Uranium induces kidney cells apoptosis via reactive oxygen species generation, endoplasmic reticulum stress and inhibition of PI3K/AKT/mTOR signaling in culture
Funding information: National Natural Science Foundation of China, Grant/Award Number: 82160627; Natural Science Foundation of Guangxi Autonomous Region, Grant/Award Number: 2020GXNFSAA297262
Abstract
Uranium (U) induces generation of excessive intracellular reactive oxygen species (ROS), which is generally considered as a possible mediator of U-triggered kidney tubular cells injury and nephrotoxicity. Our goal is designed to elucidate that the precise molecular mechanism in ROS downstream is association with U-induced NRK-52E cells apoptosis. The results show that U intoxication in NRK-52E cells reduced cell activity and triggered apoptosis, as demonstrated by flow cytometry and apoptotic marker cleaved Caspase-3 expression. U exposure triggered endoplasmic reticulum (ER) stress, which is involvement of apoptosis determined by marker molecules including GRP78, PERK, IRE1, ATF6, CHOP, cleaved Caspase-12, and Caspase-3. Administration of antioxidant N-acetylcysteine (NAC) effectively blocked U-triggered ROS generation, ER stress, and apoptosis. U contamination evidently decreased the expression of phosphorylation PI3K, AKT, and mTOR and ratios of their respective phosphorylation to the corresponding total proteins. Application of a PI3K activator IGF-1 significantly abolished these adverse effects of U intoxication on PI3K/AKT/mTOR signaling and subsequently abrogated U-triggered apoptosis. NAC also effectively reversed down-regulation of phosphorylated PI3K induced by U exposure. Taken together, these data strongly suggest that U treatment induces NRK-52E cells apoptosis through ROS production, ER stress, and down-regulation of PI3K/AKT/mTOR signaling. Targeting ROS formation-, ER stress-, and PI3K/AKT/mTOR pathway-mediated apoptosis could be a novel approach for attenuating U-triggered nephrotoxicity.
1 INTRODUCTION
Uranium (U) is recognized as a hazardous environmental contaminant. U can accumulate in some tissues or organs via ingestion, inhalation, or skin contact and caused various organs toxicity including the kidney toxicity. U-induced nephrotoxicity in mammalian models has been extensively characterized in vivo.1 U-induced excessive reactive oxygen species (ROS) is thought as a probable mediator for U nephrotoxicity.2 ROS-mediated apoptosis and necrosis are induced in the kidney proximal tubule cells after long periods or high levels of U exposure. The human, rat and pig kidney cells are isolated from the kidney tubular proximal origins and adopted to explore the apoptotic mechanisms reflecting U nephrotoxicity data in vivo.3, 4 In vitro, U-induced apoptosis is determined in some renal tubular cell lines of various species such as the rat proximal tubular cells (NRK-52E line) under 200–700 μM depleted uranyl (VI) nitrate and human kidney epithelioid cells (HK-2 line) under 200 μM depleted uranium (DU) concentration, which are involved in mitochondria-dependent apoptotic pathway.5 The toxicogenomics data show that 100–500 μM depleted uranyl (VI) acetate altered the gene expression which was associated with oxidative stress, apoptosis, and cellular homeostasis in the human embryonic renal cells (HEK293 line). Other molecular mechanisms mediating ROS-triggered pro-apoptotic pathway are not elaborated completely in U-induced nephrotoxicity.
Endoplasmic reticulum (ER) is one of important organelles involved in the protein synthesis and post-translational modification as well as intracellular Ca2+ store. ER homeostasis may be perturbed by various environmental stimuli such as ROS, heavy metals and toxins, and subsequently ER stress appears while the unfolded and/or misfolded proteins are produced and accumulated in amounts in ER lumen.6 These events usually are associated with an adaptive response termed unfolded protein response (UPR). UPR is involved in ER resident trans- membrane proteins including activating PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and transcription factor 6 (ATF6). Under the normal condition, these proteins share no functions by combining with an ER chaperone 78 kDa glucose-regulated protein (GRP78). Once upon the cellular stress, GRP78 breaks away from the ER membrane to combine the unfolded or misfolded proteins and subsequently free PERK, IRE1, and ATF6 can trigger the signaling transduction processes to resort ER function.7 Although UPR normally helps in cell survival by removing unfolded or misfolded proteins, a long time or overmuch ER stress could induce C/EBP homologous protein (CHOP) expression and Caspase-12 activation and ultimately initiate downstream Caspase-3-dependent apoptotic pathway.8-10 ER stress is known to be association with the induction of apoptosis, which occurs during nephrotoxicity or kidney cells cytotoxicity induced by mercury, cadmium, and lead in various animal or cell models.11 Ca2+ metabolism is perturbed by 2 or 4 mg/kg of uranyl (VI) acetate in the mouse kidney tissues.12 Uranyl (VI) acetate (5–50 μM) increases Ca2+ releasing in the human HEK293 cells with high levels of apoptosis. Despite these observations, in detail information is lacking about the controlling of ER stress on U-triggered kidney cells apoptosis.
PI3K/AKT/mTOR13 signaling plays a protective function against apoptosis of various cell types.14 Previous reports indicate that Akt promoted cell survival and prohibited apoptosis in the kidney tissues and cells in response to heavy metals.15 The early activation (before 7th day) of PI3K/AKT signaling alleviates mice proximal tubular cells apoptosis triggered by 2–4 mg/kg of uranyl (VI) nitrate. But, long period and/or excessive exogenous stimuli such as heavy metals, malnutrition, hypoxia and oxidative stress can inhibit the activity of PI3K, which in turn down-regulates the activation of downstream AKT. AKT inactivation further down-regulates phosphorylation of a variety of downstream targeting protein kinases including mTOR, suppresses cell proliferation, induces apoptosis by multiple pathways,13 and finally promotes cell death.16-18 Inactivation of PI3K/AKT/mTOR pathway is reported to mediate apoptosis in various cell types triggered by different toxicants such as triclosan, nickel nanoparticles, and glycation end products.
Here, our study is designed to determine whether ROS formation, ER stress and PI3K/AKT/mTOR signaling medicates U-triggered kidney cells apoptosis in vitro. This will help in elucidating the molecular mechanism during apoptotic signaling process in U-induced nephrotoxicity.
2 MATERIALS AND METHODS
2.1 Main experimental reagents
ROS Detection Kit (No.C10422) and BCA Protein Detection Kit (No.23227) were obtained from Thermo Scientific (USA). Specific monoclonal antibodies against p-PERK, p-IRE1, ATF6, GRP78, cleaved Caspase-3, and β-actin were bought from Hyclone (USA). Specific monoclonal antibodies against t-mTOR, t-AKT, t-PI3K, p-mTOR, p-AKT, p-PI3K, CHOP, and cleaved Caspase-12 were purchased from Santa Cruz Biotechnology (USA). MTT powder, IGF-1, N-acetylcysteine (NAC), and Annexin V-FITC/PI Apoptosis Detection Kit (No.APOAF-20TST) were bought from Sigma (USA). Depleted uranyl (VI) acetate was purchased from Shanghai Jizhi Company (Shanghai, China).19 U solution was prepared based on the recently published method.
2.2 Cell culture
After the cells were inoculated into the petri dish containing DMEM medium, the petri dish was placed in a constant temperature carbon dioxide incubator at 37°C for the NRK-52E cells growing. When the coverage area of kidney cells was approximately 80% of the total bottom area of the dish, we aspirated the culture medium, washed with PBS solution three times, and dropped trypsin digestion solution into the dish for 2 min. Next, the adherent cells were scraped from the bottom of the dish to form the cellular suspension. After centrifugation at 1500 rpm for 3 min, the cellular suspension was inoculated in the new medium and again put in a constant temperature carbon dioxide incubator at 37°C for continued cultivation. According to the purpose of our experiment, normal kidney cells were inoculated in the 6-well or 96-well plates with various experimental reagents for the subsequent experiments.
2.3 Experiment grouping
Previously,20 100–800 μM uranyl acetate was used to study the cytotoxicity of kidney proximal tubule cell line, and the cytotoxicity Index 50 (CI50) of uranyl acetate was identified to be approximately 500 μM for 24 h exposure. Thus, we still adopted 100–800 μM uranyl acetate to intoxicate the kidney cells for 24 h for cell viability test. Subsequently, the optimal concentrations of uranyl acetate (300–600 μM) were chosen to apply for apoptosis-detecting experiments for 24 h. One of the optimal concentrations of uranium (i.e., CI50 value, 500 μM) was adopted for studying apoptotic mechanism for varying durations (6, 12, 18, and 24 h). In some experiments, 500 μM uranyl acetate was used to intoxicate the cells for 18 h following previous exposure to 2 mM NAC for 1 h or 100 ng/ml IGF-1 for 30 min.
2.4 Test of cell viability
After they covered approximately 80% of the bottom area of the culture dish, the cells were inoculated into the 96-well plates and processed according to the above experimental protocols. After the treatments were completed, MTT solutions prepared in advance were added into the 96-well plates 10 μl per well. And then the 96-well plates were put in the incubator for 2 h. After the incubation, the supernatants in the 96-well plates were discarded and DMSO was added into the 96-well plates 100 μl per well. After shaking for 10 min, the plates were finally put into the enzyme-linked immunoassay instrument. The optical densities were detected at the 495 nm wavelength. The cell activities were expressed based on the absorbance value of OD each well.
2.5 Apoptosis determination
According to the supplier kit's instructions, approximately 1 × 106 kidney cells treated above in each tube were washed by PBS twice, centrifuged at 2500 rpm for 10 min, and resuspended with 1000 μl Annexin-binding buffers. Then, the cellular suspension in each tube was added by 10 μl V-FITC or PI, respectively. After the tubes were shaken softly, 400 μl of Annexin binding buffers were dropped into every tube. Finally, all the samples were respectively analyzed at 490 nm excitative and 535 nm emissive wavelengths at the flow cytometer. The total apoptotic proportion contained a percent of cells with fluorescent Annexin V-FITC+/PI− and V-FITC+/PI+.
2.6 ROS detection
After they were scoured gently by PBS solution in triplicate, NRK-52E cells were hatched with the DCFH-DA fluorescent probe solution prepared in advance (1 mL DMEM medium and 1 μl DCFH-DA probe) in each well in a 37°C incubator for 20 min. After the incubation was finished, we threw away the DCFH-DA fluorescent probe solution. And then the left cell suspension was washed using PBS solution three times and digested using 0.25% trypsin for 2 min. After the digestion, the cell suspensions were centrifuged with 1000 rpm for 3 min. Next, we lightly threw away the supernatants and resuspended NRK-52E cells with 50 μl of PBS solution in each well. Finally, the suspension kidney cells were put into a fluorescence spectrophotometer with the 488 nm excitative and 525 nm emissive wavelengths for measurement. ROS levels were expressed as the percentages of control kidney cells.
2.7 Western blots analysis
The processed NRK-52E cells were lysed by RIPA lysate and centrifuged in a high-speed refrigerated centrifuge to obtain the protein samples. The BCA protein determination method was applied for the protein quantitative standard curve and calculated the protein concentrations. SDS-PAGE was performed to separate the protein samples according to the molecular sizes, and then the proteins were shifted onto the PVDF membrane. After 5% skimmed milk was used to seal the cut-off membrane for 2.5 h, the membrane was hatched for 12 h at 4°C with diluted monoclonal antibodies against t-PI3K (1:1200), t-mTOR (1:1200), t-AKT (1:1200), β-actin (1:1000), p-PI3K (1:1200), p-mTOR (1:1200), p-AKT (1:1200), GRP78 (1:1200), p-PERK (1:1200), p-IRE1 (1:1200), ATF6 (1:500), CHOP (1:1200), Caspase-12 (1:1200), and Caspase-3 (1:1500), respectively. Subsequently, the membrane was hatched with HRP-linked second antibody for 2 h and then placed in a dark small environment for development. Finally, the protein band maps obtained in our experiment were analyzed using the Image J software.
2.8 Statistical analysis
The IBM SPSS Statistics23 software was utilized for data analysis. Kolmogorov–Smirnov test was performed to checkout normal distribution and Levene's test was done to examine homogeneity of variance. When both normal distribution and equal variance were fulfilled simultaneously, we performed data analysis for the single factor-treated experiments using one-way analysis of variance (ANOVA). Using least-significant difference (LSD), we compared the differences between two different groups in various factor treated experiments. When equal variance was not assumed despite the data to have fulfilled normal distribution, the data were analyzed by Dunnett's T3 post-hoc tests. All the original experiments data were shown as mean ± standard deviation (X ± SD) from at least three independent experiments. A statistically significant difference was finally adopted under the value of p < .05.
3 RESULTS
3.1 U-induced NRK-52E cells cytotoxicity
To screen the valid U concentration with the cytotoxic effects on NRK-52E cells, cell activities were detected using MTT assay after different U concentrations (100–800 μM) were adopted to treat the cells for 24 h. These cells exhibited a concentration-dependent sensitivity to U treatment compared to control cells (Figure 1). The cytotoxic effect was firstly observed at 300 μM. At the concentration of approximately 500 μM (CI50 value), cell viability was decreased by 50% during a 24 h-culture period. Kidney cells almost lost viability at 700–800 μM. Therefore, 300–600 μM U concentrations were used to elaborate the molecular mechanism involved in U-triggered NRK-52E cells apoptosis in the subsequent experiments.
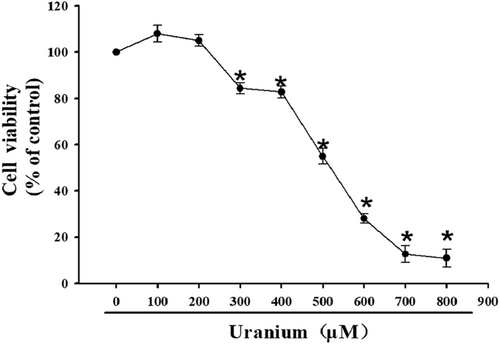
3.2 U triggered NRK-52E cells apoptosis
After Annexin V-FITC/PI staining, U-triggered kidney cells apoptosis was detected and analyzed using flow cytometry. As indicated in Figure 2A–F, apoptosis firstly occurred with 300 μM U for 24 h. U intoxication caused a concentration-dependent increase of total apoptosis ratios compared to control cells. The total apoptosis rate was approximately 13.9% at 500 μM (CI50 value) U for 24 h. Because Caspase-3 is generally considered an apoptosis marker protein, activated Caspase-3 expression was detected and analyzed using Western blots. In response to 300–600 μM U exposure, cleaved Caspase-3 expression significantly augmented compared to control cells (Figure 2G–H). The results imply that U triggered NRK-52E cells apoptosis through Caspase-3-assoicated singling.
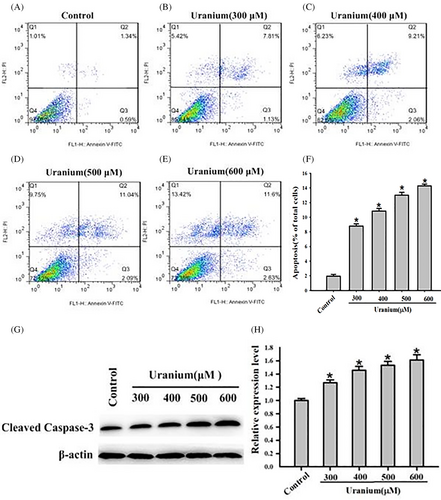
3.3 U triggered ER stress in NRK-52E cells
Considering that apoptosis is close association with ER stress, we adopted the apoptotic optimal U concentration (500 μM) to intoxicate NRK-52E cells for varying durations (6, 12, 18, and 24 h). To demonstrate whether ER stress could be induced under these conditions, we assessed the expression of several crucial ER stress biomarkers. When compared to control cells, the expression of ATF6 p-IRE1, p-PERK and GRP78 significantly increased at 12–24 h with a time-depended pattern (Figure 3A,B), indicating that U intoxication activated three major branches of UPR.
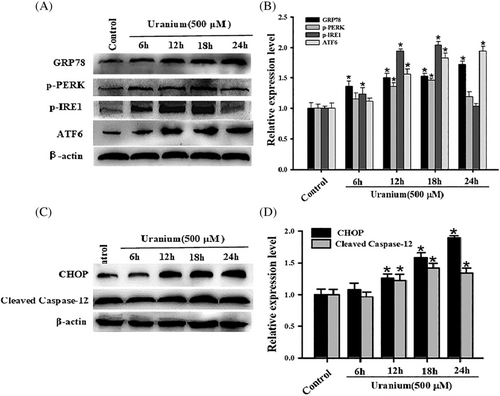
We also detected the expressing levels of activated Caspase-12 and CHOP proteins, which are generally thought as ER stress-mediated apoptosis biomarkers. As shown in Figure 3C,D, their expression significantly increased in a time-dependent pattern from 12 to 24 h in response to U intoxication compared with control cells. The results indicate that cleaved Caspase-12 and CHOP could be involvement of U-induced NRK-52E cells apoptosis.
3.4 U-induced ROS could trigger ER stress-mediated NRK-52E cells apoptosis
To elucidate whether ROS could be involved in U-triggered NRK-52E cells apoptosis mediated by ER stress, we examined the response of kidney cells to U exposure after the following pretreatment with NAC (an ROS scavenger). This was achieved by detecting apoptosis as well as valuating changes in ER stress- and apoptosis-associated biomarkers expression. As expected in Figure 4, U treatment induced ROS production and kidney cells apoptosis and decreased the expression of GRP78, CHOP, activated Caspase-12, and Caspase-3. However, NAC effectively inhibited U-induced ROS generation compared with control cells. Simultaneously, NAC evidently alleviated U-triggered NRK-52E cells apoptosis compared with U-treated cells. Application of NAC concomitantly rescued U-up-regulated expression of above ER stress- and apoptosis-associated marker proteins when compared with U-intoxicated cells. Taken together, it is concluded that ROS decreasing alleviated U-triggered NRK-52E cells apoptosis involved in ER stress.
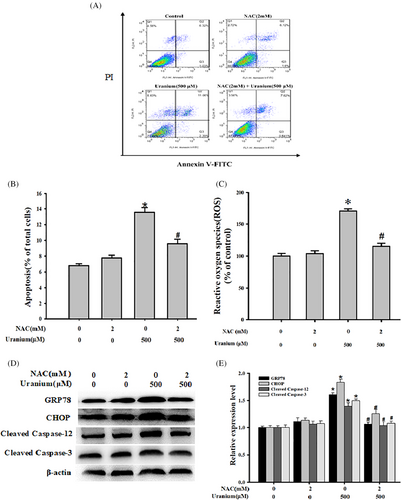
3.5 U down-regulated PI3K/AKT/mTOR pathway
To understand whether U-induced NRK-52E apoptosis could be association with a cell survival signal called PI3K/AKT/mTOR signaling, the marker proteins expression in this signaling was detected for varying durations (6, 12, 18, and 24 h). As indicated in Figure 5, the expression of phosphorylated PI3K, AKT, and mTOR all tended to decrease with the time increase of U exposure compared with control cells. The ratios of their respective phosphorylation to the corresponding total proteins all significantly reduced with time dependently when compared to U-treatment group. In contrast, we observed no difference of the expression contents in their total proteins at any time points. These results imply that PI3K/AKT/mTOR signaling was disrupted on U exposure in NRK-52E cells.
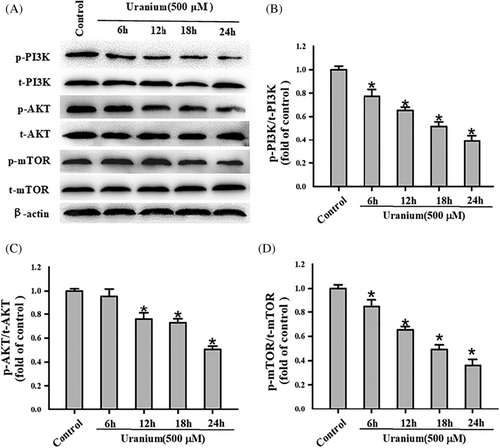
3.6 U-inhibiting PI3K/AKT/mTOR signaling could mediate NRK-52E cells apoptosis
To further determine whether PI3K/AKT/mTOR pathway could mediate U-triggered apoptosis, the marker protein expression in this signaling was determined in the presence or absence of a PI3K activator IGF-1 in U-treated NRK-52E cells. As expected in Figure 6, U exposure evidently down regulated the relative expression of respective phosphorylation proteins and ratios of their respective phosphorylation to the corresponding total proteins compared to control cells. But, administration of IGF-1 effectively reversed U-decreasing the relative expressing contents of phosphorylation PI3K, AKT, and mTOR and ratios of their respective phosphorylation to the corresponding total proteins compared with U-treated cells. Moreover, application of IGF-1 concomitantly abrogated U-triggered kidney cell apoptosis in comparison to U-intoxicated group. Our results indicate that PI3K/AKT/mTOR signaling could be involvement of U-triggered NRK-52E cells apoptosis.
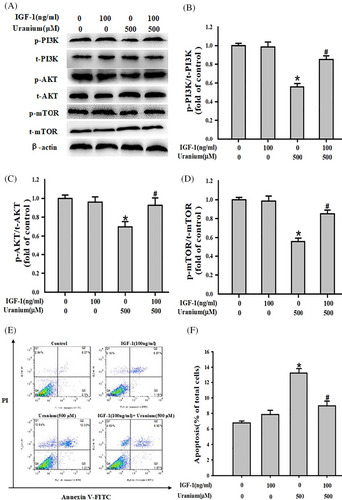
3.7 U-induced ROS decreased phosphorylation PI3K expression
To understand whether ROS could affect the level of phosphorylated PI3K in U-intoxicated NRK-52E cells, we assessed the ratio of phosphorylation PI3K to total PI3K in the presence or absence of an ROS scavenger NAC. As expected in Figure 7, U treatment effectively decreased the content of phosphorylated PI3K and ratio of p-PI3K/t-PI3K compared with control group. But, co-incubation of kidney cells with NAC significantly reversed U-down-regulated level of phosphorylation PI3K and ratio of p-PI3K/t-PI3K in comparison to U-intoxicated cells. The results implicate that U-induced ROS in NRK-52E cells could mediate suppression of PI3K signaling.
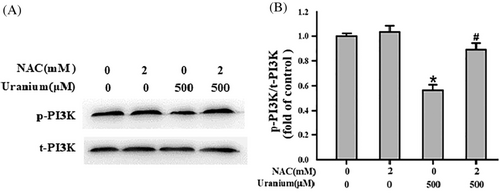
4 DISCUSSION
Previously,21-24 the effect of U at the cellular level was performed in diverse cell lines. The U sensitivity appears to be considerably different in these cell types, with the lowest CI50 (100 μM DU) in the mouse macrophage cells (J774 line) and highest CI50 (650 μM uranyl nitrate) in the porcine renal proximal tubular cells (LLC-PK1 line). One common characteristic is that the cytotoxic effects of U on various cell types are apparently mediated by apoptosis.23, 25, 26 The threshold value (300 μM) of acute cytotoxicity for U concentration is mentioned in various proximal tubular cell lines such as the pig LLC-PK1, human HK-2, and HEK-293 cells.27 These cytotoxic effects could reflect the potential environmental data of U-triggered renal injury. In this study, U can induce apoptotic death of NRK-52E cells in high cytotoxic concentrations (300–600 μM).3 Our results are similar to those from NRK-52E cells exposed by 200–700 μM uranyl (VI) nitrate.
Distinct28 apoptotic signaling is known to include the intrinsic (mitochondrial, Caspase-9), extrinsic (death ligand, Caspase-8) and ER stress-dependent apoptosis pathways, which all are involved in an apoptosis-inducing factor (i.e., Caspase-3) in the apoptotic cells. Actually, Caspase-9- and Caspase-8-dependent apoptotic signaling has been implicated in various cell types including the kidney cells, which are treated by different cytotoxic U concentrations.3, 4, 24 For example, through detecting the Caspase-9 activities and cytochrome C leakage from mitochondria into the cytosol, mitochondria-dependent apoptosis pathway is reported to mediate apoptosis in the rat lung epithelial cells (LE line) treated with 250–1000 μM uranyl (VI) acetate, human HK-2 cells contaminated by 500 μM DU, and rat NRK-52E cells intoxicated by 200–700 μM uranyl (VI) nitrate.3 But, uranyl (VI) nitrate induces NRK-52E cells apoptosis by activating extrinsic (Caspase-8) apoptosis pathway under higher cytotoxic concentrations (700–800 μM).
ER is one of the major intracellular Ca2+ stores, which is closely associated with apoptosis.29 ER stress could be induced when Ca2+ content in ER is depleted.12 Releasing of Ca2+ from ER mediates uranyl (VI) acetate (5–50 μM)-induced human HEK293 cells apoptosis by detecting the expression of type 2 IP3 receptor, proapoptotic factors Bax and Caspase-3. As an ER-specific caspase, Caspase-12 activation could result in Caspase-3 activation, finally leading to apoptosis.7 This pathway is triggered by various stimulus types such as heavy metals, irradiation, and drugs. In our experiments, it was observed that U intoxication triggered ER stress evidenced by several ER stress marker proteins expression. Similarly, U treatment concomitantly increased the expression of an apoptosis marker protein and apoptosis rates. But, the expression of ER stress- and apoptosis-associated markers and apoptosis ratios were attenuated by an ROS scavenger (NAC). Our results suggest that ER stress-related signaling could mediate Caspase-3-dependent apoptosis in U-treated NRK-52E cells. Thus, it seems likely that Caspase-8, Caspase-9, or Caspase-12-dependent apoptosis signaling all exist in the apoptotic process in U-intoxicated NRK-52E cells, indicating that U-triggered activation of caspase is very specific in this cell line.20, 30, 31 These effects could depend on multiple factors such as concentration, exposure duration, isotope composition, intracellular localization and speciation, temperature, pH and culture medium, which are involved in NRK-52E cells cytotoxicity.
One32 of the major mechanisms behind U-induced nephrotoxicity has been attributed to oxidative stress and ROS generation.7 ROS is not only an inducer of apoptosis in heavy metals-triggered stress cells but also a key element of the events which can cause the proteins to misfold in ER and eventually trigger ER stress. In our study, U-triggered ROS production, ER stress and apoptosis rates were evidently attenuated by NAC, indicating that oxidative stress can share a potential function in U-induced ER stress.33 Recent report suggests that an exogenous antioxidant H2S alleviated 500 μM U-triggered NRK-52E cells apoptosis involved in ER stress as an experimental group. It seems that administration of various free radical scavengers or antioxidants may be effective in attenuating U-induced proximal tubular cells damages by modifying ER function, showing that suitable antioxidants could counter action on UPR resulting in preventing ER stress.34, 35 ER stress is known to locate at the downstream for oxidative stress during cigarette smoke- or TNF-a-triggered apoptosis.8, 36 Of note, ROS is required for ER stress and apoptosis in various cell types treated with arsenic, cadmium and mercury. The similar mechanism how ER stress is involved in apoptosis at the downstream for oxidative stress may participate in renal tissue injury induced by heavy metal or other toxics.
PI3K/AKT/mTOR37 signaling axis can be activated by different types of cellular stimuli or toxic insults including ROS, which is closely associated with inhibition of apoptosis. Down- regulation in PI3K/AKT/mTOR signaling is prompted by cadmium, arsenic and mercury,37-39 and up-regulation of this cell survival signaling can inhibit these heavy metals-induced toxicities. In our study, U intoxication reduced the relative levels for phosphorylation PI3K, AKT, and mTOR. However, administration of a PI3K activator IGF-1 effectively reversed these adverse effects and concomitantly reduced U-triggered NRK-52E cells apoptosis, indicating that U suppressed PI3K/AKT/mTOR axis and subsequently up-regulated the apoptosis ratios.15 This result is similar to another study that later inactivation (after 7th day) of PI3K/AKT signaling promotes the mice proximal tubular cell apoptosis induced by uranyl nitrate (2–4 mg/kg). Concurrently, our study further implies that co-incubation of kidney cells with NAC effectively rescued the inhibitory effect of U on phosphoylation of PI3K, showing that ROS participated in U-induced apoptosis mediated by PI3K/AKT/mTOR signaling.3 It is reported that U-induced ROS triggers intrinsic apoptosis pathway in various cell types including NRK-52E cells treated with U (200–700 μM).17, 40, 41 Related studies indicate that inhibition of PI3K/AKT/mTOR signaling activated Caspase-3 -mediated apoptosis. Hence, a possibility could exist that ROS-co-targeting PI3K/Akt/mTOR signaling and mitochondrial apoptosis are induced in U-treated NRK-52E cells.
In summary, U induces NRK-52E cells apoptosis in vitro, which is involved in ROS production, ER stress, and down-regulation of PI3K/AKT/mTOR signaling. Targeting these apoptosis pathways could become a valid strategy for preventing and treating the kidney dysfunction associated with U exposure.
ACKNOWLEDGMENT
Our research is supported by National Natural Science Foundation of China (No. 82160627) and Natural Science Foundation of Guangxi Autonomous Region (No. 2020GXNFSAA297262).
CONFLICT OF INTEREST
All the authors solemnly state this article has no potential controversy of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




