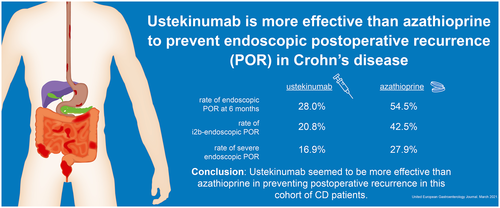
Ustekinumab is more effective than azathioprine to prevent endoscopic postoperative recurrence in Crohn's disease
Abstract
Background
Preventing postoperative recurrence (POR) is a major concern in Crohn's disease (CD). While azathioprine is an option, no data is available on ustekinumab efficacy in this situation.
Aims
We compared the effectiveness of ustekinumab versus azathioprine in preventing endoscopic POR in CD.
Methods
We retrospectively collected data from all consecutive CD patients treated with ustekinumab after intestinal resection in 9 centers. The control group (azathioprine alone) was composed of patients who participated in a randomized controlled trial conducted in the same centers comparing azathioprine alone or in combination with curcumin. Propensity score analyses (inversed probability of treatment weighting = IPTW) were applied to compare the two groups. The primary endpoint was endoscopic POR (Rutgeerts' index ≥ i2) at 6 months.
Results
Overall, 32 patients were included in the ustekinumab group and 31 in the azathioprine group. The propensity score analysis was adjusted on the main risk factors (smoking, fistulizing phenotype, prior bowel resection, resection length >30 cm and ≥2 biologics before surgery) and thiopurines or ustekinumab exposure prior to surgery making the two arms comparable (∣d∣ < 0.2). After IPTW, the rate of endoscopic POR at 6 months was lower in patients treated with ustekinumab compared to azathioprine (28.0% vs. 54.5%, p = 0.029). After IPTW, the rates of i2b-endoscopic POR (Rutgeerts' index ≥ i2b) and severe endoscopic POR (Rutgeerts' index ≥ i3) were 20.8% versus 42.5% (p = 0.066) and 16.9% versus 27.9% (p = 0.24), in the ustekinumab and azathioprine groups, respectively.
Conclusion
Ustekinumab seemed to be more effective than azathioprine in preventing POR in this cohort of CD patients.
Graphical Abstract
INTRODUCTION
Key Summary
-
Summarize the established knowledge on this subject.
- •
Endoscopic postoperative recurrence (POR) occurs in 75% of the patients with Crohn's disease (CD) within the first year after surgery in referral centers.
- •
Azathioprine is an option to prevent endoscopic POR in CD but its efficacy remains limited.
- •
Ustekinumab is effective to induce and maintain clinical remission in patients with luminal CD.
- •
-
What are the significant and/or new findings of this study?
- •
Ustekinumab was more effective than azathioprine to prevent endoscopic POR in patients with CD.
- •
Ustekinumab could be an interesting option to prevent endoscopic POR in patients with CD.
- •
Ustekinumab, an antagonist of the p40 subunit shared by the interleukin-12 (IL-12) and IL-23, showed a higher effectiveness than placebo to achieve clinical remission or endoscopic improvement in patients with luminal moderate-to-severe CD.17-20 The effectiveness of ustekinumab is unknow to prevent POR in CD patients.
We, therefore, conducted a retrospective multicenter study to assess whether ustekinumab is more effective than azathioprine to prevent endoscopic POR in patients with CD.
PATIENTS AND METHODS
Ethical considerations
The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements including patient informed consent. The study was approved by the local Ethics Committees (2020/CE14; #AU1109; IRB 16-0061; last approval: 14 April 2020). All authors had access to the study data and reviewed and approved the final manuscript.
Study design
We performed a multicenter retrospective study in nine IBD academic centers. Two groups of patients were compared: patients receiving ustekinumab in the first group, and patients receiving azathioprine in the second group (control arm). Inclusion criteria were: patients with CD older than 18 years old who had undergone a surgical resection for ileal, ileocolonic, or colonic CD with ileocolonic anastomosis. All the macroscopic lesions had to be removed during the surgery. The anastomosis had to be reachable by ileocolonoscopy.
Patients treated with azathioprine
From a randomized controlled trial where patients were recruited between October 2014 and January 2018, we included all the patients who were randomized in the control group to receive oral azathioprine 2.5 mg/kg/day with a concomitant placebo for 6 months.9 Enrollment and treatment initiation had to occur within 15 days following bowel resection or following stoma closure when a diverting ostomy had been necessary.9 Adherence to medications was evaluated, either by pill counts when patients brought back unused medications at each visit or by patient's interview. Therapeutic drug monitoring was performed in each patient by measurement of 6-thioguanine (6-TGN).
Patients treated with ustekinumab
We asked the centers who were involved in the randomized controlled trial9 for screening their database to identify all CD patients who have been treated with ustekinumab to prevent endoscopic POR. Patients with prior exposure to ustekinumab before surgery were also included. One additional center was solicited to reach a sufficient number of patients (see sample size calculation). All these patients received intravenous induction. The doses of initial intravenous infusion of ustekinumab were adjusted on body weight (260 mg if < 55 kg, 390 mg if between 56 and 80 kg and 520 mg if > 80 kg). All the subsequent subcutaneous injections were performed using a dose of 90 mg. We excluded all the patients who started ustekinumab more than 90 days after intestinal resection or following stoma closure, and patients with persistent macroscopic lesions after surgery (Figure 1).

Flow chart summarizing the inclusion of the patients in the ustekinumab group
Study endpoints
The primary endpoint was endoscopic POR evaluated 6 months (M6) after intestinal resection or following stoma closure, defined as Rutgeerts' index ≥ i2.
The secondary endpoints were alternative definitions of endoscopic POR using a Rutgeerts' index ≥ i2b (i2b-endoscopic POR),21, 22 and Rutgeerts' index ≥ i3 (severe endoscopic POR) as cut-off values.
The endoscopic procedures at M6 were scored by independent central readers in the control group. For the patients treated with ustekinumab, all endoscopic evaluations were performed by local readers who were experienced in assessing Rutgeerts' index in clinical trials.
Sample size calculation
The rate of endoscopic POR was already known (58.1%) in the azathioprine group (control arm). As no data were currently available on the efficacy of ustekinumab to prevent endoscopic POR, we hypothesized that ustekinumab could obtain the same results than anti-TNF agents (rate of endoscopic POR occurring from 0% to 22.4%).11-13 Then, at least 31 patients were required in the ustekinumab group to have a 80% chance of detecting, as significant at the 5% level, a decrease in the primary outcome measure from 58% in the control group to 25% in experimental group.
Data managing and statistical analyses
Study data were collected and managed using REDCap electronic data capture tools hosted at Clermont-Ferrand University Hospital.23
The potential differences in baseline characteristics between the treated group and the control group were tested with the Student t-test or the Mann–Whitney test for continuous variables (assumption of normality assessed using the Shapiro–Wilk test and homoscedasticity evaluated using Fisher–Snedecor's test), and the χ2 or the Fisher's exact tests for categorical variables.
The choice of treatment is usually influenced by the patients' characteristics in retrospective studies increasing the risk of indication bias.24 We performed a propensity score analysis to adjust for these differences and to be as close as possible to the situation of a randomized trial.24 A propensity score describes the probability for a patient to receive a treatment according to his/her characteristics. We used inverse probability of treatment weighting (IPTW) to perform the propensity score analyses. Each participant was then assigned an inverse weighting of the probability to receive or not ustekinumab, estimated by the propensity score.24 Thus, the weight of patients with high likelihood to receive ustekinumab based on their observable characteristics was reduced while the weight of patients who were unlikely to receive ustekinumab was increased. The aim of this method was to make as comparable as possible our two groups (same chance of being treated by one or the other treatment for each patient). The validity of the matching was tested by calculating standardized differences (|d|), with |d| > 0.2 considered an imbalance. Our propensity score model included the main usual risk factors of endoscopic POR3, 5, 16, 25, 26: smoking status, fistulizing phenotype (B3 according to the Montreal classification), prior bowel resection, resection length more than 30 cm, and more than two biologics before undergoing intestinal resection. It also included previous exposure to azathioprine or ustekinumab, which could negatively impact the effectiveness of the two investigated drugs. The other characteristics were described and compared check that there was no imbalance between the two groups. An effect size above 0.2 was considered significant.
To perform an exploratory analysis to determine the potential factors associated with endoscopic POR in patients treated with ustekinumab, aforementioned univariate tests were carried out. Results were estimated using logistic regression and expressed using odds ratios and 95% confidence intervals.
A two-sided p < 0.05 was considered statistically significant. All analyses were performed with Stata (version 15; StataCorp) software.
RESULTS
Study population
Overall, 31 patients with CD receiving azathioprine were included in the control group.9 Their characteristics are mentioned in Table 1. The median level of 6-TGN was 365 pmol (207–532) at M3 and 260 pmol (130–378) at M6. In parallel, 32 patients were included in the ustekinumab group (Figure 1). Their baseline characteristics are detailed in Table 1. Overall, 93.7% (30/32) and 83.9% (26/31) of the patients were considered as high-risk patients (at least one risk factor of POR among active smoking, fistulizing phenotype, resection length > 30 cm, prior bowel resection, and more than two biologics before surgery) in the ustekinumab and azathioprine group, respectively. We observed a higher proportion of patients exposed to ustekinumab prior to surgery (34.4% vs. 9.7%, p = 0.018) in the ustekinumab group. In addition, there were nonsignificant trends for higher rate of patients treated with vedolizumab (28.1% vs. 5.6%, p = 0.06) and more than two biologics before surgery in the ustekinumab group (40.6% vs. 19.4%, p = 0.07) (Table 1).
| Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|
| Azathioprine group n = 31 | Ustekinumab group n = 32 | p Value | Azathioprine group n = 31 | Ustekinumab group n = 32 | |d| | |
| Age at inclusion, (years), mean ± SD | 37.6 ± 13.8 | 36.8 ± 13.2 | 0.59 | 36.7 ± 12.5 | 37.7 ± 14.3 | 0.07 |
| Female gender, n (%) | 25 (80.6%) | 23 (71.9%) | 0.53 | 77.5% | 74.0% | 0.08 |
| Active smokers, n (%) | 13 (41.9%) | 11 (34.4%) | 0.54 | 42.2% | 38.5% | 0.07 |
| Prior bowel resection, n (%) | 14 (45.1%) | 17 (53.1%) | 0.53 | 45.5% | 49.6% | 0.08 |
| Montreal classification | ||||||
| CD location | ||||||
| L1, n (%) | 16 (51.6%) | 17 (53.1%) | 0.59 | 53.9% | 57.8% | 0.08 |
| L2, n (%) | 1 (3.2%) | 0 (0.0%) | – | 2.2% | 0.0% | 0.21 |
| L3, n (%) | 14 (45.2%) | 15 (46.9%) | – | 43.9% | 42.2% | 0.03 |
| CD behavior | ||||||
| B1, n (%) | 4 (12.9%) | 3 (9.4%) | 0.16 | 9.0% | 8.4% | 0.02 |
| B2, n (%) | 16 (51.6%) | 10 (31.2%) | – | 42.3% | 40.6% | 0.04 |
| B3, n (%) | 21 (35.5%) | 19 (59.4%) | – | 48.8% | 51.0% | 0.05 |
| Perianal lesions, n (%) | 5 (16.1%) | 8 (25.0%) | 0.38 | 16.7% | 21.0% | 0.10 |
| Mean length of ileal resection (cm), mean ± SD | 22.7 ± 18.8 | 23.7 ± 11.5 | 0.46 | 24.5 ± 18.9 | 24.0 ± 11.7 | 0.03 |
| Ileal resection length > 30 cm | 9 (29.0%) | 10 (31.2%) | 0.84 | 33.3% | 31.3% | 0.04 |
| Medications prior to surgery | ||||||
| Thiopurines, n (%) | 19 (61.3%) | 21 (65.6%) | 0.72 | 61.8% | 59.5% | 0.05 |
| Anti-TNF agents, n (%) | 27 (87.1%) | 30 (93.7%) | 0.77 | 87.8% | 93.1% | 0.18 |
| Infliximab, n (%) | 17 (54.8%) | 23 (71.9%) | 0.16 | 57.7% | 68.8% | 0.23 |
| Adalimumab, n (%) | 24 (77.4%) | 25 (78.1%) | 0.95 | 79.9% | 74.6% | 0.12 |
| Golimumab, n (%) | 2 (6.5%) | 0 (0.0%) | 0.14 | 7.0% | 0.0% | 0.39 |
| Ustekinumab, n (%) | 3 (9.7%) | 11 (34.4%) | 0.018 | 21.9% | 22.8% | 0.02 |
| Type of ustekinumab failure before surgery | ||||||
| Primary failure, n (%) | NA | 5/11 (45.5%) | – | – | – | – |
| Secondary loss of response, n (%) | NA | 6/11 (54.5%) | – | – | – | – |
| Maximal ustekinumab optimization | ||||||
| 90 mg every 8 weeks, n (%) | NA | 4/11 (36.4%) | – | – | – | – |
| 90 mg every 4 weeks, n (%) | NA | 7/11 (63.6%) | – | – | – | – |
| Vedolizumab, n (%) | 1 (5.6%) | 9 (28.1%) | 0.06 | 16.9% | 27.6% | 0.26 |
| Number of biologics before surgery | 0.33 | |||||
| >2 biologics | 6 (19.4%) | 13 (40.6%) | 0.07 | 29.9% | 31.4% | 0.03 |
| None, n (%) | 4 (12.9%) | 1 (3.1%) | – | – | – | – |
| 1, n (%) | 13 (41.9%) | 11 (34.4%) | – | – | – | – |
| 2, n (%) | 8 (25.8%) | 7 (21.9%) | – | – | – | – |
| 3, n (%) | 4 (12.9%) | 9 (28.1%) | – | – | – | – |
| 4, n (%) | 2 (6.5%) | 4 (12.5%) | – | – | – | – |
- Note: |d| = standardized difference (difference is not significant when |d| < 0.20).
- Abbreviations: CD, Crohn's disease; IPTW, inverse probability of treatment weighting; n, number; SD, standard deviation; TNF, tumor necrosis factor.
The characteristics of the population after propensity score modeling were also given in Table 1. After adjustment, the two groups were similar except for pre-operative use of infliximab (68.8% vs. 57.7%, |d| = 0.23) and vedolizumab (27.6% vs. 16.9%, |d| = 0.26) for the patients treated with ustekinumab and azathioprine, respectively. However, these two groups were comparable concerning anti-TNF exposure before surgery (93.1% vs. 87.8%, |d| = 0.18). As expected, the two groups were similar for all the factors included in the propensity score analysis: smoking status, fistulizing phenotype (B3 according to Montreal classification), prior bowel resection, resection length > 30 cm, azathioprine exposure before surgery, ustekinumab exposure prior to surgery, and the use of more than two biologics before intestinal resection (|d| < 0.20 for each variable) (Table 1).
All the patients treated with ustekinumab to prevent endoscopic POR received intravenous infusion. Ustekinumab was started with a median of 30.5 days interquartile range [IQR] [16.8–49.8 days] after the restoration of bowel continuity. All the patients had a subsequent subcutaneous injection 8 weeks later. The maintenance regimen was then every 12 weeks in one patient (3.1%), every 8 weeks in 29 patients (90.6%), and every 4 weeks in two patients (6.2%). All the patients received ustekinumab as monotherapy (no combination with azathioprine). Endoscopic evaluation was performed at M6 (scheduled visit in the randomized controlled trial) in the azathioprine group, while the median interval between the first ustekinumab infusion and colonoscopy was 6.0 months IQR [5.0–7.0 months] in the ustekinumab group.
Prevention of endoscopic postoperative recurrence
Before adjustment, the rate of endoscopic POR (defined as Rutgeerts' index ≥ i2) at 6 months (M6) was significantly lower in patients treated with ustekinumab (31.2% [10/32]) compared to those receiving azathioprine (58.1% [18/31]) (p = 0.032) (Figure 2). Using the propensity score analysis (IPTW), the rate of endoscopic POR was lower in the ustekinumab group (28.0% vs. 54.5%, p = 0.029) (Figure 2).
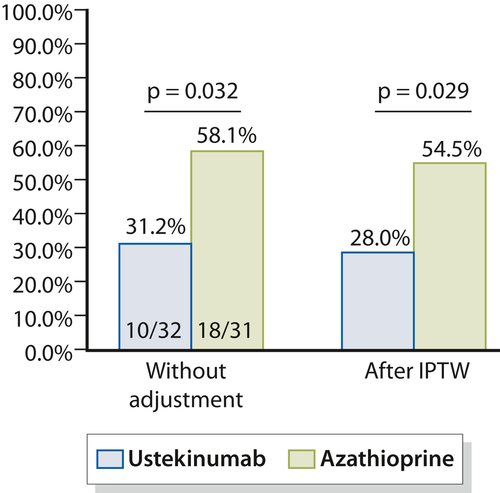
Endoscopic postoperative recurrence (≥i2) in 32 patients treated with ustekinumab and 31 patients treated with azathioprine
We performed a sensitivity analysis using Rutgeerts' index ≥ i2b to define endoscopic POR (i2b-endoscopic POR). Without adjustment, the proportion of patients experiencing i2b-endoscopic POR was numerically lower in patients treated with ustekinumab (21.9% vs. 41.9%) without reaching statistical significance (p = 0.091) (Figure 3a). After IPTW, this trend was reinforced with 20.8% of i2b-endoscopic POR in the ustekinumab arm compared to 42.5% in the azathioprine arm (p = 0.066) (Figure 3a).
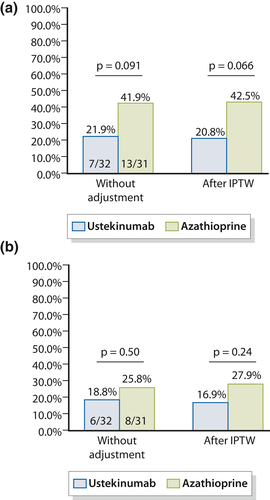
Endoscopic postoperative recurrence (≥i2b) (a) and severe endoscopic postoperative recurrence (≥i3) (b) in 32 patients treated with ustekinumab and 31 patients treated with azathioprine
We did not observe any significant difference regarding the rate of severe endoscopic POR (Rutgeerts' index ≥ i3) between the two groups (ustekinumab group = 18.8% and azathioprine group = 25.8%, p = 0.50) (Figure 3b). After propensity score analysis, the rates of severe endoscopic POR were 16.9% and 27.9%, for ustekinumab and azathioprine, respectively (p = 0.24) (Figure 3b).
Safety
No adverse event was observed in patients treated with ustekinumab in this study.
DISCUSSION
In this study, we reported the first data on ustekinumab therapy to prevent endoscopic POR in patients with CD. In a multicenter retrospective study, we showed that ustekinumab was more effective than azathioprine in this situation.
Rather than simply describing the rate of endoscopic POR in patients treated with ustekinumab, which would have been difficult to interpret, we used a control group. Recently, we reported the results of a randomized controlled trial comparing curcumin to placebo in patients treated with azathioprine to prevent endoscopic POR.9 We used the group of patients receiving azathioprine and a placebo as a control group to evaluate the effectiveness of ustekinumab to prevent POR. Although we performed a sample size calculation based on the number of patients included in the azathioprine group, we aimed to include all the consecutive patients in the same centers to make the retrospective analyses as powered as possible. As we did not reach the expected sample size (n = 31 patients), we included four consecutive patients from one additional center27-31 (Figure 1).
As expected, the baseline characteristics differed between the two groups of patients. The patients treated with ustekinumab after surgery presented with more refractory CD as underlined by the use of a higher number of biologics including a higher exposure to ustekinumab and vedolizumab prior to intestinal resection. It is why we performed a propensity score analysis to make the two populations as similar as possible to tend toward the ideal situation of a randomized controlled trial. In the calculation of the propensity score, we included the main risk factors of endoscopic POR , that is, active smoking, prior bowel resection, penetrating disease, resection length > 30 cm and preoperative failure to at least two biologics.3, 5, 16, 27 It is noteworthy that we also included the preoperative use of thiopurines and ustekinumab to further reduce the risk of bias. Then, the two arms were comparable except for preoperative use of vedolizumab (higher in the ustekinumab group).
There was no published data on this topic so far. In our study, we found that ustekinumab was more effective than azathioprine in preventing endoscopic POR (defined as Rutgeerts ≥ i2a). After propensity score analysis, we found a twofold lower rate (28.0% vs. 54.5%, p = 0.029) in the ustekinumab group. In addition, the patients included in the azathioprine group received concomitant placebo,9 which could have potentially increased azathioprine efficacy due to a placebo effect. Our study showed, for the first time, that ustekinumab is an effective option to prevent endoscopic POR and that it might be preferred over azathioprine, especially in patients previously exposed to several biologics. Even though it did not reach statistical significance, we observed a numerically twofold lower rate of i2b-recurrence in the ustekinumab group (after IPTW: 20.8% vs. 42.5, p = 0.066). We observed the same trend with a reduction of 10% regarding severe endoscopic POR (≥i3) in the patients receiving ustekinumab (after IPTW: 16.9% vs. 27.9%, p = 0.24).
Even though azathioprine remains an option, its efficacy remains limited as preventive medication after surgery in CD.3, 6-8, 16 The rate of endoscopic POR in our patients treated with azathioprine (58.1%) was comparable to the data from the medical literature ranging from 21% to 63%.16 D'Haens et al.32 reported a rate of endoscopic POR of 55% in patients with concomitant use of metronidazole. In a substudy of the POCER trial, 45% of the patients on thiopurines had endoscopic POR at 6 months. In a subanalysis of the TOPPIC trial including 124 patients with endoscopic evaluation, 29 (43%) of 67 patients in the mercaptopurine group had endoscopic recurrence with a Rutgeerts score of i2 or greater, which was not more effective than placebo (28/57, 49%) (p = 0.38).33 In our study, the high rate of endoscopic POR could be partly explained by the inclusion of more severe patients (83.9% had at least one risk factor of POR in our control group).
Other biologics have already been compared to azathioprine to prevent endoscopic POR. Several small sample size studies suggested a higher effectiveness of anti-TNF compared to thiopurines. In a small randomized controlled trial (n = 51 patients), Savarino et al.15 reported that adalimumab was more effective (6% vs. 65% of endoscopic POR). In the same way, the proportion of endoscopic POR at 6 months was lower in patients treated with adalimumab (21%) compared to those receiving azathioprine (45%) in a substudy of the POCER trial (n = 101 patients).13 In contrast, a Spanish study did not find any significant difference between adalimumab (42%) and azathioprine (59%) (p = 0.12) among 91 patients who received concomitant metronidazole therapy.
The main limitations of our study were the relatively small sample size, the retrospective data collection and the lack of central reading for the ustekinumab group. Nevertheless, the potential bias induced by the retrospective design were limited by the high standardization of the postoperative management including systematic endoscopic evaluation at 6 months with experienced IBD endoscopists. Its novelty (first study on ustekinumab to prevent POR in CD), the well-characterized control group retrieved from a randomized controlled trial and the use of a propensity score analysis as well as the multicenter design are the main strengths of our study.
In this cohort study, we found that ustekinumab seemed to be more effective than azathioprine to prevent endoscopic POR in patients with CD. Hence, ustekinumab could be an interesting option in this situation. This needs to be confirmed by large prospective studies.
USTEK POST-OP STUDY GROUP
Marion Goutte, Dilek Coban, Marie Dodel, Maud Reymond, Michel Dapoigny, Elisa Sollelis, Mathilde Boube, (Clermont-Ferrand), Florian Poullenot, Pauline Riviere (Bordeaux), Gilles Boschetti (Lyon), Jérôme Filippi (Nice), Emilie Del Tedesco, Pauline Veyrard (Saint-Etienne), Maria Nachury, Pauline Wils (Lille), Camille Zallot (Nancy), Clara Yzet, and Franck Brazier (Amiens).
ACKNOWLEDGMENTS
The authors thank the “François Aupetit” Association (AFA Crohn-RCH) and the CHU Clermont-Ferrand (DRCI) for its recurrent support.
CONFLICT OF INTEREST
Anthony Buisson: Consulting fees for Abbvie, Amgen, Biogen, Janssen, MSD, Pfizer, Roche, Takeda, and Tillots. Lecture fees for Abbvie, Amgen, Biogen, Janssen, Mayoly Spindler, MSD, Norgine Pfizer, Roche, Takeda, and Tillots. Stéphane Nancey: Consulting fees from Merck, Abbvie, Takeda, Ferring, Norgine, Vifor Pharma, Novartis, Janssen Cilag, Hospira, Takeda, and HAC-Pharma. David T. Rubin: Consultant fees for Abbvie, Abgenomics, Allergan, Inc., Amgen, Celgene Corporation, Forward Pharma, Genentech/Roche, Janssen Pharmaceuticals, Merck & Co., Inc., Miraca Life Sciences, Napo Pharmaceuticals, Pfizer, Salix Pharmaceuticals, Inc., Samsung Bioepis, Sandoz Pharmaceuticals, Shire, Takeda and grant support from Abbvie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, Takeda and UCB Pharma. Xavier Hebuterne: Abbvie, Abivax, Alphasigma, Arena, Astellas, Baxter, Bristol Myers Squibb, Cellgen, Gilead, Eli Lilly, Enterome, Ferring, Fresenius-Kabi, InDex Pharmaceuticals, Janssen, MSD, Nutricia, Pfizer, Roche, Sanofi-Advantis, SAlix, Sangamo, Servier, Takeda, Theravance, TillotAbbvie, MSD, Pfizer, Janssen Cilag, and Takeda. Benjamin Pariente: Consulting or lecture fees: AbbVie, MSD, Ferring, Takeda, Janssen, Biogaran, Pfizer, Biogen, Sandoz, Tillots, Lilly. Mathurin Fumery: Consulting or lecture fees for Abbvie, Amgen, Biogen, Gilead, Janssen, MSD, Pfizer, Ferring, Tillots, Celgene, Boehringer, and Takeda. David Laharie: Consulting and lecture fees from AbbVie, Ferring, Janssen Cilag, MSD, Pfizer, and Takeda. Xavier Roblin: Abbvie, MSD, Pfizer, Janssen Cilag, and Takeda. Laurent Peyrin-Biroulet: Consulting fees from Merck, Abbvie, Janssen, Genentech, Ferring, Norgine, Tillots, Vifor, Shire, Therakos, Pharmacosmos, Pilege, BMS, UCB-Pharma, Hospira, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, Pfizer, and HAC-Pharma. Lecture fees from Merck, Abbvie, Takeda, Janssen Cilag, Ferring, Norgine, Tillots, Vifor, Therakos, HAC-Pharma, and Mitsubishi. Lucine Vuitton: Lecture fees from Abbvie, Ferring, Mayoli, MSD, Pfizer, Janssen and; Takeda; consulting fees from Abbvie, Ferring, Gilead, Janssen and Takeda; research grants from MSD, Pfizer and Takeda. Other authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Guarantor of the article: Anthony Buisson. Anthony Buisson: Conception and design of the study; analysis and interpretation of data; drafting of the manuscript; approval of the final version of the manuscript. Luc Manlay: Conception and design of the study; generation, collection, assembly, analysis and interpretation of data; revision of the manuscript; approval of the final version of the manuscript. Stéphane Nancey, David T. Rubin, Xavier Hebuterne, Benjamin Pariente, Mathurin Fumery, David Laharie, Xavier Roblin, Gilles Bommelaer, Bruno Pereira, Laurent Peyrin-Biroulet: Generation, collection, assembly of data; substantial revision of the manuscript; approval of the final version of the manuscript. Bruno Pereira: Conception and design of the study; statistical analyses; revision of the manuscript; approval of the final version of the manuscript. Lucine Vuitton: Conception and design of the study; analysis and interpretation of data; substantial revision of the manuscript; approval of the final version of the manuscript. All authors approved the final version of the article, including the authorship list.
Open Research
DATA AVAILABILITY
The author has provided the required Data Availability Statement, and if applicable, included functional and accurate links to said data therein.