D-galactose-induced toxicity associated senescence mitigated by alpinate oxyphyllae fructus fortified adipose-derived mesenchymal stem cells
Wei-Wen Kuo and Chih-Yang Huang have contributed equally.
Funding information: China Medical University, Taiwan, Grant/Award Number: CMU107-TC-03
Abstract
This study addresses the effect of D-galactose-induced toxicity associated senescence mitigated by alpinate oxyphyllae fructus (AOF; Alpinia oxyphylla Miq) extracts fortified with adipose-derived mesenchymal stem cells (ADMSCs) in rats. Male 18 week-old Wistar Kyoto (WKY) rats were used in this study. We analyzed cardiac fibrosis by Masson's trichrome staining. The tissue sections were dyed using hematoxylin and eosin (H&E). Tissue sections were stained for the restoration of Nrf2 expression in treatment groups by immunohistochemistry. Immunohistochemistry and western blotting analysis showed that AOF with ADMSCs could significantly reduce aging-induced oxidative stress in D-galactose-induced aging rat hearts by inducing Nrf2 pathway. Reduction in ROS resulted in the suppression of inflammatory signals (p-NF-κB and IL-6). Histopathological studies were showed an increased interstitium and collagen accumulation in aging-induced heart sections. However, AOF and ADMSCs treated hearts were recovered from cardiac remodeling. Furthermore, hypertrophy and fibrosis associated markers were also significantly reduced (P < .05) in treatment groups. We speculate that ADMSCs might activate certain paracrine factors, which could target the upstream activator of aging associated cardiac complications and AOF might provide homing for these stem cells.
1 INTRODUCTION
Aging, an inevitable phase of life, is more vulnerable to diseases especially cardiovascular diseases (CVDs).1 Aging heart undergoes various structural remodeling and functional changes. Aging is known to trigger by increased reactive oxygen species (ROS) production, which facilitates remodeling of heart, declined cardiac output and ultimately results in heart failure.2 Oxidative stress and inflammation are the two predominant mechanisms associated with CVDs3 and acting as upstream regulators of myocardial remodeling, which include cardiac hypertrophy and fibrosis in aging heart.4
Accumulation of ROS leads to oxidative stress. In aging heart, ROS are produced predominantly by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mitochondrial electron transport chain.5, 6 Increased oxidative stress is responsible for cascade of reactions including inflammation in aging heart, which is known as “Inflammaging”.7 Inflammation activated cells trigger certain cytokines, which damage DNA and some inflammatory cytokines like IL-4, IL-6, and IL-13 exhibit a concrete association with cardiac remodeling.8, 9 Accumulating evidences suggest that low-grade systemic inflammation and cardiac remodeling are inter-twinned. Cardiac insults trigger inflammatory responses to protect heart; however, prolonged inflammation results in structural remodeling/damage, which progresses to fibrosis in heart.10 Matrix metalloproteinases (MMPs) mediate the degradation of extra cellular matrix. Tissue inhibitors of metalloproteinases (TIMPs) determine the activity of MMPs.11 In aging, the homeostasis of MMPs and TIMPs is altered which result in cardiac fibrosis.12
Chronic inflammation is linked to cardiac fibrosis and hypertrophy and ultimately to heart dysfunction.13 Systemic inflammation facilitates the progression of hypertrophy.14 Hypertrophy is one of the complications that aging heart undergoes.15 Inflammation triggered cells activate certain cytokines like TNF-α which promote cardiac hypertrophy.16
As the elderly population has been growing every year, it is rationale to discover medications to attenuate/reverse cardiac complications-associated with aging.15 Under physiological or pathological conditions, stem cells substitute injured cells in heart.17 Aging heart undergoes oxidative stress, which triggers ROS. Increased ROS might lead to the loss/impairment of stem cells, which eventually accelerate the pathogenesis in aging heart.18 Stem cells play a significant role in organogenesis and maintaining homeostasis all over the life, retain the ability to migrate long distances and target pathological conditions and express therapeutic genes.19 Mesenchymal stem cells (MSCs) modulate inflammatory responses, which could control the activation of immune cells such as macrophages.20 Therefore, the supplementation of stem cells is a promising arena in anti-aging treatment. Hence, stem cell therapy has a promising role in the restoration of aging-associated cardiac damages. Preconditioning of MSCs is one of the approaches to increase the performance of MSCs in stem cell therapy.21 Stem cells treatment to restore/repair heart would require enrichment of stem cells by either chemical compounds or natural extracts.
Alpinate oxyphyllae fructus (AOF; Alpinia oxyphylla Miq) is a well-known Chinese medicine belongs to the Zingiberaceae family, which has been demonstrated for treating ulcerations, gastralgia, diarrhea, dementia, and tumors. It has been used as a traditional Chinese medicine for strengthening the brain, reducing saliva, alleviating stomach pain, and warming the kidney. Our recent studies demonstrated that AOF extracts showed the anti-apoptotic and pro-survival effects in a D-galactose-induced aging heart.22, 23 Furthermore, it has been reported that AOF extracts possess anti-oxidative stress and anti-aging properties.24, 25 However, the beneficial effect of AOF on the ROS-mediated cardiac remodeling remains unclear. In the present study, we have evaluated the protective effects of AOF extracts with preconditioned adipose-derived mesenchymal stem cells (ADMSCs) on aging associated cardiac remodeling in D-galactose-induced toxicity associated senescence rat hearts.
2 MATERIALS AND METHODS
2.1 Preparation of AOF extracts and preconditioning
Fragments of AOF were obtained from Shin-Long Pharmaceutical Company (Taichung, Taiwan, ROC). The AOF fragments (150 g) were extracted with 600 mL of distilled water for 2 h. The extract was passed through Whatman filter paper at decreased pressure. The filtrate was stored until become powder. Approximately 7.4% yield was obtained. The powder was then dissolved in distilled water and stock solution was prepared. The extract was filter sterilized before administered to animals. AOF preconditioning was performed by treating 100 μg/mL of AOF for 8 h on ADMSCs cultured for 16 h.
2.2 Isolation and characterization of ADMSCs
The stem cells were isolated and characterized as mentioned in our previous reports.24 The epididymal fat tissue was isolated and digested at 37°C using type-2 collagenase enzyme (0.2% in PBS). The isolated cells were cultured in Dulbecco's modification of Eagle's medium (DMEM; Invitrogen, Carlsbad, California) containing 10% Fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin and streptomycin (Invitrogen), 100 g/mL streptomycin (Invitrogen), and 2 mM L-glutamine (Invitrogen) at 37°C with 5% CO2.
2.3 Animals and experimental design
The Institutional Animal Care and Use Committee (IACUC) of the China Medical University (No. 101-263-B) has approved the experimental protocols for animal use and handling. All animals maintained in the facilities were treated according to the principles of laboratory animal care. Male Wistar Kyoto (WKY) rats (18 weeks old) were used in this study. All rats were housed in a temperature-controlled room (23 ± 2°C) with a 12:12-h light-dark cycle, and water and rat chow were provided ad libitum. After a 2-weekacclimation period, the rats were segregated into six groups. Group I was normal control group (NC). The other five were induced aging groups injected with D-galactose (150 mg/kg/day for 4 weeks) (IA); in induced aging groups, the Group III was orally administered with AOF extract of 100 mg/kg/day (AO) and the Group IV administered intravenously with ADMSCs of 107 cells (SI), Group V received AOF with ADMSCs (AOS) and Group VI received AOF extracts preconditioned with ADMSCs (ATS). Rats were sacrificed at the end of the treatment, and heart tissue was immediately collected and stored at −80°C until further use.
2.4 Cardiac characteristics
The whole hearts of animals were weighed after being excised and cleaned with PBS. The left ventricle tissues were isolated and weighed. The tibia length was measured by the electronic digital Vernier caliper to adjust the whole heart weight. The ratios of the whole heart weight to the tibia length and the left ventricle weight to tibia length were calculated.
2.5 Masson trichrome staining
Masson's trichrome stain was applied on tissue sections to observe the morphological changes in the heart sections. The sections were stained for collagen fibres (blue staining) using the Masson's trichrome method (Sigma-Aldrich, St. Louis, Missouri), with the images observed under the microscope (OLYMPUS Microscope, Tokyo, Japan).
2.6 Hematoxylin-Eosin staining
The tissue sections were dyed using hematoxylin and eosin (H&E). After the hearts were excised, tissue sections were stained using hematoxylin and eosin (H&E). Sections were dewaxed by immersion in xylene and dyed with hematoxylin for 5 min; the sections were washed three times in double-distilled water (DDW) and soaked in 85% alcohol for 2 min. The sections were dyed with eosin for 5 min and dehydrated through graded alcohols (100%, 95%, and 75%). Finally, the sections were soaked in 100% alcohol for 5 min and two times in xylene for 1 min. The sections were then sorted and photographically analyzed using a microscope (OLYMPUS Microscope, Tokyo, Japan).
2.7 Immunohistochemistry
Tissue sections were stained for visualizing Nrf2expression. Impact DAB Peroxidase kit was purchased from Vector Laboratories, California. Sections were dried at 60°C overnight and then de-paraffinized in xylene (X3), and rehydrated in ethanol gradient (95%, 75%, and 50%). Sections were kept in antigen retrieving buffer for 15 min at 95°C. After a brief PBS wash, it was treated with 3% H2O2 for 5 min. After X3 wash, sections were blocked with 2.5% horse serum for 1 h. After PBS wash, it was incubated with anti-Nrf2 antibody (1:100) overnight at 4°C. Then, the sections were incubated with the appropriate secondary antibodies (Santa Cruz Biotechnology, Dallas, Texas) for 15 min at room temperature and then stained with DAB for 5 min. Further, hematoxylin was added and kept for 5 min. The sections were then viewed by using microscopy (magnification: X 200; OLYMPUS Microscope, Tokyo, Japan).
2.8 Tissue extraction
The left ventricle (LV) tissues were isolated and washed three times in PBS buffer and then weighed. Approximately 100 g tissue was added by 1 mL lysis buffer (0.1% SDS, 0.5% Na-deoxycholate, 1% NP-40, 2m MEDTA, 50mM TrisHCL, 50 mM NaF, and 150 mM NaCl) into the mixture. The tissue was homogenized for 20 min and centrifuged at 1200 rpm at 4°C. After stirring and centrifugation at 12,500 rpm, a clean upper layer suspension was extracted. The homogenization was repeated, and a clean upper layer suspension was extracted.
2.9 Western blotting
The protein concentration of cardiac tissue extracts were determined by the Lowry protein assay. The samples (40 μg/lane) were separated by 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, Maryland). The membranes were blocked in 5% milk in TBS buffer for 2 h with rotation. After washed with TBS buffer three times, membranes were incubated overnight at 4°C with primary antibody. The immunoblots were washed with TBS buffer three times for 10 min each and then incubated with the secondary antibody for 1 h at room temperature. The signals were visualized with an enhanced chemiluminescence (ECL) reagent (Santa Cruz Biotechnology, Santa Cruz, California). Relative density of the blots was quantified using Image J software (NIH, Bethesda, Maryland).
2.10 Statistical analysis
All the experimental data are expressed as mean ± S.D. Comparison between two-groups were performed with Student's t-tests; statistical comparisons between multiple groups were performed by one-way ANOVA. In all cases, a value of P < 0.05 was considered as statistically significant; the ones at P < 0.01 were considered at higher statistical significance.
3 RESULTS
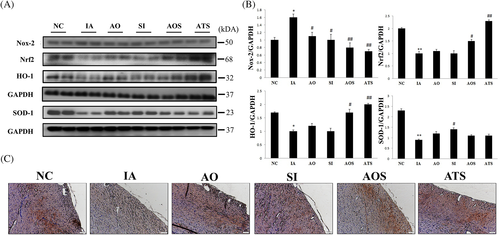
3.1 AOF and ADMSCs synergistically reduce oxidative stress in aging hearts
In aging, ROS is produced increasingly and regulates cardiovascular decline. Therefore, expression modulations of ROS markers in D-galactose and treatment rats were compared. Oxidative stress associated markers (Rac-1, Nox-2) were upregulated, Nrf2 and anti-oxidants (HO-1, SOD-1) were downregulated in aging rat hearts whereas in treatment groups, Nrf2 and anti-oxidants were significantly upregulated (Figure 1A,B). Further, immunohistochemistry confirmed the restoration of Nrf2 in treatment groups (Figure 1C). In both the experiments, AOF group (AO) showed better recovery against oxidative stress than ADMSCs treated group (SI), however, AO and SI synergistically revive heart from aging-induced oxidative stress. These findings suggest that ADMSCs and AOF synergistically reduce oxidative stress in aging hearts.
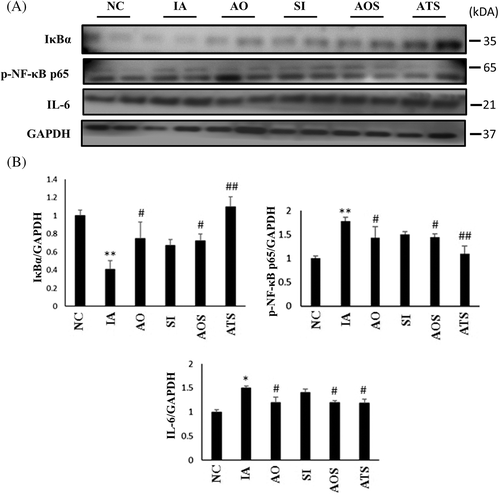
3.2 Reduction of inflammation by AOF and ADMSCs
We evaluated the link between oxidation and inflammation in aging, thus the modulation inflicted on the expression levels of inflammatory markers was analyzed. IκB kinase (IKK) and its target protein, p-NF-κB together mediate inflammatory responses. Hence, we focused on the expressions of IkB and p-NF-κB p65 (Figure 2A). The expression of IkB was reduced in D-galactose group (IA) and restored in treatment groups whereas p-NF-κB p65 was upregulated in IA group and significantly reduced (P < .05) in treatment groups. Moreover, IL-6, an aging associated inflammatory cytokine was also reduced in treatment groups (Figure 2B). These findings suggest that AOF and ADMSCs treated groups potentially reduced the aging associated inflammatory responses.
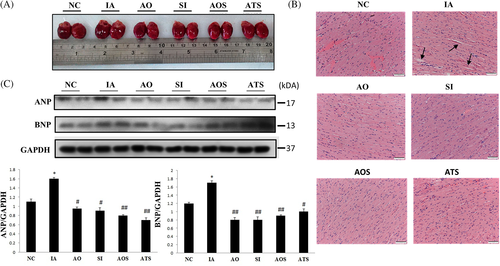
3.3 Aging-induced cardiac remodeling is salvaged by stem cells with AOF extract
Aging heart undergoes cardiac remodeling which affects contractility and arrhythmogenicity of heart. Therefore, we decided to study whether our treatments could restore aging-induced cardiac hypertrophy and cardiac fibrosis.
3.3.1 Body weight and cardiac features
Since aging heart undergoes remodeling, we analyzed the appearance and weight of hearts among experimental groups. Induced aging rat heart (IA) was evidently increased when compared to control and treatment group hearts (Figure 3A). Rat heart weight and cardiac functional parameters were analyzed to compare differences between groups. Left ventricle (LV) weight was also significantly increased in IA group (P < .05). However in treatment groups, LV weight was significantly reduced (P < .05; Table 1). This result suggests that aging-induced increase in size and weight of heart was partially restored by AOF, ADMSCs and in combination treatments.
| NC | IA | AO | SI | AOS | ATS | |
|---|---|---|---|---|---|---|
| BW (g) | 325 ± 10.80 | 335 ± 23.80 | 326 ± 9.46 | 328.75 ± 19.31 | 325.1 ± 17.32 | 327.5 ± 16.45 |
| WHW (g) | 1.13 ± 0.04 | 1.31 ± 0.09* | 1.11 ± 0.08** | 1.18 ± 0.04** | 1.09 ± 0.07** | 1.10 ± 0.04** |
| LVW (g) | 0.84 ± 0.03 | 0.96 ± 0.05* | 0.79 ± 0.06** | 0.82 ± 0.03** | 0.78 ± 0.03*** | 0.82 ± 0.04** |
| WHW/Tibia (102) | 2.66 ± 0.06 | 3.10 ± 0.09* | 2.53 ± 0.06*** | 2.80 ± 0.02** | 2.55 ± 0.04*** | 2.59 ± 0.03*** |
| LVW/Tibia (102) | 1.97 ± 0.06 | 2.28 ± 0.08* | 1.89 ± 0.06** | 1.94 ± 0.09** | 1.85 ± 0.08*** | 1.91 ± 0.05** |
- Note: Values are mean ± SD; n = 4 at least in each group. P < .05 compared to the control group, **P < .05, and ***P < .01 compared to the induced aging group, respectively.
- Abbreviations: AO, oral administration of AOF; AOS, AOF with ADMSCs; ATS, AOF preconditioned with ADMSCs; BW, body weight; IA, induced aging rats; LVW, left ventricular weight, LVW/tibia, left ventricular weight normalized by tibia length; NC, normal control; SI, intravenous administration of ADMSCs; WHW, whole heart weight; WHW/tibia, whole heart weight normalized by tibia length.
3.3.2 Effects on cardiac hypertrophy
In aging heart, occurrence of left ventricular hypertrophy (LVH) is more than in healthy adult. Thus, we investigated cardiac hypertrophy at both cellular and molecular levels in aging-induced and treatment groups. H and E staining showed interstitial space between cells in IA group, which was evidently reduced in AOS and ATS groups (Figure 3B). Hypertrophy markers were also significantly upregulated in IA group (Figure 3C). The result indicates that aging hearts undergo LV hypertrophy, AOF and ADMSCs could partially restore aging-induced cardiac hypertrophy.
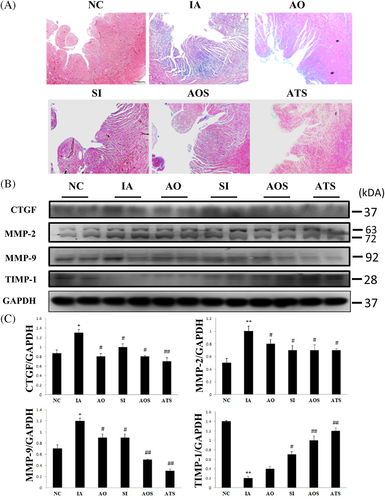
3.3.3 Effects on cardiac fibrosis
In aging heart, fibrosis occurs due to the deposition of extra cellular matrix, expression of fibrotic markers and downregulation of fibrotic inhibitors. We analyzed cardiac fibrosis by Masson's trichrome staining and western blotting. Masson's trichrome staining showed the deposition of collagen in IA group, which was markedly reduced in AOF and ADMSCs treated groups (ATS) (Figure 4A). Similarly, western blotting also confirmed the upregulation of pro-fibrotic markers (CTGF, MMP-2, and MMP-9) and downregulation of inhibitors of MMPs like (TIMP-1, -4) in aging group, which was significantly changed in stem cells and AOF treated group (AOS and ATS) (Figure 4B,C). These results suggest that stem cells synergistically with AOF extract ameliorate aging-induced cardiac fibrosis.
4 DISCUSSION
Cardiac aging is a basic physiological process, which leads to functional decline of heart. Maintaining an aging heart healthy is a challenging task for physicians and researchers. Recent understandings in the molecular mechanisms behind cardiac aging have been resulting in the discovery of new promising drugs/therapies. Stem cell therapy has been considered as a promising therapeutic strategy to treat aging associated cardiac diseases and clinical trials are underway.26, 27 Adipose-stem cells are attractive in stem cell therapy because of their copious presence and availability. The capability of ADMSCs to remarkably decrease the level of cardiac remodeling and simultaneously to enhance cardiac performance has been established.28 Therapeutic strategies based on treatments with growth factors or stem cells are an alternative option to the counter measures used at present. Stem cells therapy to restore/repair heart would require enrichment of stem cells by either chemical compounds or natural extracts. In the present study, a Chinese medicinal plant extract was used to potentiate the activity of ADMSCs to treat aging-induced rat heart.
Oxidative stress is the primary process that activates aging and one of the mediators of cardiovascular diseases. NADPH oxidase (NOX) is the prime cause of ROS production, mainly NOX-1 and -2. The released superoxide would efficiently oxidize endogenous antioxidants and create oxidative stress. On the other hand, Nrf2 regulates cellular resistance to oxidants by activating anti-oxidants such as SOD-1, SOD-2 and other scavengers of ROS like HO-1 29, 30 while Nrf2 assists with maintaining ROS homeostasis under physiological conditions. In this study, we observed that ADMSCs along with AOF extract partially alleviated the effects of oxidative stress by activating Nrf2 pathway.
In aging heart, inflammation occurs because of prevailing oxidative stress. Chronic inflammation leads to pathological conditions. NF-κB, a transcription factor, is responsible for the activation of inflammatory factors including interleukeins (ILs).31 NF-kB is retained inactive in the cytoplasm by inhibitory κB proteins (IκBs), which comprise different subunits p50, p52, RelA, RelB, and c-Rel. Upon stimulation, IkB phosphorylation mediated by IkB kinase (IKK) results in its ubiquitination and nuclear translocation of NF-kB.32, 33 Inhibitory κB proteins (IκBα) controls the activation of NF-κB by binding. During ROS, IκBα is phosphorylated and degraded, thus unbound NF-κB enters into nucleus and activates inflammatory responses. Here, we found that aging-induced p-NF-κB activation was reduced by the recovery of IκBα in ADMSCs and AOF treatments. IL-6 is one of the inflammatory cytokines associated with aging is also reduced as a consequence of p-NF-κB inactivation.
Excessive inflammatory responses might lead to pathological cardiovascular remodeling.34 Extreme interactions among inflammatory cells, fibroblasts and extracellular matrix (ECM) facilitates pathological fibrosis.35 IL-6, an inflammatory cytokine participates in the initiation of fibroblast proliferation. CTGF is over-expressed during myocardial fibrosis also the expression of MMP-2,-9 are up-regulated in G-galactose-induced aging rat models.24 MMPs are tightly regulated by their inhibitors (TIMPs). Here, we showed the upregulation of pro-fibrotic markers like CTGF, MMP-2 and MMP-9 and the downregulation of inhibitor (TIMP-1) in aging group. However, ADMSCs and AOF could suppress the expression of CTGF and MMPs and enhanced the expression of TIMP-1. Earlier, we have demonstrated the anti-fibrotic activities of AOF. MSCs possess anti-fibrotic effects by their capability to degrade excessive ECM.36 Hence, the rationale behind the study using AOF and ADMSCs together is also justified.
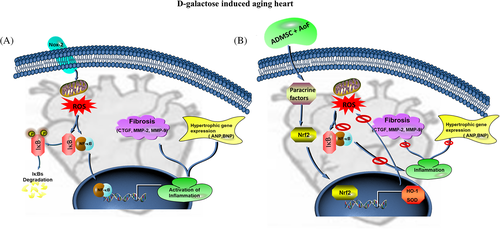
Cardiac aging is a normal physiological process which leads to functional decline of heart. Cardiovascular complications are very common among elderly persons. Stem cells play a major role in the regeneration of damaged tissues under stressful conditions. In conditions like aging, tissue regeneration potential is critically affected due to deterioration in the function of stem cells. Cardiac fibrosis is reportedly accompanied by cardiac hypertrophy in aging heart leading to decline cardiac performance. We observed an increase in the size and LV weight in the aging heart. Furthermore, interstitial space between cells was increased and western blotting also confirmed the upregulation of hypertrophic markers (ANP and BNP) in aging heart. These structural changes and collagen accumulation together decelerate aging heart. Targeting the upstream regulators of cardiac remodeling would be a beneficial therapeutic approach in anti-aging treatment. Therefore, we investigated the synergistic effects of a medicinal plant extract and ADMSCs on oxidative stress, a chief mechanism that regulates and accelerates cardiac aging (Figure 5). Stem cells might activate/secrete paracrine factors, which could trigger the production of beneficial proteins. AOF extract might provide homing to the stem cells so that stem cells could secrete such paracrine factors to protect/recover aging heart in a sustainable manner. To our surprise, both AOF oral with ADMSCs (AOS), and AOF preconditioned ADMSCs (ATS) groups showed almost similar effects on D-galactose-induced toxicity associated senescence. Hence, we speculate that AOF and ADMSCs synergistically enhance the cardio-protective properties of each other.
5 CONCLUSION
The present study was conducted to investigate the synergistic effect of Alpinate oxyphyllae fructus (AoF; Alpinia oxyphylla Miq) extracts with adipose-derived mesenchymal stem cells (ADMSCs) on aging associated cardiac remodeling in rats. The study findings reveal that ADMSCs might activate certain paracrine factors, which could target the upstream activator of aging associated cardiac complications and AOF might provide homing for these stem cells. Hence, synergistic effects of AOF and ADMSCs together might possess therapeutic values against cardiac aging.
ACKNOWLEDGMENTS
This study is supported by a grant from China medical University (CMU107-TC-03), Taiwan.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




