degrading Variovorax boronicumulans strain c24. Microbiology Resource Announcements, 9, 597–520. https://doi.org/10.1128/MRA.00597-20
- Baldwin, B. R., Nakatsu, C. H., & Nies, L. (2003). Detection and enumeration of aromatic oxygenase genes by multiplex and real-time PCR. Applied and Environmental Microbiology, 69, 3350–3358. https://doi.org/10.1128/AEM.69.6.3350-3358.2003
- Bao, Y., He, J., & Li, Y. (2013). Facile and efficient synthesis of hyperbranched polyesters based on renewable castor oil. Polymer International, 62, 1457–1464. https://doi.org/10.1002/pi.4440
- Becker, J. G. (2020). The microbial ecology and bioremediation of chlorinated ethene-contaminated environments. In Women in water quality (pp. 153–171). Springer. https://doi.org/10.1007/978-3-030-17819-2_9
10.1007/978-3-030-17819-2_9 Google Scholar
- Blanford, W. J., Pecoraro, M. P., Heinrichs, R., & Boving, T. B. (2018). Enhanced reductive de-chlorination of a solvent contaminated aquifer through addition and apparent fermentation of cyclodextrin. Journal of Contaminant Hydrology, 208, 68–78. https://doi.org/10.1016/j.jconhyd.2017.10.006
- Borden, R. C., Richardson, S. D., & Bodour, A. A. (2019). Enhanced reductive dechlorination of trichloroethene in an acidic DNAPL impacted aquifer. Journal of Environmental Management, 237, 617–628. https://doi.org/10.1016/j.jenvman.2018.12.093
- Brusseau, M. L. (2019). Soil and groundwater remediation. In Environmental and pollution science (pp. 329–354). Elsevier. https://doi.org/10.1016/B978-0-12-814719-1.00019-7
10.1016/B978-0-12-814719-1.00019-7 Google Scholar
- Castaño, A., Prosenkov, A., Baragaño, D., Otaegui, N., Sastre, H., Rodríguez-Valdés, E., Gallego, J. L. R., & Peláez, A. I. (2021). Effects of in situ remediation with nanoscale zero valence iron on the physicochemical conditions and bacterial communities of groundwater contaminated with arsenic. Frontiers in Microbiology, 12, 580. https://doi.org/10.3389/fmicb.2021.643589
- Chang, Y., Okeke, B., Hatsu, M., & Takamizawa, K. (2001). In vitro dehalogenation of tetrachloroethylene (PCE) by cell-free extracts of Clostridium bifermentans DPH-1. Bioresource Technology, 78, 141–147. https://doi.org/10.1016/S0960-8524(01)00005-0
- Chen, W.-Y., Wu, J. H., & Chu, S. C. (2020). Deciphering microbiomes in anaerobic reactors with superior trichloroethylene dechlorination performance at low pH conditions. Environmental Pollution, 257, 113567. https://doi.org/10.1016/j.envpol.2019.113567
- Comas, D., Wagner, J., & Tomás, M. (2006). Creaming stability of oil in water (O/W) emulsions: Influence of pH on soybean protein–lecithin interaction. Food Hydrocolloids, 20, 990–996. https://doi.org/10.1016/j.foodhyd.2005.11.006
- Corseuil, H. X., Monier, A. L., Gomes, A. P., Chiaranda, H. S., do Rosario, M., & Alvarez, P. J. (2011). Biodegradation of soybean and castor oil biodiesel: Implications on the natural attenuation of monoaromatic hydrocarbons in groundwater. Groundwater Monitoring & Remediation, 31, 111–118. https://doi.org/10.1111/j.1745-6592.2011.01333.x
- de Guzmán, G. T. N., Hapeman, C. J., Millner, P. D., Torrents, A., Jackson, D., & Kjellerup, B. V. (2018). Presence of organohalide-respiring bacteria in and around a permeable reactive barrier at a trichloroethylene-contaminated superfund site. Environmental Pollution, 243, 766–776. https://doi.org/10.1016/j.envpol.2018.08.095
- Dong, J., Yu, D., Li, Y., Li, B., & Bao, Q. (2019). Transport and release of electron donors and alkalinity during reductive dechlorination by combined emulsified vegetable oil and colloidal Mg (OH) 2: Laboratory sand column and microcosm tests. Journal of Contaminant Hydrology, 225, 103501. https://doi.org/10.1016/j.jconhyd.2019.103501
- Dugat-Bony, E., Biderre Petit, C., Jaziri, F., David, M. M., Denonfoux, J., Lyon, D. Y., Richard, J. Y., Curvers, C., Boucher, D., & Vogel, T. M. (2012). In situ TCE degradation mediated by complex dehalorespiring communities during biostimulation processes. Microbial Biotechnology, 5, 642–653. https://doi.org/10.1111/j.1751-7915.2012.00339.x
- Esfe, M. H., Bahiraei, M., & Mahian, O. (2018). Experimental study for developing an accurate model to predict viscosity of CuO–ethylene glycol nanofluid using genetic algorithm based neural network. Powder Technology, 338, 383–390. https://doi.org/10.1016/j.powtec.2018.07.013
- Futamata, H., Nagano, Y., Watanabe, K., & Hiraishi, A. (2005). Unique kinetic properties of phenol-degrading Variovorax strains responsible for efficient trichloroethylene degradation in a chemostat enrichment culture. Applied and Environmental Microbiology, 71, 904–911. https://doi.org/10.1128/AEM.71.2.904-911.2005
- Guo, Y., Liu, J. X., Lu, Y., Zhu, W. Denman, S., & McSweeney, C. (2008). Effect of tea saponin on methanogenesis, microbial community structure and expression of mcrA gene, in cultures of rumen micro-organisms.. Letters in Applied Microbiology, 47, 421–426.
- Gupta, A., Eral, H. B., Hatton, T. A., & Doyle, P. S. (2016). Nanoemulsions: Formation, properties and applications. Soft Matter, 12, 2826–2841. https://doi.org/10.1039/C5SM02958A
- Haluska, A. A., Schaefer, C. E., Cho, J., Lavorgna, G. M., & Annable, M. D. (2019). Long-term mass flux assessment of a DNAPL source area treated using bioremediation. Journal of Contaminant Hydrology, 227, 103516. https://doi.org/10.1016/j.jconhyd.2019.103516
- Hao, M., Qiu, M., Yang, H., Hu, B., & Wang, X. (2021). Recent advances on preparation and environmental applications of MOF-derived carbons in catalysis. Science of the Total Environment, 760, 143333. https://doi.org/10.1016/j.scitotenv.2020.143333
- He, B. N., He, J. T., Wang, F., Lian, Y. Q., & Zhao, Y. K. (2018). Migration, clogging, and carbon source release of nano emulsified vegetable oil in porous media, evaluated by column experiments. Bioremediation Journal, 22, 53–62. https://doi.org/10.1080/10889868.2018.1476451
- Heavner, G. L., Mansfeldt, C. B., Wilkins, M. J., Nicora, C. D., Debs, G. E., Edwards, E. A., & Richardson, R. E. (2019). Detection of organohalide-respiring enzyme biomarkers at a bioaugmented TCE-contaminated field site. Frontiers in Microbiology, 10, 1433. https://doi.org/10.3389/fmicb.2019.01433
- Heidari, H., Sedighi, M., Zamir, S. M., & Shojaosadati, S. A. (2017). Bisphenol a degradation by Ralstonia eutropha in the absence and presence of phenol. International Biodeterioration & Biodegradation, 119, 37–42. https://doi.org/10.1016/j.ibiod.2016.10.052
- Hermon, L., Hellal, J., Denonfoux, J., Vuilleumier, S., Imfeld, G., Urien, C., Ferreira, S., & Joulian, C. (2019). Functional genes and bacterial communities during organohalide respiration of chloroethenes in microcosms of multi-contaminated groundwater. Frontiers in Microbiology, 10, 89. https://doi.org/10.3389/fmicb.2019.00089
- Hostettmann, K., & Marston, A. (2005). Saponins. Cambridge University Press. https://doi.org/10.1016/B0-12-369397-7/00548-3
- Kao, C. M., Sheu, Y. T., Ou, J. H., & Lin, W. H. (2019). Application of slow-release materials for in situ and passive remediation of contaminated groundwater. In Sustainable remediation of contaminated soil and groundwater: Materials, processes, and assessment (pp. 169–199). Butterworth-Heinemann.
- Kim, B. H., Cho, D. H., Sung, Y. B., Ahn, C. Y., Yoon, B. D., Koh, S. C., Oh, H. M., & Kim, H. S. (2010). Analysis of microbial community during the anaerobic dechlorination of PCE/TCE by DGGE. Microbiology and Biotechnology Letters, 38, 448–454.
- Kim, U., Parker, J. C., & Borden, R. C. (2019). Stochastic cost-optimization and risk assessment of in situ chemical oxidation for dense non-aqueous phase liquid (DNAPL) source remediation. Stochastic Environmental Research and Risk Assessment, 33, 73–89. https://doi.org/10.1007/s00477-018-1633-y
- Kregiel, D., Berlowska, J., Witonska, I., Antolak, H., Proestos, C., Babic, M., Babic, L., & Zhang, B. (2017). Saponin-based, biological-active surfactants from plants. Application and Characterization of Surfactants, 6, 184–205. https://doi.org/10.5772/68062
10.5772/68062 Google Scholar
- Kruse, S., Türkowsky, D., Birkigt, J., Matturro, B., Franke, S., Jehmlich, N., von Bergen, M., Westermann, M., Rossetti, S., & Nijenhuis, I. (2021). Interspecies metabolite transfer in a co-culture of Dehalococcoides and Sulfurospirillum leads to rapid and complete tetrachloroethene dechlorination. The ISME Journal, 15, 1794–1809.
- Lee, L., & Norton, I. T. (2013). Comparing droplet breakup for a high-pressure valve homogeniser and a microfluidizer for the potential production of food-grade nanoemulsions. Journal of Food Engineering, 114, 158–163. https://doi.org/10.1016/j.jfoodeng.2012.08.009
- Li, C., Zhang, X., Lu, Y., Fan, Z., Wang, T., & Zhang, G. (2020). Cometabolic degradation of p-chloroaniline by the genus Brevibacillus bacteria with extra carbon sources. Journal of Hazardous Materials, 383, 121198. https://doi.org/10.1016/j.jhazmat.2019.121198
- Li, H., Zhang, S. Y., Wang, X. L., Yang, J., Gu, J. D., Zhu, R. L., Wang, P., Lin, K. F., & Liu, Y. D. (2015). Aerobic biodegradation of trichloroethylene and phenol co-contaminants in groundwater by a bacterial community using hydrogen peroxide as the sole oxygen source. Environmental Technology, 36, 667–674. https://doi.org/10.1080/09593330.2014.957730
- Li, J., Hu, A., Bai, S., Yang, X., Sun, Q., Liao, X., & Yu, C. P. (2021). Characterization and performance of lactate-feeding consortia for reductive dechlorination of trichloroethene. Microorganisms, 9, 751. https://doi.org/10.3390/microorganisms9040751
- Li, Q., Chen, Z., Wang, H., Yang, H., Wen, T., Wang, S., Hu, B., & Wang, X. (2021). Removal of organic compounds by nanoscale zero-valent iron and its composites. Science of the Total Environment, 792, 148546. https://doi.org/10.1016/j.scitotenv.2021.148546
- Li, Y., Zhang, Y., Yang, S., Xue, Y., Liu, J., Wang, M., Liu, S., & Chen, Y. (2021). Citrate ligand-enhanced microscale zero-valent aluminum corrosion for carbon tetrachloride degradation with high electron utilization efficiency. Science of the Total Environment, 783, 146999. https://doi.org/10.1016/j.scitotenv.2021.146999
- Liang, L., Xi, F., Tan, W., Meng, X., Hu, B., & Wang, X. (2021). Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar, 3, 255–281. https://doi.org/10.1007/s42773-021-00101-6
- Lien, P., Yang, Z., Chang, Y., Tu, Y., & Kao, C. (2016). Enhanced bioremediation of TCE-contaminated groundwater with coexistence of fuel oil: Effectiveness and mechanism study. Chemical Engineering Journal, 289, 525–536. https://doi.org/10.1016/j.cej.2016.01.011
- Lin, W. H., Chen, C. C., Sheu, Y. T., Tsang, D. C., Lo, K. H., & Kao, C. M. (2020). Growth inhibition of sulfate-reducing bacteria for trichloroethylene dechlorination enhancement. Environmental Research, 187, 109629. https://doi.org/10.1016/j.envres.2020.109629
- Liu, X., Ma, R., Zhuang, L., Hu, B., Chen, J., Liu, X., & Wang, X. (2021). Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Critical Reviews in Environmental Science and Technology, 51, 751–790. https://doi.org/10.1080/10643389.2020.1734433
- Lo, K. H., Lu, C. W., Lin, W. H., Chien, C. C., Chen, S. C., & Kao, C. M. (2020). Enhanced reductive dechlorination of trichloroethene with immobilized Clostridium butyricum in silica gel. Chemosphere, 238, 124596. https://doi.org/10.1016/j.chemosphere.2019.124596
- Luo, S., Chen, S., Cao, W., Lin, W., Sheu, Y., & Kao, C. (2019). Application of γ-PGA as the primary carbon source to bioremediate a TCE-polluted aquifer: A pilot-scale study. Chemosphere, 237, 124449. https://doi.org/10.1016/j.chemosphere.2019.124449
- Luo, X., Zhou, Y., Bai, L., Liu, F., Zhang, R., Zhang, Z., Zheng, B., Deng, Y., & McClements, D. J. (2017). Production of highly concentrated oil-in-water emulsions using dual-channel microfluidization: Use of individual and mixed natural emulsifiers (saponin and lecithin). Food Research International, 96, 103–112. https://doi.org/10.1016/j.foodres.2017.03.013
- Margareta, W., Nagarajan, D., Chang, J.-S., & Lee, D.-J. (2020). Dark fermentative hydrogen production using macroalgae (Ulva sp.) as the renewable feedstock. Applied Energy, 262, 114574. https://doi.org/10.1016/j.apenergy.2020.114574
- Matteucci, F., Sprocati, A., Alisi, C., Chiavarini, S., & Ercole, C. (2018). Microbial degradation of chlorinated solvents: A microcosm study and a microbial genetic analysis to remediate a contaminated area in Central Italy. International Journal of Environmental Bioremediation & Biodegradation, 110, 1–12. https://doi.org/10.29011/IJBB-110/100010
10.29011/IJBB-110/100010 Google Scholar
- Mikkola, V. (2012). Impact of concentration, particle size and thermal conductivity on effective convective heat transfer of nanofluids. MS Thesis. Aalto University.
- Mikkola, V. (2015). Impact of concentration, particle size and thermal conductivity on effective convective heat transfer performance of nanofluids (pp. 1–83). Aalto University.
- Němeček, J., Steinová, J., Špánek, R., Pluhař, T., Pokorný, P., Najmanová, P., Knytl, V., & Černík, M. (2018). Thermally enhanced in situ bioremediation of groundwater contaminated with chlorinated solvents—A field test. Science of the Total Environment, 622, 743–755. https://doi.org/10.1016/j.scitotenv.2017.12.047
- Neumann, A., Scholz Muramatsu, H., & Diekert, G. (1994). Tetrachloroethene metabolism of Dehalospirillum multivorans. Archives of Microbiology, 162, 295–301. https://doi.org/10.1007/BF00301854
- Newatia, N., Chawla, A., Prasad, S., & Choksi, H. H. (2014). Techno-economical analysis of biodiesel production using castor oil: An Indian approach. Carbon, 87, 77.
- Ohl, S. W., Klaseboer, E., & Khoo, B. C. (2015). Bubbles with shock waves and ultrasound: A review. Interface Focus, 5(5), 20150019. https://doi.org/10.1098/rsfs.2015.0019
- Prajapat, G., Jain, S., & Agrawal, A. (2019). Microbial diversity and dynamics in hydrocarbon resource environments. In T. Satyanarayana, B. N. Johri, & S. K. Das (Eds.), Microbial diversity in ecosystem sustainability and biotechnological applications: (Vol. 1, pp. 533–571). Microbial Diversity in Normal & Extreme Environments. Springer Singapore; https://doi.org/10.1007/978-981-13-8315-1_17
10.1007/978-981-13-8315-1_17 Google Scholar
- Puentes Jácome, L. A., Wang, P. H., Molenda, O., Li, Y. X., Islam, M. A., & Edwards, E. A. (2019). Sustained dechlorination of vinyl chloride to ethene in Dehalococcoides-enriched cultures grown without addition of exogenous vitamins and at low pH. Environmental Science & Technology, 53, 11364–11374. https://doi.org/10.1021/acs.est.9b02339
- Qiu, M., Hu, B., Chen, Z., Yang, H., Zhuang, L., & Wang, X. (2021). Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar, 3, 117–123. https://doi.org/10.1007/s42773-021-00098-y
- Rice, E., Baird, R., Eaton, A., Odor, T., By, D., & Carbon, T. O. (2017). Standard methods for the examination of water and wastewater. American Public Health Association (APHA).
- Ritalahti, K. M., Amos, B. K., Sung, Y., Wu, Q., Koenigsberg, S. S., & Löffler, F. E. (2006). Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Applied and Environmental Microbiology, 72, 2765–2774. https://doi.org/10.1128/AEM.72.4.2765-2774.2006
- Rowlands, D. (2004). Development of optimal pH for degradation of chlorinated solvents by the KB-1 anaerobic bacterial culture. Prepared for Geosyntec Consultants/SiREM.
- Saiyari, D. M., Chuang, H. P., Senoro, D. B., Lin, T. F., Whang, L. M., Chiu, Y. T., & Chen, Y. H. (2018). A review in the current developments of genus Dehalococcoides, its consortia and kinetics for bioremediation options of contaminated groundwater. Sustainable Environment Research, 28, 149–157. https://doi.org/10.1016/j.serj.2018.01.006
- Salman, M., Gerhard, J. I., Major, D. W., Pironi, P., & Hadden, R. (2015). Remediation of trichloroethylene-contaminated soils by star technology using vegetable oil smoldering. Journal of Hazardous Materials, 285, 346–355. https://doi.org/10.1016/j.jhazmat.2014.11.042
- Schiefler, A. A., Tobler, D. J., Overheu, N. D., & Tuxen, N. (2018). Extent of natural attenuation of chlorinated ethenes at a contaminated site in Denmark. Energy Procedia, 146, 188–193. https://doi.org/10.1016/j.egypro.2018.07.024
- Sheu, Y., Lien, P., Chen, K., Ou, J., & Kao, C. (2016). Application of NZVI-contained emulsified substrate to bioremediate PCE-contaminated groundwater—A pilot-scale study. Chemical Engineering Journal, 304, 714–727. https://doi.org/10.1016/j.cej.2016.06.126
- Sheu, Y. T., Chen, S., Chien, C., Chen, C., & Kao, C. (2015). Application of a long-lasting colloidal substrate with pH and hydrogen sulfide control capabilities to remediate TCE-contaminated groundwater. Journal of Hazardous Materials, 284, 222–232. https://doi.org/10.1016/j.jhazmat.2014.11.023
- Sheu, Y. T., Tsang, D. C., Dong, C. D., Chen, C. W., Luo, S. G., & Kao, C. M. (2018). Enhanced bioremediation of TCE-contaminated groundwater using gamma poly-glutamic acid as the primary substrate. Journal of Cleaner Production, 178, 108–118. https://doi.org/10.1016/j.jclepro.2017.12.212
- Sood, P., Modgil, R., Sood, M., & Chuhan, P. (2012). Anti-nutrient profile of different Chenopodium cultivars leaves. Annals Food Sci. Technol, 13, 68–74.
- Steffan, R. J., & Schaefer, C. E. (2016). Current and future bioremediation applications: Bioremediation from a practical and regulatory perspective. In Organohalide-respiring bacteria (pp. 517–540). Springer. https://doi.org/10.1007/978-3-662-49875-0_22
10.1007/978-3-662-49875-0_22 Google Scholar
- Stoll, V. S., & Blanchard, J. S. (2009). Buffers: Principles and practice. Methods in Enzymology, 463, 43–56. https://doi.org/10.1016/S0076-6879(09)63006-8
- Su, C., Puls, R. W., Krug, T. A., Watling, M. T., O'Hara, S. K., Quinn, J. W., & Ruiz, N. E. (2017). Long-term performance evaluation of groundwater chlorinated solvents remediation using nanoscale emulsified zerovalent iron at a superfund site, applying nanotechnology for environmental sustainability (pp. 92–111). IGI Global.
- Sudheesh, A. (2018). Bioaugmentation of chlorinated solvents in groundwater using Dehalococcoides. International Journal of Advance Research, Ideas and Innovations in Technology, 4, 238–248.
- Sun, Y., Jing, R., Zheng, F., Zhang, S., Jiao, W., & Wang, F. (2019). Evaluating phytotoxicity of bare and starch-stabilized zero-valent iron nanoparticles in mung bean. Chemosphere, 236, 124336. https://doi.org/10.1016/j.chemosphere.2019.07.067
- Thakur, S., & Karak, N. (2013). Castor oil-based hyperbranched polyurethanes as advanced surface coating materials. Progress in Organic Coatings, 76, 157–164. https://doi.org/10.1016/j.porgcoat.2012.09.001
- Thulasi, D., Muralidhar, M., Saraswathy, R., & Kumar, J. A. (2017). Temporal and spatial distribution of sulfate reducing bacteria in shrimp culture pond sediment. Asian Journal of Environment & Ecology, 3, 1–16. https://doi.org/10.9734/AJEE/2017/35287
10.9734/AJEE/2017/35287 Google Scholar
- Tolvanen, K. E., Santala, V. P., & Karp, M. T. (2010). [FeFe]-hydrogenase gene quantification and melting curve analysis from hydrogen-fermenting bioreactor samples. International Journal of Hydrogen Energy, 35, 3433–3439. https://doi.org/10.1016/j.ijhydene.2010.01.132
- Tran, T., & Rousseau, D. (2013). Stabilization of acidic soy protein-based dispersions and emulsions by soy soluble polysaccharides. Food Hydrocolloids, 30, 382–392. https://doi.org/10.1016/j.foodhyd.2012.06.001
- Ushikubo, F., & Cunha, R. (2014). Stability mechanisms of liquid water-in-oil emulsions. Food Hydrocolloids, 34, 145–153. https://doi.org/10.1016/j.foodhyd.2012.11.016
- Wen, L. L., Li, Y., Zhu, L., & Zhao, H. P. (2020 Apr 1). Influence of non-dechlorinating microbes on trichloroethene reduction based on vitamin B12 synthesis in anaerobic cultures. Environmental Pollution, 259, 113947.
- Wen, L. L., Zhang, Y., Chen, J. X., Zhang, Z. X., Yi, Y. Y., Tang, Y., Rittmann, B. E., & Zhao, H. P. (2017). The dechlorination of TCE by a perchlorate reducing consortium. Chemical Engineering Journal, 313, 1215–1221. https://doi.org/10.1016/j.cej.2016.11.021
- Wu, W. M., Carley, J., Green, S. J., Luo, J., Kelly, S. D., Nostrand, J. V., Lowe, K., Mehlhorn, T., Carroll, S., & Boonchayanant, B. (2010). Effects of nitrate on the stability of uranium in a bioreduced region of the subsurface. Environmental Science & Technology, 44, 5104–5111. https://doi.org/10.1021/es1000837
- Yang, Y. (2012). Exploring anaerobic reductive dechlorination at low pH environments. University of Tennessee.
- Yang, Y., Cápiro, N. L., Marcet, T. F., Yan, J., Pennell, K. D., & Löffler, F. E. (2017). Organohalide respiration with chlorinated Ethenes under low pH conditions. Environmental Science & Technology, 51, 8579–8588. https://doi.org/10.1021/acs.est.7b01510
- Yang, Y., Cápiro, N. L., Yan, J., Marcet, T. F., Pennell, K. D., & Löffler, F. E. (2017). Resilience and recovery of Dehalococcoides mccartyi following low pH exposure. FEMS Microbiology Ecology, 93, fix130. https://doi.org/10.1093/femsec/fix130
- Yao, L., Yang, H., Chen, Z., Qiu, M., Hu, B., & Wang, X. (2021). Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater. Chemosphere, 273, 128576. https://doi.org/10.1016/j.chemosphere.2020.128576
- Zalesak, M., Ruzicka, J., Vicha, R., & Dvorackova, M. (2021). Examining aerobic degradation of chloroethenes mixture in consortium composed of Comamonas testosteroni RF2 and Mycobacterium aurum L1. Chemosphere, 269, 128770. https://doi.org/10.1016/j.chemosphere.2020.128770
- Zhang, S., You, J., Kennes, C., Cheng, Z., Ye, J., Chen, D., Chen, J., & Wang, L. (2018). Current advances of VOCs degradation by bioelectrochemical systems: A review. Chemical Engineering Journal, 334, 2625–2637. https://doi.org/10.1016/j.cej.2017.11.014
- Zhou, Z., Liu, Y. G., Liu, S. B., Liu, H. Y., Zeng, G. M., Tan, X. F., Yang, C. P., Ding, Y., Yan, Z. L., & Cai, X. X. (2017). Sorption performance and mechanisms of arsenic (V) removal by magnetic gelatin-modified biochar. Chemical Engineering Journal, 314, 223–231. https://doi.org/10.1016/j.cej.2016.12.113
- Zhu, Z., Wen, Y., Yi, J., Cao, Y., Liu, F., & McClements, D. J. (2019). Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. Journal of Colloid and Interface Science, 536, 80–87. https://doi.org/10.1016/j.jcis.2018.10.024