Tricetin suppresses human oral cancer cell migration by reducing matrix metalloproteinase-9 expression through the mitogen-activated protein kinase signaling pathway
Funding information: This study was financially supported by grants from Changhua Show-Chwan Memorial Hospital Research Grant. This study was supported by the grant from Changhua Christian Hospital (102-CCH-IRP-042). This work was also supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST-103-2632-B-040-001, MOST-104-2632-B-040-001, and MOST-105-2632-B-040-001)
Abstract
Tricetin is a flavonoid derivative and a potent anti-inflammatory and anticancer agent. However, the molecular mechanism underlying the effects of tricetin on human oral cancer cell migration remains unclear. The cell migration and invasion abilities of three oral cancer cell lines (SCC-9, HSC-3, and OECM-1) were analyzed using Boyden chamber migration assays. Our results demonstrated that tricetin attenuates 12-O-tetradecanoylphorbol-13-acetate-induced SCC-9, HSC-3, and OECM-1 cell invasiveness and migration by reducing matrix metalloproteinase (MMP)-9 enzyme activity. The reverse transcription polymerase chain reaction and luciferase reporter assay revealed that tricetin downregulates the mRNA expression and promoter activity of MMP-9. In addition, Western blot analysis revealed that tricetin significantly reduced the levels of phosphorylated c-Jun N-terminal kinase (JNK) 1/2 and p38 levels but not those of extracellular signal-regulated kinase 1/2. In conclusion, this study demonstrated that tricetin suppresses MMP-9 enzymatic activity by downregulating the p38/JNK1/2 pathway and might be a beneficial chemopreventive agent.
1 INTRODUCTION
Oral cancer has the highest prevalence and mortality rate of all cancers in Taiwan, and oral squamous cell carcinoma (OSCC) represents more than 90% of all oral cancers.1 After treatments such as surgery, chemotherapy, and radiotherapy, the 5-year survival rate in patients with OSCC is approximately 50%.2 The significant poor prognostic factors for OSCC include invasive advanced stage and metastatic tumors, such as regional cervical lymph node metastasis and distal metastasis.3-5 Increasing evidence indicates that standard treatments combined with chemoprevention may be highly effective for treating oral cancer.6
The use of plant products against malignant invasive progression of various diseases has recently attracted attention.7, 8 Flavonoids are cytoprotective compounds widely distributed in vegetables and dietary plants. To date, most flavonoids are safe for human use. Studies have reported that some flavonoids exert antimetastatic effects on oral cancer cells and play a crucial role in oral cancer prevention and therapy.9, 10 Tricetin (5,7,3′,4′,5′-pentahydroxyflavone; Figure 1A) is a flavonoid derivative found in Myrtaceae pollen and Eucalyptus honey.11, 12 Studies have reported that tricetin has potential anti-inflammatory and anticancer abilities.13-16 However, the molecular mechanisms underlying tricetin-induced antimalignant effects vary with cell types. A study reported that tricetin arrests breast cancer cells at the G2/M phase by activating the p53-dependent pathway.14 Furthermore, tricetin induced apoptosis by triggering the mitochondrial and death receptor 5 apoptotic pathway in human liver cancer cells.15 Moreover, our previous study revealed that tricetin suppresses the matrix metalloproteinase (MMP)-2-mediated metastasis of glioblastoma multiforme (GBM) cells by inhibiting the transcriptional activity of specificity protein 1 (Sp-1).16
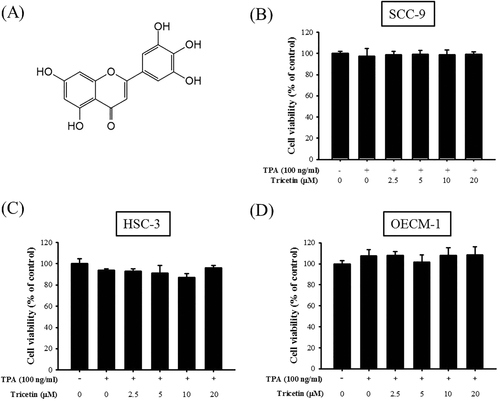
Effect of tricetin on cell viability. A, Structure of tricetin. B, SCC-9; C, HSC-3; and D, OECM-1 cells were treated with various concentrations (0, 2.5, 5, 10, and 20 μM) of tricetin in the presence or absence of TPA (100 ng/mL) for 24 h before being subjected to an MTT assay for cell viability. The values represented the means ± SD of at least three independent experiments
MMPs are a family of zinc-containing endopeptidases that can induce or enhance tumor progression, including cell growth, angiogenesis, invasion, and metastasis.17-20 Numerous studies have demonstrated that patients with OSCC having positive MMP-9 expression have significantly poorer overall survival than those with negative MMP-9 expression, and MMP-9 expression is positively correlated with lymph node metastasis and advanced tumor stage groups.21-25 MMP-9 expression can be regulated through signaling pathways such as mitogen-activated protein kinase (MAPK) signaling pathways, growth factors, cytokines, or chemicals.26-29 However, the effects of tricetin on oral cancer cell lines are yet to be investigated. Therefore, the present study elucidated the underlying molecular mechanism of tricetin-mediated suppression of cell migration and invasion of 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated human oral cancer cell lines.
2 MATERIALS AND METHODS
2.1 Cell culture
SCC-9 (Human squamous carcinoma of the tongue) and HSC-3 human oral cancer cell lines were purchased from the American Type Culture Collection (Manassas, VA) and Japanese Collection of Research Bioresources (Osaka, Japan), respectively. Both cell lines were cultured in Dulbecco's modified Eagle's medium, accompanied by a nutrient mixture comprising F-12 Ham's medium, as previously described.30 Oral epidermal carcinoma cell line OECM-1 cells were originally established by Dr. Meng's group31 and maintained in RPMI supplemented with 10% FBS. All cell cultures were maintained in 5% CO2 at 37°C.
2.2 Cell viability assay
SCC-9 cells (5 × 104 cells/well) were seeded in 24-well plates and treated with tricetin (0, 2.5, 5, 10, and 20 μM) and TPA (100 ng/mL) at 37°C for 24 h. The media were removed and cells were washed with phosphate buffered saline (PBS), followed by incubation with 20 μL of 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/mL; Sigma chemical Co., St. Louis, MO) for 4 h. The viable cell number per dish is directly proportional to the amount of formazan formed by dehydrogenases in the mitochondria within live cells. The formazan was dissolved in isopropanol and analyzed spectrophotometrically at 563 nm.
2.3 Cell migration and cell invasion assay
SCC-9 cell migration and invasion assays were performed according to the methods described by Yang et al.32 HSC-3 and OECM-1 cells were harvested and seeded to a Transwell (Costar, Corning, NY) at 4 × 104 cells per well as previously described.28 The number of migrated cells was counted under a light microscope at a 100× magnification.
2.4 Gelatin zymography assay
The enzyme activity of MMP-9 was analyzed through gelatin zymography as previously described.33 Briefly, after treatment with tricetin (0, 2.5, 5, 10, and 20 μM) and TPA (100 ng/mL) at 37°C for 24 h, the conditioned media were collected, mixed with a loading buffer, and electrophoresed on a 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel containing 0.1% gelatin as previously described.33
2.5 Western blot analysis
The cell lysates were separated in a 10% polyacrylamide gel and transferred onto a nitrocellulose membrane as described previously.33 The blot was subsequently incubated with 5% nonfat milk in tris-buffered saline (20 mM Tris, 137 mM NaCl, pH 7.6) for 1 h to block nonspecific binding and then overnight with polyclonal antibodies against three MAPKs (ERK 1/2, JNK 1/2, and p38) (Cell Signaling Technology, Inc. Danvers, MA) as described previously.33
2.6 RNA isolation, cDNA synthesis, and real-time polymerase chain reaction
The total RNA was extracted from SCC-9 cells (1 × 106) using Trizol reagent (Invitrogen, Carlsbad, CA). According to the manufacturer's instructions, 2 μg of RNA was reverse-transcribed into cDNA using the SuperScript III First-Strand Synthesis Supermix kit (Invitrogen, Carlsbad, CA). MMP-9 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers and corresponding probes were designed using commercial software (ABI PRISM Sequence Detection System; Applied Biosystems Division, Foster City, CA) as described previously.33
2.7 Promoter activity assay
The promoter sequence of the human MMP-9 gene was cloned into the pGL3 plasmid (pGL3-MMP-9; Promega, Madison, WI), yielding the recombinant vector pGL3-MMP-9 containing the SV40 promoter-controlled firefly luciferase open reading frame. In addition, the β-galactosidase plasmid was used as an internal control vector. Cells were cotransfected with the β-galactosidase plasmid and pGL3-MMP-9 constructs and lysed after 24 h. Subsequently, luciferase and β-galactosidase activities were assayed according to manufacturer's protocol (Promega, Madison, WI).
2.8 Statistical analysis
All values are expressed as means ± SDs. Statistical analysis was performed using SPSS (version 16, Chicago, IL). The experimental and control groups were compared using the Student's t-test, and P < .05 was considered statistically significant.
3 RESULTS
3.1 Tricetin does not exert cytotoxic effects on the viability of oral cancer cell lines
The cytotoxic effects of various concentrations of tricetin (2.5, 5, 10, and 20 μM) on SCC-9, HSC-3, and OECM-1 cell lines were assessed using the MTT assay. Tricetin did not exert significant cytotoxic effects on the viability of SCC-9, HSC-3, and OECM-1 cell lines (Figure 1B–D). In addition, TPA alone did not exert significant cytotoxic effects on cell viability compared with parental cells. These results indicated that TPA or tricetin did not alter SCC-9, HSC-3, and OECM-1 cell viability. Therefore, different tricetin concentrations were used in the subsequent experiments.
3.2 Tricetin inhibits oral cancer cell migration and invasion
The Boyden chamber assays were performed to evaluate the effects of tricetin on SCC-9 cell migration and invasion. Tricetin suppressed TPA-induced SSC-9 cell migration and invasion in a dose-dependent manner (Figure 2A,B) and significantly reduced (P < .05) HSC-3 and OECM-1 cell migration and invasion at a concentration of >20 μM (Figure 2C–F).
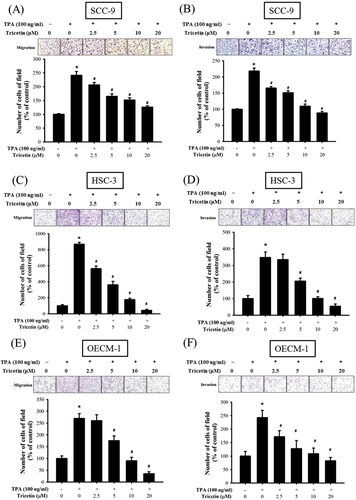
Effect of tricetin on migration and invasion of SCC-9, HSC-3, and OECM-1 cells. C and D, After being treated with tricetin at a concentration of 0, 2.5, 5, 10, and 20 μM in the presence or absence of TPA (100 ng/mL) for 24 h, The cell migration and invasion were measured using a Boyden chamber for 24 h with polycarbonate filters. The number of cells which invaded the underside of the porous polycarbonate was counted for assessing the migration and invasion abilities of SCC-9 (A and B), HSC-3 (C and D), and OECM-1 (E and F) cells. The values represented the means ± SD of at least three independent experiments. *P < .05, compared with the vehicle group. #P < .05 compared with the TPA treatment group. [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Tricetin inhibits MMP-9 activity by reducing MMP-9 mRNA expression
According to the aforementioned data, tricetin suppressed SCC-9, HSC-3, and OECM-1 cell migration and invasion. To investigate the effects of tricetin on TPA-induced MMP-9 activity, the gelatin zymography assay was used to assess MMP-9 enzyme activity. The MMP-9 enzyme activity increased substantially after TPA treatment (Figure 3A–C). Moreover, high tricetin concentrations suppressed the MMP-9 enzyme activity. Subsequently, the mRNA expression of MMP-9 was investigated through reverse transcription (RT)-PCR. The RT-PCR results revealed a 4.5-fold increase in the MMP-9 mRNA expression in TPA-treated SCC-9 cells compared with parental SCC-9 cells (Figure 3D). The MMP-9 mRNA expression decreased gradually (4.5-fold to 1.3-fold) with increasing tricetin concentrations. Meanwhile, a luciferase reporter assay was performed to validate the aforementioned finding. The MMP-9 promoter construct (pGL3–MMP-9) was transfected into TPA-treated SSC-9 cells, and the results confirmed that tricetin gradually suppresses MMP-9 promoter activity in a dose-dependent manner (Figure 3E). According to the aforementioned data, the MMP-9 mRNA expression depends on its promoter activity, and tricetin inhibits MMP-9 promoter activity, thus resulting in downregulated MMP-9 mRNA expression.
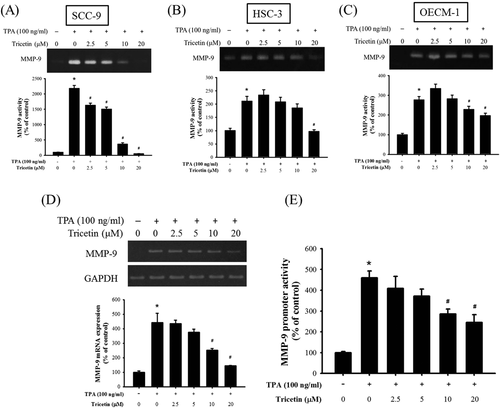
Effect of tricetin on the enzymatic activity, mRNA level and promoter activity of MMP-9. For A, SCC-9; B, HSC-3; and C, OECM-1 cells, the gelatin zymography assay was applied for measurement of MMP-9 activity on Tricetin (0, 2.5, 5, 10, and 20 μM) in the presence or absence of TPA (100 ng/mL) for 24 h. D, Semiquantitative RT-PCR assay were performed to compare MMP-9 mRNA levels in the SCC-9 cell. The values represented the means ± SD of at least three independent experiments. E, SCC-9 cells were transfected with pGL3 basic or a MMP-9 promoter/reporter plasmid, and then treated with various concentrations (0, 2.5, 5, 10, and 20 μM) of tricetin in the presence or absence of TPA (100 ng/mL). After 24 h incubation, luciferase activities were determined and normalized to β-galactosidase activity. The values represented the means ± SD of at least three independent experiments. *P < .05, compared with the vehicle group. #P < .05 compared with the TPA treatment group
3.4 Tricetin regulates the MAPK signaling pathway
We investigated the molecular mechanism underlying the inhibitory effects of tricetin and assessed the expression of MAPK pathway-related proteins, such as p-ERK, p-JNK 1/2, and p-p38, through Western blot analysis. TPA significantly induced the phosphorylation of ERK, JNK 1/2, and p38 in SCC-9 cells (Figure 4A–C). Notably, after treatment with tricetin (20 μM), the expression levels of p-JNK 1/2 and p-p38 decreased, whereas those of p-ERK remained unchanged in SCC-9 cells. These results suggested that tricetin inhibited SCC-9 cell migration by suppressing the p38/JNK-MMP-9 axis signaling pathway.
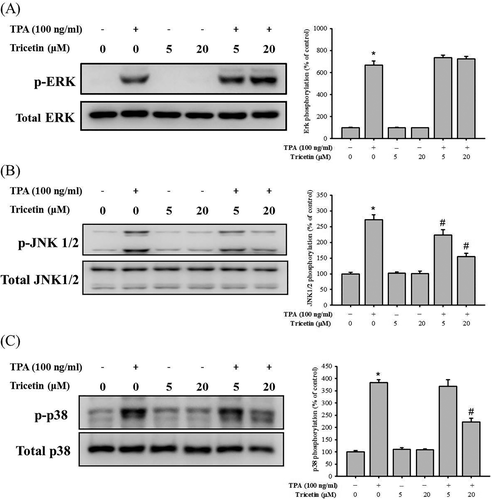
Effect of tricetin on the MAPKs pathway. After a 24 h culture in various concentrations of Tricetin (0, 5, and 20 μM) in the presence or absence of TPA (100 ng/mL), the lysates of SCC-9 cells were subjected to SDS-PAGE followed by Western blots with anti-ERK (A), anti-JNK (B), and anti-p38 (C) (total and phosphorylated) antibodies. The values represented the means ± SD of at least three independent experiments. *P < .05, compared with the vehicle group. #P < .05 compared with the TPA treatment group
4 DISCUSSION
Oral cancer is one of the leading cancer types worldwide. Furthermore, because of a low 5-year survival rate, highly effective therapies are required for oral cancer. Flavonoids have antioxidant, anti-inflammation, and anticancer properties,34 and tricetin is a flavonoid derivative. Recent studies have indicated that tricetin inhibits severely invasive tumor metastasis, including liver cancer15 and GBM.16 In this study, the treatment value of tricetin was evaluated, and the results indicated that tricetin (1) did not exert cytotoxic effects on oral cancer cell viability; (2) inhibited SCC-9, HSC-3, and OECM-1 cell migration and invasion; (3) suppressed MMP-9 mRNA expression; and (4) regulated the p38/JNK signaling pathway by reducing p-JNK 1/2 and p-p38 phosphorylation.
The major cause of death in patients with OSCC is cancer metastasis.20 The critical step of metastasis is the degradation of the basement membrane through the activation of several important proteolytic enzymes, causing extracellular matrix (ECM) remodeling.20, 35 MMP-2 and MMP-9 are the two major proteolytic enzymes involved in ECM degradation and have been reported to be overexpressed in various human cancers, including lung cancer, breast cancer, and oral cancer.20, 21, 36 Therefore, MMP-2 and MMP-9 overexpression can be reliable tumor progression markers. According to our data, tricetin can attenuate TPA-induced MMP-9 enzyme activity and mRNA expression in oral cancer cells (Figure 3). Chao et al. proposed that tricetin inhibits GBM cell migration and invasion by reducing MMP-2 expression.16 Regarding other flavonoid derivatives, Lin et al. and Gao et al. have demonstrated that resveratrol reduced TPA-induced MMP-9 expression in SCC-9 cells and cerebral ischemia reperfusion in mice, respectively.29, 37 Moreover, our previous reports shown that Rubus idaeus extract and Leucaena leucocephala extract exerted an inhibitory effect of migration and invasion in oral cancer cells.38, 39 Therefore, flavonoid derivatives, such as tricetin, can inhibit cancer cell malignancy; however, the molecular mechanisms underlying such inhibitory effects vary with cell types.
Numerous studies have reported that MMP expression is regulated by the MAPK signaling pathway.40 ERK, JNK1/2, and p38 are considered to be the major components of the MAPK signaling pathway. However, the relationship between the MAPK signaling pathway and tricetin treatment is yet to be investigated. Chao et al. provided evidence that tricetin inhibits tumor progression by interfering with Sp-1–MMP-2 promoter binding and altering Sp-1 expression in GBM cells. Furthermore, a combined treatment of tricetin and ERK inhibitors yields greater antitumor effects than tricetin alone.16 Hsieh et al.41 reports that dehydroandrographolide reduces MMP-2 expression by downregulating MAPK signaling pathways in oral cancer cells. This is consistent with our result that tricetin suppressed the e phosphorylation of JNK 1/2 and p38 (Figure 4).
This study is the first to report that tricetin effectively inhibits TPA-induced oral cancer cell migration by reducing MMP-9 enzymatic activity and mRNA expression. In addition, we elucidated the possible signaling pathways regulated by tricetin. These results suggest that tricetin can be considered a safe chemopreventive and chemotherapeutic agent for human oral cancer treatment.
ACKNOWLEDGMENTS
This study was financially supported by grants from Changhua Show-Chwan Memorial Hospital Research Grant. This study was supported by the grant from Changhua Christian Hospital (102-CCH-IRP-042). This work was also supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST-103–2632-B-040–001, MOST-104–2632-B-040–001 and MOST-105–2632-B-040–001).




