Lactational exposure to hydroxylated polychlorinated biphenyl (OH-PCB 106) causes hyperactivity in male rat pups by aberrant increase in dopamine and its receptor
Abstract
Polychlorinated biphenyls (PCBs) are recognized as persistent environmental pollutants that may cause adverse health problems. Despite extensive investigations of PCB in neural function, little is known about behavioral traits by PCB exposure and its neurochemical mechanism. Here, we report the behavioral study of a rat pup that was exposed to hydroxylated-PCB 106 (OH-PCB 106; 4-hydroxy-2',3,3',4',5'-pentachlorobiphenyl) through maternal milk. The different groups of mothers received via gavage corn oil vehicle, 0.5, 5, or 50 mg/kg body weight of OH-PCB 106 every second day from day 3 to 13 after delivery. The exposure did not affect the body weight of the dams or the physical development of the newborn pups in both sexes. Male rats exposed to OH-PCB 106 showed hyperactivity that was characterized by increased locomotor activity in novel environment and circadian period. Interestingly, OH-PCB 106-exposed rat pups displayed abnormally high levels of dopamine and D2 dopamine receptor (D2DR), but not D1DR and D5DR, in the striatum, an important center for the coordination of behavior. These findings indicate that OH-PCB 106 has a significant neurotoxic effect on rat behavior, which may be associated with increased D2DR mediated signals. © 2012 Wiley Periodicals, Inc. Environ Toxicol 29: 876–883, 2014.
INTRODUCTION
Polychlorinated biphenyls (PCBs) belong to a broad family of organohalogen chemicals known as polycyclic chlorinated hydrocarbons and are among the most persistent environmental contaminants. These compounds comprise 209 congeners, each of which is chlorinated to various degrees (Safe,1994; Tilson and Kodavanti,1997). Although the manufacture of PCBs was discontinued in 1972, significant quantities still remain in the environment (Erickson,2001). Any organism may be exposed continuously since PCBs accumulate within food chains, water, soil, and air. Once absorbed, they are retained in the liver and adipose tissue because of their high lipophilicity. Moreover, they easily cross the placenta and are secreted into the breast milk, making the contamination heritable (Faroon et al.,2001).
Although the molecular and cellular mechanisms of PCB action have not yet been fully understood, accumulating evidence shows that PCBs induce neurotoxicity through alteration of signal transduction and gene expression. Aroclor 1254, a commercial mixture of PCB congeners, reduces intracellular dopamine and causes cell death by depleting intracellular Ca2+ stores and alters nitric oxide production in an immortalized catecholaminergic cell-line (Kang et al.,2002, 2004). Aroclor 1254 also alters intracellular calcium buffering and protein kinase C translocation in rat cerebellar granule cells (Tilson and Kodavanti,1998), perturbs Ca2+ homeostasis and increases phosphorylation of cyclic AMP responsive element-binding protein in cultured rat cortical neurons (Inglefield et al.,2001). Moreover, the non-coplanar congener PCB 95 (2,2',3,5',6- pentachlorobiphenyl) disrupts intracellular Ca2+ signaling in PC12 cells by interacting with the FK506 binding protein/ryanodine receptor complex (Wong et al.,2001). We previously reported that PCB potentially influences acid-sensitivity through alteration of the membrane potential of acid-sensitive neurons in the medulla oblongata, which could affect the regulation of respiration (Okada et al.,2005). Furthermore, we found that exposure to hydroxylated-PCB (OH-PCB) 106 (4-hydroxy-2',3,3',4',5'-pentachlorobiphenyl) induces c-Jun expression and c-Jun NH2-terminal kinases (JNK) phosphorylation in PC12 cells (Shimokawa et al.,2006), which may be mediated by membrane depolarization via activation of Na+ channels and subsequent extracellular Ca2+ influx through voltage-gated Ca2+ channels. We also showed that exposure to OH-PCB 106 induces Ca2+ oscillations and alters the membrane potential in part by inducing extracellular influx of Ca2+ and/or release of Ca2+ from intracellular Ca2+ stores in cultured rat cortical neurons (Londoño et al., 2009). These results indicate that PCBs may alter intracellular calcium homeostasis and membrane potential, which may be at least in part responsible for their neurotoxicity.
It is accepted that several PCB congeners affect animal behavior. Holene et al. reported that pre and postnatal exposure to low doses of the mono-ortho-substituted PCB 118 (2,3',4,4',5-pentachlorobiphenyl) and the co-planar PCB 126 (3,3',4,4',5-pentachlorobiphenyl) induce poor visual discrimination and hyperactivity in male rats (Holene et al.,1995) and that postnatal exposure to di-ortho-substituted PCB 153 (2,2',4,4',5,5'-hexachlorobiphenyl) and the co-planar PCB 126 cause behavioral hyperactivity in neonate rats through maternal milk (Holene et al.,1998). Schantz et al. (1995) found a delayed spatial alternation task in a T-maze following pre and postnatal exposure to the ortho-substituted PCB 28 (2,4,4'-trichlorobiphenyl), 118 and 153. Exposure to PCB causes the alteration of active avoidance learning and retention of a visual discrimination (Lilienthal and Winnecke,1991). However, there are only few reports that linked neurochemical mechanism behind behavioral alteration by PCB exposure.
In this study, we assessed the behavioral effects of postnatal exposure to OH-PCB 106 through maternal milk in newborn pups, and revealed a part of neurochemical mechanisms of behavioral alteration by OH-PCB 106. Here, we show that postnatal exposure to OH-PCB causes hyperactivity by the aberrant expression of dopamine and its receptor.
MATERIALS AND METHODS
Reagents and Antibodies
Since OH-PCB is a major metabolite of PCB in mammals and our previous studies have shown with the hydroxylated form of PCB (OH-PCB 106) (Miyazaki et al.,2004; Shimokawa et al.,2006; Londoño et al., 2009), we used OH-PCB 106 in this study. OH-PCB 106 was purchased from AccuStandard Chemicals (HPCB-5005S, purity 99%, New Haven, CT). It was dissolved in pure corn oil, which did not affect either physiological condition or lactation of rats at the concentration used in this study (0.5 mL/administration/rat). Reverse transcriptase and PCR enzyme for reverse transcription-polymerase chain reaction (RT-PCR) were obtained from Takara Bio (Shiga, Japan) and Roche (Basel, Switzerland), respectively. The mouse monoclonal anti-D2 dopamine receptor (D2DR) antibody (clone B-10) and rabbit polyclonal anti-β-actin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Cell Signaling Technology (Beverly, MA), respectively.
Animals and Treatment
The Wistar rats used in this study were purchased from Japan SLC (Hamamatsu, Japan). The animal experimentation protocol used for this study was approved by the Animal Care and Experimentation Committee, Gunma University Showa Campus. Rats were kept in the animal facilities under standard laboratory conditions (12:12 light–dark cycle, lights on at 6:00 a.m., 24°C, 55% relative humidity; water and standard rat chow ad libitum). Virgin females were mated with males at 12–15 weeks of age. They were singly housed when found to be pregnant and allowed to give birth to pups. The day of delivery was designated as postnatal day 0 (P0).
On postnatal day 3 (P3), the litters were culled to six newborn pups and sex-balanced (three male and three female pups/dam). The 12 dams and their litters were randomly divided into four groups. Every 2 days from P3 to P13 (P3, 5, 7, 9, 11, 13), the dams were given by gavage 0.5, 5, or 50 mg/kg body weight (BW) of OH-PCB 106 dissolved in 0.5 mL of pure corn oil, or only pure corn oil (controls). The dose of OH-PCB 106 was determined based on the previous study showing behavioral alteration by other PCB congener (Holene et al.,1998). Pups were separated from dams at P21.
Open Field Test
All rats at P28 were tested one by one in an open field apparatus. The detection unit of motor activity in rats consists of a 45 × 45 × 20 (width × length × height) cm frame containing a total of 16 × 16 crossed infrared beams at intervals of 2.5 cm, located on the sides (LE 8811, Panlab, S.L.U., Barcelona, Spain). Motor activity is detected on the basis of the analysis of the position and frequency with which the experimental rats break the infrared beams. The data were analyzed with using the Acti-Track program (Panlab, S.L.U.) At the beginning of the 5 min session, animals were placed in the center of the arena. Locomotor activity and rearing were continuously monitored for 5 min and the data of locomotor activity were analyzed as 1 min bins. These tests were conducted between 09:00 and 10:00.
Measurement of Circadian Activity
An infrared sensor was placed over each cage (45 cm × 50 cm × 45 cm) to monitor circadian locomotor activity. The rat's displacements were individually measured in the cage under standard laboratory conditions as described above. The infrared beam crossings was continuously recorded and sent to an online data-acquisition system (NS-ASS01, O'hara, Tokyo, Japan). Rat at P28 was placed in the cages at 10:00 and the circadian locomotor activity was measured for 72 h. The first and last 24 h were excluded from the analysis as a habituation period and an excessive habituation. The activity of each rat was recorded as the longitudinal data shown every 10 min by a rhythm analysis program (O'hara Co. Ltd.). The floor of each cage was covered with sawdust and there was food and water on disposal.
Isolation of Striatum from Brain
Animals at P31 were anesthetized with diethyl ether and sacrificed by cervical translocation at 10:00. Whole brains were removed, washed in ice-cold PBS, and dissected into hemispheres. The striatum was taken from the both hemispheres according to the procedure of Glowinski and Iversen (1966). Striata isolated from the right and left hemisphere were used for the detection of mRNA and protein levels of dopamine receptor, respectively.
Semiquantitative RT-PCR
For measurement of the dopamine receptors mRNA levels in the striatum, semiquantitative RT-PCR was performed. RNA was extracted from the striata using an RNeasy kit (Qiagen, Hilden, Germany). For reverse transcription, cDNA was synthesized from 500 ng of total RNA as described in the Transcriptor First Strand cDNA Synthesis kit (Takara Bio) using the oligo dT and random primers. In one reaction, 2.5 μL of the reverse-transcribed cDNA, 0.5 μM sense and antisense primers, 200 μM dNTPs, and 0.125 μL Taq polymerase (Roche) were added in a final volume of 25 μL. To define the linear range for PCR amplification, the optimal number of PCR cycles was decided. All PCR products were detected and analyzed by the Electrophoresis Documentation and Analysis System 290 (Kodak, Norwalk, CT). The PCR results for each sample were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level as an internal control. All experiments were repeated three times to confirm the consistency of results. Specific primers used for the RT-PCR are as follows: D1DR sense primer 5'-TATCTCCAGCCCTTTCCAGTATGA-3' and antisense primer 5'-ATTCCACCAGCCTCTTCCTTCTTC-3', D2DR sense primer 5'-AAGCGTCGGAAGCGGGTCAAC-3' and antisense primer 5'-GCCAAGCCAACAATCAAGGTG-3', D5DR sense primer 5'-GAAGGGAGGACGGAGAACTGTGAC-3' and antisense primer 5'-TTGGGGGTGAGAGGTGAGATTTTG-3' (Karadaghy et al.,1997), GAPDH sense primer 5'-CAAGGCCGAGAATGGGAAGCT-3' and antisense primer 5'-GATGATGACCCTTTTGGCTCC-3'.
Western Blot
For analysis of the expression of D2DR in the rat striatum, Western blotting was performed. The dissected striata were weighted, homogenized in the membrane protein extraction reagent (Cell-LyEX MP, Toyo-B-Net, Tokyo, Japan) with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2.5 mg/mL of leupeptin, 10 mg/mL of aprotinin), and 1 mM sodium orthovanadate (a phosphatase inhibitor). The tissue lysates were separated by centrifugation for 15 min at 15,000 g at 4°C, and protein concentrations in the supernatant were measured (Bradford protein assay, Bio-Rad, Hercules, CA). Equal amounts of protein were resolved by SDS-PAGE, transferred to a nitrocellulose filter and immunoblotted with anti-D2DR antibody (1:1000). The antigen–antibody complexes were detected by chemiluminescence with an ECL system (GE Healthcare, Buckinghamshire, UK) and visualized with a Lumi-Imager imaging analyzer (Roche, Basel, Switzerland). The intensity of bands was quantified using an image analysis software (LumiAnalyst, Roche). Blots were reprobed with an anti-β-actin antibody (1:1000) for monitoring the quantity and integrity of protein.
Measurement of Dopamine Contents
Sample preparations were carried out according to the procedure of Shimokawa et al. (2010). Briefly, dissected striata at P31 were weighed before homogenizing with perchloric acid (0.2 M) in Teflon/glass homogenizer. The homogenate was centrifuged at 4°C for 15 min at 20,000 g. The supernatant was adjusted to pH 3 with 1 M sodium acetate and filtered before injecting into the HPLC system. Dopamine level was determined using HPLC coupled to electro-chemical (HPLC-EC) detection. Ten microliter samples were injected into a reverse-phase analytical column (Eicompak, SC-5ODS, 3 mm inner diameter, Eicom Corp, Kyoto, Japan) perfused at a flow rate of 0.5 mL/min with a mobile phase containing: 83% 0.1 M citric acid-sodium acetate buffer (pH 3.5), 17% methanol, 170 mg/L octyl sodium sulfate, 5 mg/L EDTA·2NA. Dopamine was detected with a graphite carbon detector electrode maintained at +750 mV relative to an Ag/AgCl reference electrode. The output from the working electrode was integrated using EPC-300 software (Eicom). Chromatographic peaks were identified/quantified by reference to known concentrations of standards.
Statistical Analysis
An analysis of variance (ANOVA) was used to compare the distribution of the values between groups; post-hoc individual comparisons were made using Bonferroni's test. Data are presented as mean ± SEM. The accepted level of significance was p < 0.05.
RESULTS
No Obvious Abnormality in Body Weight Gain of Newborn Pups by OH-PCB 106
The weight of newborn pups was recorded every 2 days from P3 to P13 when OH-PCB 106 or vehicle was administered to dams. There were neither signs of toxicity in the dams during the treatment period nor weight-gain differences between the groups of dams from delivery until weaning (data not shown). There were no group differences in newborn pup's body weight (BW) gain in both sexes during the maternal treatment period (Fig. 1). Therefore, administration of OH-PCB 106 to dams does not cause any obvious abnormalities in weight-gain of newborn pups.
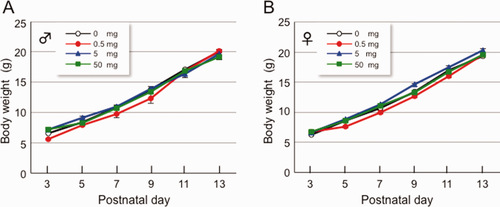
Effect of lactational exposure to OH-PCB 106 on BW of newborn pups. OH-PCB 106 was administered orally (0, 0.5, 5, or 50 mg OH-PCB/kg BW) through a gastric tube at P3, P5, P7, P9, P11, and P13. Body weight (A, male; B, female) was measured at P3, P5, P7, P9, P11, and P13. Data are expressed as mean ± SEM for nine newborn pups/sex/dose group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
OH-PCB 106-exposed Pups Are Hyperactive in a Novel Environment
We next evaluated locomotor activity in a novel environment using the open field test. A pup was placed in the center of the arena and allowed to move freely for 5 min. The 5 mg/kg BW of OH-PCB 106-exposed male pups displayed a high level of locomotor activity throughout the test period compared to control males [Fig. 2(A)]. The 0.5 and 5 mg/kg BW of OH-PCB 106-exposed pups in male had significantly higher total locomotor activity for 5 min [Fig. 2(C)]. There were no differences in distance traveled in the 50 mg/kg BW in male and all doses in female of OH-PCB 106-exposed pups compared with control [Fig. 2(A–D)].
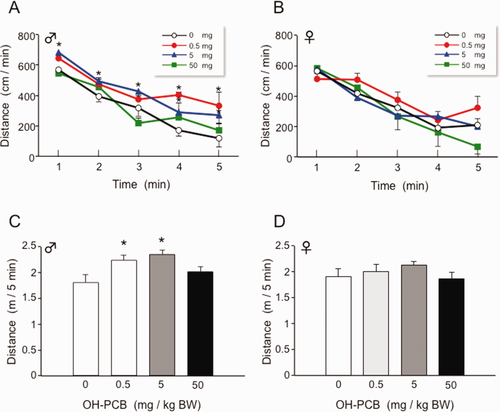
Effect of lactaional exposure to OH-PCB 106 on open field locomotor activity of pups. Distance traveled of male (A) and female (B) pups was continuously monitored for 5 min at P28 and data were analyzed as 1 min bins. Data are expressed as mean ± SEM for nine pups/sex/dose group. *p < 0.05 compared with control value (corn oil) at same time point. Total locomotor activities of male (C) and female (D) pups during 5 min period are also shown. Data are expressed as mean ± SEM for nine pups/sex/dose group. *p < 0.05 compared with control value (corn oil). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Exposure to OH-PCB 106 Induces Spontaneous Hyperlocomotion
The circadian locomotor activity of OH-PCB 106-exposed pups was measured for 72 h. Motor activity of both OH-PCB106-exposed and control pups varied according to the light/dark cycle, increasing significantly during the dark phase (Fig. 3). OH-PCB 106-exposed pups exhibited pronounced increase of locomotor activity when evaluated during a 24 h continuous session, under a 12 h light/dark cycle. OH-PCB 106-exposed pups have maintained locomotor circadian rhythm as in control pups, but the level of circadian activity increases significantly, compared to that of control male.
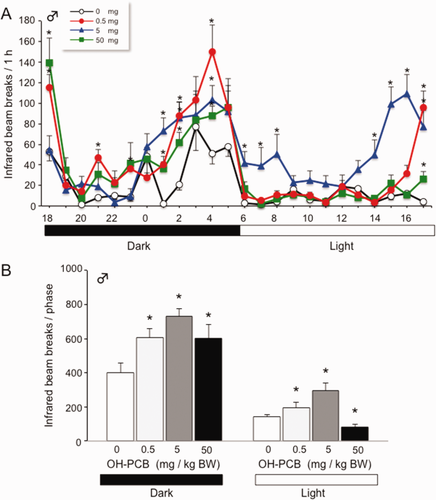
Lactational exposure to OH-PCB 106 causes increased circadian locomotor activity in male pups. (A) Locomotor activity for pups was measured at P28–P31 (for 72 h). Data are expressed as mean ± SEM for nine pups/dose group of hourly measurement during 24 h period. *p < 0.05 compared with control value (corn oil) at same time point. (B) Total locomotor activity of male pups during the dark and light phases. Data are expressed as mean ± SEM for nine pups/dose group in a dark or light phase during 12 h. *p < 0.05 compared with control value (corn oil) at same phase. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Exposure to OH-PCB 106 Leads to Aberrant Increase in Dopamine and Its Receptor
On the basis of multiple studies reporting involvement of dopaminergic signaling in the regulation of movement, learning, reward-seeking behavior, and motivation (Yao et al.,2008), we next investigated whether OH-PCB 106 could alter dopamine signaling in the striatum, an important center for the coordination of animal behavior. We first analyzed dopamine levels in the striatum of OH-PCB 106-exposed pups, using HPLC electrochemical detection. Interestingly, we found that the animals that were exposed low-dose (0.5 mg/kg BW) OH-PCB 106 displayed significantly increased levels of dopamine compared with control (approximately 65% increase, ANOVA and Bonferroni's test, *p < 0.05) [Fig. 4(A)]. Augmentation of dopamine levels may have multiple explanations, but in the light of our previous study reporting important function for dopamine receptors in the hyperactivity (Shimokawa et al.,2010), we then analyzed whether exposure to OH-PCB 106 affects the expression of D2DR in the striatum. When exposing the newborn pups to OH-PCB 106, both mRNA and protein levels of D2DR were increased significantly, compared to control [Fig. 4(B,C)]. Taken together, these data show that the dopamine signal in the striatum is altered in OH-PCB 106-exposed pups. The exposure to OH-PCB 106 is thus strongly increased in the intensity of locomotor activity through the excess of dopamine signal.
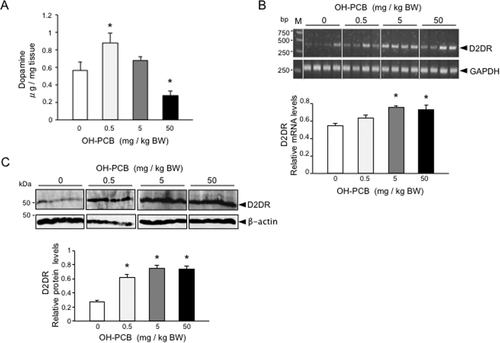
Lactational exposure to OH-PCB 106 alters dopaminergic signal in the striatum of pups. (A) Effect of lactational OH-PCB 106 exposure on striatal dopamine level of pups. The content of striatal dopamine was determined by high performance liquid chromatography using an electrochemical (HPLC-EC) detector. Data are expressed as mean ± SEM for five pups/dose group. *p < 0.05 compared with control value (corn oil). (B) Expression of striatal D2DR mRNA of pups following lactational OH-PCB 106 exposure. The mRNA levels of striatal D2DR of pups were analyzed by semiquantitative RT-PCR. Values of D2DR mRNAs are expressed as relative optical density (OD) levels, which are normalized with the corresponding density of GAPDH mRNA. Fluorescent bands in the upper part of the panel show the representative amplified band of each group. The positions of standard DNA molecular weight (bp) are indicated at the left. Data are expressed as mean ± SEM for four pups/dose group. *p < 0.05 compared with control value (corn oil). (C) The protein levels of striatal D2DR of pups were analyzed by Western blotting. Values of D2DR are expressed as relative OD levels, which are normalized with the corresponding density of β-actin. Bands in the upper part of the panel show the representative immunoblots of each group. The position of standard molecular masses (kDa) are indicated at the left. Data are expressed as mean ± SEM for four pups/dose group. *p < 0.05 compared with control value (corn oil).
No Obvious Alterations in Expression of D1 and D5 Dopamine Receptor by Exposure to OH-PCB 106
Mammalian genomes have been reported to encode at least five different dopamine receptor sub-types, all belonging to the seven transmembrane G protein-coupled receptor (GPCR) superfamily (Yao et al.,2008). The receptors are entitled D1–D5 and fall into two distinct classes based on sequence and functional similarities, the D1-like (D1 and D5) and D2-like (D2, D3, D4) dopamine receptors. Even though D1 dopamine receptors (D1DRs) are also expressed in the striatum, D1DR and D2DR are in general segregated into distinct striatal neurons. We therefore asked whether the exposure to OH-PCB 106 to rats may alter D1DR and D5DR expression in the striatum. No difference of D1DR [Fig. 5(A)] or D5DR [Fig. 5(B)] expression between OH-PCB 106-exposure pups and control rats was observed. Taken together, these results strongly indicate that exposure to OH-PCB 106 leads to D2DR-specific expression in the striatum.
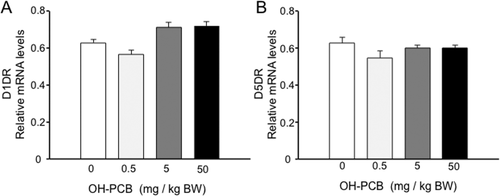
No obvious alterations in expression of D1DR and D5DR mRNAs by lactational exposure to OH-PCB 106. The mRNA levels of striatal dopamine receptors of pups were analyzed by semiquantitative RT-PCR. Values of D1DR (A) and D5DR (B) mRNAs are expressed as relative optical density (OD) levels, which are normalized with the corresponding density of GAPDH mRNA. Data are expressed as mean ± SEM for four pups/dose group. *p < 0.05 compared with control value (corn oil).
DISCUSSION
In this study, we showed that postnatal exposure to OH-PCB 106 through maternal milk induced hyperactivity phenotypes in male pups, characterized by increased locomotor activity in novel environment (Fig. 2) and circadian period (Fig. 3). Such hyperactivity could be due to aberrant dopamine signaling in the CNS, i.e., the increases of dopamine contents and dopamine receptor expression (Fig. 4). This study provides the first evidence that dopamine as well as its receptor is involved in OH-PCB 106 induced hyperactivity.
The neurological mechanism of OH-PCB 106-induced hypoactivity is highly complexed and may involve multiple of pathways. Holene et al. (1988) reported that postnatal exposure to di-ortho-substituted PCB congener 153 or the co-planar PCB congener 126 through maternal milk causes hyperactivity in neonate rats. Moreover, Seegal et al. (2002) showed that the concentrations of dialysate dopamine in the striatum were significantly elevated after 3 days of exposure to a mixture of PCBs (Aroclor 1254); however, striatal tissue dopamine concentrations were not significantly altered. Recently, Dreiem et al. (2010) reported that mixture of PCB congeners elevated dopamine release and reduced synaptosomal dopamine and the dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) concentrations, since PCBs inhibit the function of plasma membrane dopamine transporters (DAT) and vesicular monoamine transporters (VMAT) (Bemis and Seegal,2004; Mariussen and Fonnum,2001). Interestingly, elevations in serum PCB concentrations, due to long-term occupational exposure, are inversely associated with striatal DAT density in women (Seegal et al.,2010). Taken together, these results indicate that the exposure to PCB causes at least the alteration of dopamine dynamics in the brain.
Interestingly, in this study, we found that OH-PCB 106 affects the expression of D2DR, but not D1DR and D5DR, in rat striatum. Recently, we found that absence of CIN85, an adaptor/scaffold protein localized to the post-synaptic compartment of striatal neurons in which it co-clusters with D2DRs, gives rise to increased striatal dopamine and D2DR levels (Shimokawa et al.,2010). As a result, CIN85 knockout mice show hyperactivity phenotypes, characterized by increased physical activity and exploratory behavior. Regarding the site of action of OH-PCB 106 have not been clarified, it will be interesting to identify whether OH-PCB 106 also affects CIN85 activity following OH-PCB 106 exposure. On the other hand, the hyperactive behavior evoked by alterations in other pathways (such as glutamatergic and serotonergic cascades) or by administration of chemical compounds (such as haloperidol and clozapine), also indirectly affects dopaminergic signaling. It should be noted that such hyperactive phenotype may be associated with increased levels of high-affinity state D2DRs (D2High) in the striatum and nucleus accumbens (Seeman et al.,2006). Thus, taken together with present study, D2DR may play central role in controlling in locomotor behavior.
In conclusion, OH-PCB 106 disrupts D2DR-mediated signaling in the striatum, which is involved in controlling locomotor behavior in rats. It is of interest to perform further studies to analyze the relationship among signal transduction of OH-PCB 106, receptor–ligand kinetics of dopamine and behavior.
We are thankful to other members of our laboratory for helpful discussions and critical comments on the manuscript.




