Inhibitory effects of Leucaena leucocephala on the metastasis and invasion of human oral cancer cells
Funding information: Ministry of Science and Technology, Taiwan, Grant Numbers: MOST-103-2632-B-040-001, MOST-104-2632-B-040-001, and MOST-105-2632-B-040-001; Changhua Christian Hospital, Taiwan, Grant numbers: 103-CCH-IRP-042 and 104-CCH-IRP-061
Abstract
Oral cancer is one of the most common cancers worldwide, and metastasis is recognized as a major factor causing its low survival rate. The inhibition of metastasis progress and the improvement of the survival rate for oral cancer are critical research objectives. Leucaena leucocephala from the mimosa branch Leucaena genus is native to Central and South America and has been used as a traditional remedy for treating various disorders. Previous studies have demonstrated antioxidant, anti-inflammatory as well as anticancer properties of L. leucocephala plant materials. However, the molecular mechanism underlying the anticancer effect induced by L. leucocephala remains unclear. In this study, we investigated the effect of L. leucocephala extract (LLE) on SCC-9 and SAS oral cancer cells and examined the potential inhibitory mechanisms involved. The results indicated that LLE attenuated the migration and invasion abilities of both SCC-9 and SAS cells by reducing the activity and protein expression of matrix metalloproteinases-2 (MMP-2). Regarding mitogen-activated protein kinase (MAPK) pathways, the phosphorylation of ERK1/2 and p38 exhibited a significant inhibitory effect in the presence of LLE. The application of ERK inhibitor and p38 inhibitor confirmed that both signalling transduction pathways were involved in the inhibition of cell metastasis. These data indicate that L. leucocephala could be a potent therapeutic agent for the prevention and treatment of oral cancer and a prominent plant source for anticancer research in the future.
1 Introduction
Oral cancer is a subclass of head and neck cancer with substantial morbidity and mortality, and the worldwide incidence is estimated to be ∼275,000 cases annually.1 Epidemiological studies have indicated that smoking, betel nut chewing, and alcohol consumption are predominant risk factors triggering oral cancer.2, 3 Oral cancer possesses high local invasiveness and great metastatic ability in cervical lymph nodes with poor patient prognoses. The metastasis of cancer cells is the major factor leading to cancer-related deaths and typically involves multiple processes. One of the critical cytophysiological changes involved in these processes is the alteration of the adhesive capabilities of cancer cells through proteolytic activity to degrade the extracellular matrix (ECM).4, 5 The hydrolysis of ECM is catalyzed by various proteases secreted from cancer cells and results in promoting the mobility and metastasis of cancer cells.6 A major class of proteases that belong to a family of zinc-dependent endopeptidases is matrix metalloproteinases (MMPs), which are known to be involved in the degradation of ECM.4, 7 Based on their structures and substrates, the MMP family can be further divided into several subgroups including collagenases, stromelysins, gelatinases, and membrane-type MMPs.8 Initially, MMPs are synthesized in a latent inactive form and their enzymatic activities are regulated by cleaving the N-terminal domain.9, 10 The active form of MMPs is specifically regulated by the endogenous inhibitors known as tissue inhibitors of metalloproteinases (TIMPs).11 The expression imbalance of MMPs and TIMPs affects the remodeling of the ECM composition, which has been observed in various cancer diseases.12-15
Mitogen-activated protein kinases (MAPKs), an enzyme family belonging to serine-threonine protein kinases, are widely known to be involved in signal transduction pathways that regulate diverse cellular processes, such as embryogenesis, growth, proliferation, differentiation, migration, and apoptosis.16 Numerous MAPK genes have been characterized in mammals. Extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinase (JNK), and p38 are three well-known families that have been intensively examined for their roles in various human cancers. ERK signaling plays an essential role in growth by stimulating cell-cycle processes from G0/G1 to S phase.17 The deregulation of ERK signaling was observed in one-third of all human cancers16 and generally, upregulation of ERK pathway results in cancer cell proliferation. The JNK and p38 pathways belong to the stress-activated MAPK signaling pathways and are known to promote apoptosis.16 Moreover, numerous studies have well established the role of the MAPK pathway in the regulation of cancer metastasis in oral cancer.7, 18-20 Therefore, methods to regulate these pathways could be potential cancer therapy strategies.
Currently, increasing numbers of scientists attempting to extract the bioactive compounds in planta, which typically belong to plant secondary metabolites and exhibit varied pharmacological effects that can prevent or intervene in disorders.21 For example, Paclitaxel (also known as taxol) from pacific yew (Taxus brevifolia) has been applied as a standard remedy for treating various cancers including ovarian, breast, and lung cancers worldwide.22 To date, numerous novel bioactive compounds have been isolated from plant species and have been demonstrated to have potential against various human diseases. In this study, a traditional medicinal plant, Leucaena leucocephala, was investigated for its inhibitory activity on oral squamous cell carcinoma (OSCC). L. leucocephala, commonly known as lead tree, belongs to the Leguminosae family. This tree legume is native to southern Mexico and northern Central America and is now widely grown in tropical and subtropical regions including Australia, Southern India, Africa, South America, the Philippines, and Taiwan.23 Because of the high protein content in its foliage, it has been applied as livestock feed and also consumed as a vegetable in Central America, Indonesia, and Thailand.23 Various parts of the lead tree are utilized as a traditional remedy for treating disorders such as internal pain, respiration disorders,24 parasitic diseases,25 and diabetes.26 Mimosine, a nonprotein amino acid found in all parts of L. leucocephala, was demonstrated to be a potential bioactive compound involved in the antioxidant activity of the plant extract.27 Moreover, mimosine has been reported to promote the expression of a cyclin-dependent kinase inhibitor (p21) at both mRNA and protein levels, which inhibits the growth of breast cancer cells (21PT) in the late G1 phase of the cell cycle.28 Regarding the effect of L. leucocephala on human cancer, Gamal-Eldeen et al. reported that a sulfated glycosylated extract from its seeds exerted a critical antiproliferation effect on hepatocarcinoma HepG2 cell line and exhibited anticancer properties.29 However, to date, few oncology studies have investigated the anticancer properties of L. leucocephala. In the present study, we characterized the anticancer properties of L. leucocephala extract on oral cancer cell lines (SCC-9 and SAS) and revealed the inhibitory mechanism involved. The results demonstrated that L. leucocephala extract can repress cell migration and invasion ability by downregulating the MMP-2 expression. L. leucocephala extract has the potential to prevent oral cancer metastasis and to be used for secondary oral cancer treatment in the future.
2 Materials and methods
2.1 Preparation of Leucaena leucocephala extracts (LLE)
Leucaena leucocephala was purchased from herb stores in Taichung, Taiwan and the Leucaena leucocephala extracts (LLE) were prepared as described previously.30 Briefly, after rinsing and air drying, 100 g of chopped plant materials were extracted twice by 500 mL of 50% ethanol in flask which was heated in 70°C water bath for 24 h. The pooled extracts were filtered and condensed by a rotary evaporator under low pressure. After organic solvent was removed from extract, the concentrated extract was frozen at −80°C for 2–3 days and freeze-dried using a lyophilizer. The dried Leucaena leucocephala extracts was applied for following experiments.
2.2 Cell lines, culture condition, and treatments
Two human tongue squamous cell carcinoma cell lines, SCC-9 was obtained from ATCC (Manassas, VA). SAS cells were purchased from and validated by the Japanese Collection of Research Bioresources Cell Bank (JCRB, Shinjuku, Japan). And these cells were all maintained in Dulbecco's Modified Eagle Medium (DEME; Life Technologies, Grand Island, NY) supplemented with Ham's F12 Nutrient Mixture (Life Technologies, Grand Island, NY), 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), 100 μg mL−1 streptomycin, 100 U mL−1 penicillin, 0.1 mg L−1 hydrocortisone, and 1.2 g L−1 sodium bicarbonate at 37°C in a humidified 5% CO2 incubator. The dried LLE powder was dissolved in 50% dimethyl sulfoxide (DMSO) and prepared a serial stock including 0, 10, 20, 40, and 80 mg mL−1 for treatments. The ERK inhibitor (U0126; 10 μM) and p38 inhibitors (SB203580; 20 μM) were also utilized to confirm if LLE inhibition was via the MAPK pathway. Cells were pretreated with U0126 or SB203580 combined with or without LLE (20 μg mL−1) for 1 h and then incubated in different LLE concentrations for 23 h.
2.3 Cell viability assay (MTT assay)
For determination of cell viability, a colorimetric assay using tetrazolium dye, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), was performed for determining the cytotoxicity of LLE.19 SCC-9 and SAS cells (6 × 104 cells/well) were seeded in 24-well plates and treated with the indicated concentrations of LLE for 24 h under the same culture condition. The medium was removed after LLE treatment. Attached cells were washed by phosphate buffered saline (PBS) and incubated with 20 μL of 5 mg mL−1 MTT (Sigma chemical, St. Louis, MO) at 37°C for 4 h. The viable cell per well was assessed by production of formazan which was measured spectrophotometrically at 563 nm following solubilization with isopropanol.
2.4 In vitro wound closure
SCC-9 and SAS cells (6 × 104 cells/well) were plated in 6-cm petri dishes for 16 h and wounds were produced by manually scratching with a 200 μL pipette tip as described previously.20 The injured cells were then incubated in DMEM medium containing 0.5% FBS and treated with LLE (0, 10, 20, 40, and 80 μg mL−1) or combination with two inhibitors, U0126 (10 μM) and SB203580 (20 μM). Images were recorded at indicated times after treatment using a phase-contrast microscope (×100).
2.4 Cell migration and invasion assays
Methods of cell migration and invasion assays were described as Ho et al.31 After treatments with or without LLE (0, 10, 20, 40, and 80 μg mL−1) or combination with two inhibitors for 24 h, surviving cells were collected and loaded into upper Boyden chamber (Neuro Probe, Cabin John, MD) at 104 cells/well in serum-free medium. The lower chamber contained standard medium with 10% FBS and 8 μm pore-size polycarbonate membrane filters was applied for migration assay. After 24-h incubation at 37°C, the cells were fixed by 100% methanol and stained with 5% Giemsa for 3 h. The non-invasive cells were removed by wiping with a cotton swab and the invaded cells were counted under a light microscope at 200× magnification. The invasion assay was carried out as described above with a 12 μm pore-size polycarbonate membrane coating by 10 μL Matrigel (25 mg/50 mL; BD Biosciences, MA).
2.5 Assessment of MMP-2 by gelatin zymography
The activity of MMP-2 in the medium was determined by gelatin zymograph protease assays.18 The cultured medium was collected after LLE treatment (0, 10, 20, 40, and 80 μg mL−1) or combination with two inhibitors for 24 h and subjected to 0.1% gelatin-8% SDS-PAGE electrophoresis. The gels were then washed by 2.5% Triton X-100 for 30 min twice and then incubated in reaction buffer consisting of 40 mM Tris-HCl, 10 mM CaCl2, and 0.01% NaN3 at pH 8.0 for 12 h at 37°C. After incubation, gels were stained with Coomassie Brilliant Blue R-250 for 30 min and destained in solution containing 5% methanol and 7.5% acetic acid.
2.6 Western blot analysis
For preparation of total cell lysates, the treated cells were rinsed with 1× PBS twice and suspended in 200 μL RIPA buffer comprising protease inhibitors cocktail as previously described.32 Cells were then vortexed at 4°C for 30 min and centrifuged at 10,000 rpm for 10 min. After removing the insoluble pellets, the protein concentration of total cell lysates was determined by Bradford assay. Equal amounts of protein (20 μg) from each cell lysates were boiled at 99°C for 10 min and chilled on ice. Subsequently, samples were separated by SDS-PAGE on 10% polyacrylamide gels and electrotransferred onto a nitrocellulose membrane. The membranes were incubated with 5% non-fat milk (w/v) in Tris-buffered saline (20 mM Tris, 137 mM NaCl, pH 7.6) for 1 h to block nonspecific binding and immunoblotted overnight with polyclonal antibodies against MMP-2, TIMP-2 or three MAPKs (ERK1/2, JNK1/2, and p38) with the specific antibodies for unphosphorylated or phosphorylated forms of the corresponding ERK1/2, JNK1/2, and p38. The blots were then incubated with a horseradish peroxidase goat anti-rabbit or anti-mouse IgG for 1 h. After extensive washing, the band signal was detected by using enhanced chemiluminescence (ECL) commercial kit (Amersham Biosciences, Piscataway, NJ) and relative photographic density was quantitated by scanning the photographic negatives on a gel documentation and analysis system.
2.7 Statistical analysis
The statistical analyses were applied SigmaStat 2.0 (Jandel Scientific, San Rafael, CA). Difference between control and treated groups were calculated by Student's t test and P < 0.05 was considered statistically significant. All experiments were performed three times.
3 Results
3.1 Effects of LLE on cell viability of SCC-9 and SAS cells
The cytotoxic effects of LLE at various concentrations (0–80 μg mL−1) on SCC-9 and SAS cells were examined using an MTT assay. After treatments for 24 h, no significant effects of LLE at all treated concentrations were observed on cell viability of both cell lines (Figure 1A,B). Therefore, this LLE concentration range was used for subsequent experiments.
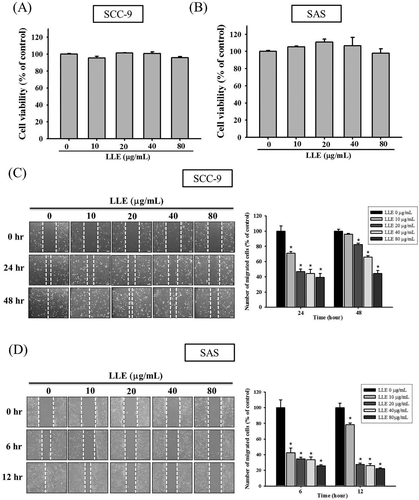
Effect of Leucaena leucocephala extract on cell viability and on motility. SCC-9 (A) and SAS (B) cells were treated with different concentrations (0, 10, 20, 40, and 80 μg mL−1) of Leucaena leucocephala extract for 24 h before being subjected to an MTT assay for cell viability. The motility of SCC-9 (C) and SAS (D) cells were assessed by in vitro wound closure assay with different concentration of Leucaena leucocephala extract (0, 10, 20, 40, and 80 μg mL−1) at different time points. A quantitative assessment of cell number in the denuded zone is the mean ± SD (n = 3). The values represented the means ± SD of at least three independent experiments. *P < 0.05, compared with the vehicle group
3.2 Effects of LLE on motility, migration, and invasion of SCC-9 and SAS cells
In a scratch-wound assay in the control group, we observed that the wounds were completely healed after 48 and 12 h in the SCC-9 and SAS cell lines, respectively. The cell motility of SCC-9 exhibited significant inhibition at all tested LLE concentrations, except for the group treated with 10 μg mL−1 for 48 h (Figure 1C). In the SAS cell line, all LLE-treated samples exhibited significant inhibition (P < 0.05), and LLE demonstrated dose- and time-dependent effects on the motility of the SAS cells (Figure 1D). By using a Boyden chamber-based assay, we demonstrated that LLE reduced the migration and invasion ability of the two cell lines significantly after 24 h (P < 0.05). LLE reduced the migration of SCC-9 and SAS cells to 44–96% and 29–80%, respectively, at various treated concentrations (0–80 μg mL−1) (Figure 2A,B). Similar inhibition trends were observed for the invasion capacity of the cell lines (Figure 2C,D). Our results indicated that LLE can prevent the motility, migration, and invasion of the two oral cancer cell lines, SCC-9 and SAS, in a concentration-dependent manner.
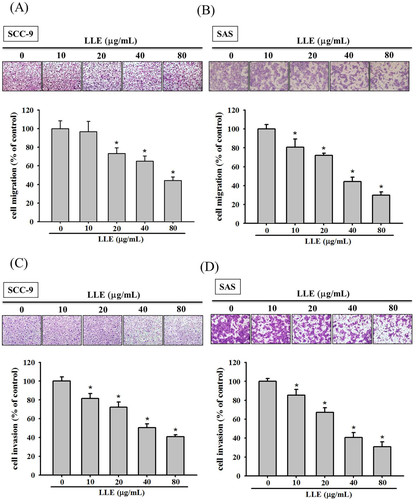
Effect of Leucaena leucocephala extract on migration and invasion of SCC-9 and SAS cells. The cell migration (A and B) and invasion (C and D) were measured using a Boyden chamber for 24 h with polycarbonate filters. The number of cells which invaded the underside of the porous polycarbonate was counted for assessing the migration and invasion abilities of SCC-9 and SAS. The values represented the means ± SD of at least three independent experiments. *P < 0.05, compared with the vehicle group [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Effect of LLE on enzyme activity and protein expression of MMP-2
To understand the role of MMP-2 in the suppression of cell migration by LLE, a gelatin zymography assay was used to characterize the MMP-2 activity. We observed that the decrease in MMP-2 activity correlated to the LLE concentration in both cell lines (Figure 3A,B). At the highest dosage used in the study (80 μg mL−1), LLE reduced the MMP-2 enzymatic activity by 55.9 and 32.1% in the SCC-9 and SAS cells, respectively. With the decrease in LLE concentration, the suppressive effect on MMP-2 activity diminished. Moreover, LLE was demonstrated to significantly downregulate MMP-2 expression at the protein level through western blotting (Figure 3C,D). The trend of MMP-2 protein expressions corresponded to its enzymatic activity. The results suggested that the antimetastatic ability of LLE was characterized by the decrease in MMP-2 activity and expression.
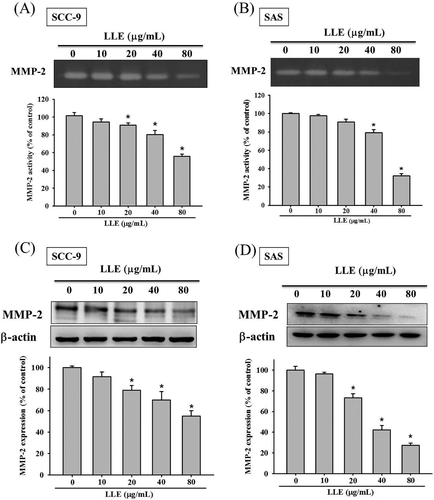
Effect of Leucaena leucocephala extract on the enzymatic activity and protein expression of MMP-2. The gelatin zymography assay was applied for measurement of MMP-2 activity on Leucaena leucocephala extract treated SCC-9 (A) and SAS (B) cells (0, 10, 20, 40, and 80 μg mL−1). The expressions of MMP-2 protein on the same treatments of SCC-9 (C) and SAS (D) cells were assessed by western blot. The values represented the means ± SD of at least three independent experiments. *P < 0.05, compared with the vehicle group
3.4 Effect of LLE on MAPK pathway in SCC-9 cell lines
To further study the regulatory mechanisms of upstream signal transduction, we examined the expression of three protein kinases, ERK1/2, JNK1/2, and p38, involved in MAPK pathway through western blotting. Experimental results are presented in Figure 4, which indicated that LLE can suppress the phosphorylation of ERK and p38 in a dose-dependent manner. At 80 μg mL−1, LLE reduced the phosphorylation level of ERK1 and p38 to 48 and 53%, respectively (Figure 4A,C). However, the amount of phosphorylated JNK1/2 proteins was not affected by LLE in all tested concentrations (Figure 4B). Thus, the results supported that the metastasis inhibition by LLE was regulated by two classes of MAPKs, ERK1/2, and p38.
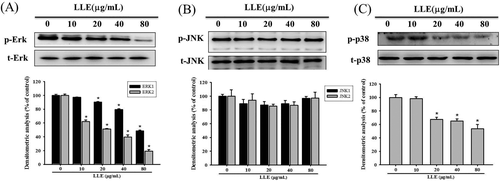
Effect of Leucaena leucocephala extract on the MAPKs pathway. After a 24 h culture in various concentrations of Leucaena leucocephala extract (0, 10, 20, 40, and 80 μg mL−1) for 24 h, the lysates of SCC-9 cells were subjected to SDS-PAGE followed by western blots with anti-ERK (A), anti-JNK (B) and anti-p38 (C) (total and phosphorylated) antibodies. The values represented the means ± SD of at least three independent experiments. *P < 0.05, compared with the vehicle group
3.5 Effect of LLE and MAPK pathway inhibitors on SCC-9 cell lines
Based on the aforementioned data, ERK and p38 inhibitors, U0126 (10 μM) and SB203580 (20 μM), were combined with LLE treatments (20 μg mL−1) for further confirmation. In vitro wound closure assay demonstrated that LLE can notably inhibit the motility of SCC-9 cells. We observed that the combination of LLE and the inhibitors enhanced the suppression effect (Figure 5A). In migration analysis, similar trends were observed through a Boyden chamber assay. Compared with a treatment that only used LLE, significant inhibition was observed when we combined LLE with each inhibitor (Figure 5B). Gelatin zymography assays also demonstrated that combination of LLE and MAPK pathway inhibitors can reduce MMP-2 enzymatic activity (Figure 5C). Moreover, our results shown that, in SCC-9 cells, the combination of LLE and the ERK inhibitor (U0126) enhanced the suppression effect of Erk phosphorylation (Figure 5D). A serial assay with MAPK pathway inhibitors indicated that both ERK1/2 and p38 were involved in LLE-conducted antimetastasis in SCC-9 cells.
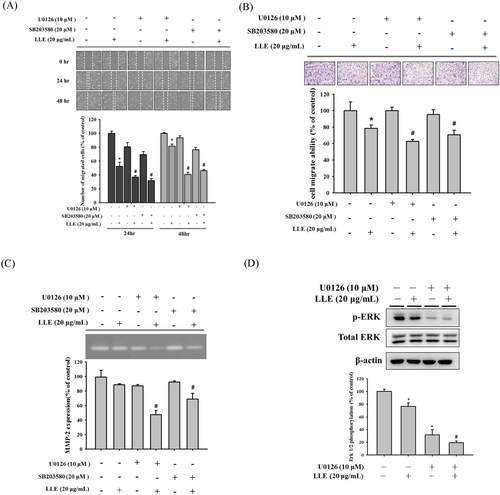
Effect of Leucaena leucocephala extract, ERK inhibitor (U0126) and p38 inhibitor (SB203580) on MMP-2 activity, motility and migration of SCC-9 cells. SCC-9 cells were pretreated with U0126 or SB203580 combined with or without 20 μg mL−1 of Leucaena leucocephala extract for 1 h and then incubated in the presence or absence of Leucaena leucocephala extract for 23 h. Analysis of (A) motility and (B) migration of SCC-9 cells were assessed as described in Methods section. (C) The gelatin zymography assay was used for measurement of MMP-2 activity. (D) The western blotting was used for measurement of Erk1/2 phosphorylation. The values represented the means ± SD of at least three independent experiments. *P < 0.05, compared with the vehicle group. #P < 0.05, compared with the Leucaena leucocephala extract treated group [Color figure can be viewed at wileyonlinelibrary.com]
4 Discussion
Recently, in Taiwan, the prevalence of oral cancer in men has significantly increased, which may be attributed to the increase in betel nut chewing.33 More than two million people in Taiwan are estimated to chew betel nut habitually, and 80% of oral cancer mortality was related to this habit. The 5-year survival rate of patients with OSCC is ∼25%,34 which is lower than that of patients with breast and colorectal cancer. The invasive and metastatic ability of this type of cancer is believed to be of the key factors causing its low survival rate.35 Therefore, the prevention of metastasis is one of several critical approaches to treating cancer diseases. The results of this study indicated that LLE (1) retards the migration and invasion of SCC-9 and SAS oral cancer cells at noncytotoxic concentrations, (2) supresses the expression of MMP-2 protein and its enzymatic activity, and (3) inhibits the phosphorylation of ERK1/2 and p38 MAPK.
During the progress of a metastasis, it is critical that cancers cells secrete the proteases for digesting ECM and allow themselves to migrate to other tissues or organs.4, 7, 36 Previous studies have revealed that two MMPs (MMP-2 and MMP-9) are highly expressed in various types of malignant tumors.20, 37, 38 Moreover, MMP-2 is one of the enzymes that demonstrate upregulated expression in invasive and metastatic cases of human oral cancer.39 Aparna et al. also reported that higher MMP-2 expression in the early stages of tongue squamous cell carcinoma was associated with distant metastasis.40 MMP-2, known as gelatinase A, has been most intensively studied and was found to be expressed in most tissues and cells.4, 41-43 In our study, we observed through a zymography assay that the inhibitory effects of MMP-2 activity follow the increase of LLE concentrate (Figure 3A,B). Loffek et al. stated that the unbalance of MMPs and their endogenous inhibitors could result in tissue remodeling or degradation of ECM.44 Therefore, the downregulation of MMP-2 might be caused by the upregulation of its endogenous inhibitor. However, the detailed verification of the regulation of its endogenous inhibitor is warranted.
In addition, several pathways are involved in the regulatory mechanism of MMP-2. The MAPK pathway is one of the most crucial signaling transduction pathways that govern the expression of MMP-2.14, 45-47 The function of MAPK is to transfer the signal from the cell membrane to the cell and to control cell growth, survival, and differentiation.48 Three main families of MAPK are well known, namely ERK, JNK, and p38. A previous study revealed that ERK1/2 was involved in the cell invasion and metastatic ability of human fibrosarcoma HT1080 cells.49 Moreover, other studies have indicated that the suppression of tumorigenesis can occur through the activation of the JNK pathway, which promotes apoptosis.50, 51 Therefore, we utilized a western blot to characterize the expression of ERK1/2, JNK1/2, and p38 after treatment with various LLE concentrations. We observed that the amount of phosphorylated ERK1/2 and p38 decreased with increases in the applied LLE concentrations (Figure 4A,C). However, no effect was observed on the phosphorylation of JNK1/2 at all treated concentrations (Figure 4B). The outcome implied that the antimetastatic effect might be induced by LLE through both ERK1/2 and p38 signal transduction pathways and might further regulate MMP-2 expression. Although the activation of p38 pathway was reported to lead to cell apoptosis and growth-inhibitory signals in general,52 the opposite effects have also been reported.53 In our study, the phosphorylation of p38 did not appear to facilitate the antimetastatic effect on oral cancer cells. However, we still cannot eliminate its potential involvement in the LLE-induced antimetastasis. To elucidate whether both ERK and p38 played an essential role in this inhibitory effect, we used the ERK and p38 inhibitors, U0126 and SB203580, to characterize the roles of ERK and p38, respectively. The gelatin zymography assay revealed that individual application of the two inhibitors can cause consistent inhibitory trends of MMP-2 expression compared with LLE-only treatment (Figure 5C). Synergistic effects were perceived in the assays of LLE plus U0126 or SB203580. Both treatments exhibited a higher suppressive effects, indicating that both ERK and p38 pathways were involved. The same treatment combinations were applied to examine the effects on motility and migration, and similar synergistic inhibition results were observed (Figure 5A,B).
In conclusion, this study demonstrated that LLE inhibited the metastasis of oral cancer cells at several key stages, such as invasion and migration, through regulation of the related protease (MMP-2). Regarding the MAPK pathway, we demonstrated that LLE can efficiently suppress ERK1/2 and p38 expression, which might downregulate MMP-2, resulting in an antimetastatic effect. Based on these results, LLE can be identified as a potential and powerful natural preventive and therapeutic agent for oral cancer metastasis. However, the key bioactive compounds in L. leucocephala that possess the antimetastatic property remain to be identified and isolated.
Acknowledgment
This work was supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST-103-2632-B-040-001; MOST-104-2632-B-040-001 and MOST-105-2632-B-040-001). This study was also supported by grants from Changhua Christian Hospital, Taiwan (103-CCH-IRP-042 and 104-CCH-IRP-061).




