β-catenin involvement in arsenite-induced VEGF expression in neuroblastoma SH-SY5Y cells
Abstract
Arsenic is a widespread contaminant in the environment especially in drinking water. Although it is a known carcinogen in human, the mechanism by which arsenic induces carcinogenesis is not well understood. Among several effects of arsenic, it has been suggested that arsenic-induced vascular endothelial growth factor (VEGF) expression plays a critical role in arsenic carcinogenesis. In the present study, we demonstrated that arsenite induced VEGF expression in neuroblastoma SH-SY5Y cells without induction of HIF-1α, a well-known transcriptional activator for VEGF suggesting that arsenite-induced VEGF expression in SH-SY5Y cells may not require HIF-1α activation. It has been reported that VEGF expression is regulated by multiple transcription factors including β-catenin. We therefore investigated whether β-catenin was involved in arsenite-induced VEGF expression in SH-SY5Y cells. Treatment of arsenite caused β-catenin accumulation in the nucleus. Additionally, arsenite treatment decreased the activity of GSK3, an enzyme that phosphorylates and targets β-catenin for degradation by proteasome, without activation of its upstream kinase, Akt. Inhibition of PI3K/Akt which negatively regulates GSK3 activity by LY294002 resulted in a decrease in arsenite-mediated β-catenin nuclear accumulation, and VEGF expression. These results suggested that β-catenin plays a role in arsenite-induced VEGF in SH-SY5Y cells, and the induction of β-catenin by arsenite is mediated by inhibition of GSK3 without activating its upstream kinase Akt. © © 2012 Wiley Periodicals, Inc. Environ Toxicol 29: 672–678, 2014.
INTRODUCTION
Arsenic, a natural metalloid element, is ubiquitously present in water, soil, and food. Exposure to arsenic from drinking ground water threatens human health worldwide especially in developing countries where people rely on ground water as a major supply for drinking water. Epidemiological studies reveal association of chronic exposure to arsenic with vascular diseases such as Blackfoot disease (States et al., 2009), diabetes (Tseng et al., 2002) as well as several types of cancer including skin, bladder, and lung (Tchounwou et al., 2003). Despite being identified as a human carcinogen, the exact mechanisms that underlie arsenic carcinogenesis are still unknown.
Among several effects of arsenic, it has been suggested that arsenic-induced vascular endothelial growth factor (VEGF) expression plays a critical role in arsenic carcinogenesis. Arsenic has been shown to stimulate VEGF expression in many cell systems such as OVCAR-3 human ovarian cancer cells (Duyndam et al., 2003), BEAS-2B human immortalized lung epithelial cells (Liu et al., 2011), B16-F10 mouse melanoma cells, HMEC-1 human microvascular endothelial cells (Kamat et al., 2005), porcine primary vascular smooth muscle cells (Soucy et al., 2004) and SH-SY5Y human neuroblastoma cells (Watcharasit et al., 2012). It is well established that HIF-1α (hypoxia-inducible factor-1α) plays a pivotal role in transcription regulation of VEGF expression upon hypoxic stress (Takahashi and Shibuya, 2005). However, the role of HIF-1α in arsenic-induced VEGF expression remains controversial. Soucy et al. (2003) reported that despite causing induction of HIF-1α, arsenic-induced VEGF was HIF-1α independent in porcine primary vascular smooth muscle cells as HIF-1α siRNA did not attenuate VEGF expression stimulation by arsenic. Similar observation was found in OVCAR-3 human ovarian cancer cells in which arsenic caused increase in HIF-1α level but not transcriptional activity (Duyndam et al., 2003). This evidence suggests that arsenic induces VEGF expression in a HIF-1α independent fashion. Conversely, Kamat et al. (2003) and Liu et al. (2003) showed that HIF-1α involves in arsenic-induced VEGF as they reported that inhibition of HIF-1α using inhibitor of HIF-1α, YC-1 and HIF-1α siRNA, respectively blocked arsenic-induced angiogenesis, a process in which VEGF is exclusively essential (Ferrara, 2009). Although the later two reports mentioned did not directly demonstrate that HIF-1α was involved in arsenic-induced VEGF, they suggested the implication of HIF-1α in arsenic action on VEGF stimulation.
β-catenin is a protein in the Wnt signaling pathway aberration of which is implicated in several types of cancer (Polakis, 2007). Level of β-catenin is mainly regulated by ubiquitin-dependent proteasome degradation. In the absence of Wnt, glycogen synthase kinase3 (GSK3) phosphorylates β-catenin at serine 33, 37 and threonine 41 resulting in increases in its ubiquitination, targeting it to degradation by ubiquitin/proteasome system. In the presence of Wnt, Wnt-receptor binding stimulates signal leading to inactivation of GSK3, hence decreasing β-catenin phosphorylation and degradation and thereby allowing it to accumulate and translocate to the nucleus (Kimelman and Xu, 2006; MacDonald et al., 2009). In the nucleus, it functions as a transcription factor by forming a complex with a transcription coregulator, Tcf/Lef (T cell factor/lymphocyte enhancer factor), and activates transcription of genes such as cyclin D1 (Shtutman et al., 1999) and c-myc (He et al., 1998). The role of β-catenin for transcription regulation of VEGF has recently been emerged. It has been demonstrated that inhibition of β-catenin by knock down β-catenin using antisense or shRNA (Easwaran et al., 2003; Thirunavukkarasu et al., 2008), and expression of constitutively active GSK3β inhibited VEGF expression (Easwaran et al., 2003; Skurk et al., 2005) while inhibition of GSK3 activity which decreases degradation of β-catenin increased expression of VEGF (Skurk et al., 2005; Silva et al., 2007).
Interestingly, β-catenin nuclear accumulation in response to arsenic has been reported (Patterson and Rice, 2007). However, the role of β-catenin in arsenic-induced VEGF expression has never been explored. Recently, we reported that low concentrations of arsenic increased VEGF secretion and triggered cell proliferation in SH-SY5Y cells (Watcharasit et al., 2012). Therefore, we investigated whether β-catenin was involved in VEGF expression induction by arsenic in SH-SY5Y cells. We found that reduction of β-catenin nuclear accumulation by activation of GSK3 by inhibition of its upstream regulator, PI3K/Akt using LY294002 attenuated VEGF induction by arsenic suggesting involvement of β-catenin in arsenic-induced VEGF expression in SH-SY5Y cells.
MATERIALS AND METHODS
Cell Culture
Human neuroblastoma SH-SY5Y cell line was obtained from the ATCC (Manassas, VA). Cells were cultured in a 1:1 mixture of Ham's F12 and MEM medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (JR Scientific, Woodland, CA), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine (Gibco, Grand Island, NY) at 37°C in a humidified 5% CO2 incubator.
VEGF ELISA
VEGF levels were measured by ELISA using a human VEGF ELISA kit (Peprotech, Rocky Hill, NJ) as instructed by the manufacturer. SH-SY5Y cells were plated onto 6-well plates at 5 × 105 cells/well, and allowed to attach overnight. The cells were put in serum free medium and treated with sodium arsenite (Sigma-Aldrich, St. Louis, MO) for 24 h. At the end of the treatment, the medium was collected for VEGF measurement. Samples were assayed in duplicate, and data were presented as % control.
RT-PCR
Cells were treated with sodium arsenite with or without 30 min pretreatment with inhibitor (LY294002, lithium, or SB216763). Total RNA was harvested using TRIZOL® Reagent (Invitrogen, Carlbad, CA) according to the manufacturer's protocol. cDNA was prepared from 1 μg of total RNA by reverse transcription using poly T primer with RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Leon-Rot, Germany) according to the manufacturer's instruction. cDNA was amplified with primer specific for VEGF (Forward: TTTCTGCTGTCTTGGGTGCATTGG, and Reverse: TCTGCATGGTGATGTTGGACTCCT) or GADPH (Forward: GAAGGTGAAGGTCGGAGTC, and Reverse: GAAGATGGTGATGGGATTTC). PCR conditions consist of denaturation at 95°C for 30 s, annealing at 57°C for 30 s, and elongation at 72°C for 30 s, for 35 cycles. The PCR products were run on a 1.8% agarose gel, and the gel was photographed using Quantity One 4.6.7 Gel Doc XR (Bio-Rad Laboratories, Hercules, CA).
Immunoblot Analysis
Cells were serum starved for 2 h before sodium arsenite treatment with or without inhibitor. At the end of the treatment time, cells were washed with cold PBS, and lysed with ice-cold lysis buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.5% NP-40, 1 mM Na3VO4, 50 mM NaF, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), and protease cocktail inhibitor (Calbiochem, Darmstadt, Germany). The cell lysates were collected in centrifuge tubes, sonicated, and then centrifuged at 16,000 × g for 15 min at 4°C. The protein concentration was determined with Bradford assay (Bio-Rad, Hercules, CA). The samples were stored at −80°C until use. For immunoblotting, cell lysate (30 μg protein) was mixed with equal volume of 2X Laemmli sample buffer (125 mM Tris, pH 6.8, 25% glycerol, 2% SDS, and 0.01% bromophenol blue), and placed in a boiling water bath for 5 min. Proteins were resolved in 7.5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were washed with TBST (20 mM Tris-buffer saline, pH 7.6, 0.1% Tween 20), and then incubated in a blocking buffer (5% nonfat dried milk in TBST) with agitation for 1 h at room temperature. The membranes were then incubated overnight with primary antibody at 4°C with agitation. Thereafter these were incubated with appropriate secondary antibody conjugated with horseradish peroxidase for 1 h at room temperature. Immunoreactive bands were detected using enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK). Anti human HIF-1α, β-tubulin, and histone antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti human β-catenin antibody was obtained from BD Bioscience (San Jose, CA). Anti human phospho-GSK3α/β (Ser 21/9), GSK3α/β, phospho-Akt (Thr437), and Akt antibodies were from Cell Signaling Technology (Denvers, MA). Anti human β-actin antibody was purchased from Sigma-Aldrich (St. Louis, MO). Goat anti-mouse and -rabbit coupled with horseradish peroxidase secondary antibodies were purchased from GE Healthcare (Buckinghamshire, UK).
Nuclear and Cytosolic Extract Preparation
Treated cells were washed with cold PBS, and lysed by incubation with extraction buffer (10 mM Tris pH 7.4, 10 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 0.05% NP-40, 1 mM Na3VO4, 50 mM NaF, 1 mM PMSF, and protease inhibitor cocktail) for 10 min on ice. The cell extracts were spun at 700xg for 10 min. The supernatant containing the cytosol was centrifuged at 100,000 × g for 30 min at 4°C. The pellet containing nuclei was washed three times with wash buffer (10 mM HEPES pH 7.4, 10 mM NaCl, 250 mM sucrose, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM Na3VO4, 50 mM NaF, 1 mM PMSF, and protease inhibitor cocktail), and centrifuged at 2,700 × g for 5 min 4°C. The resulting nuclear pellet was resuspended in 100 μL, and layered on 1 mL of sucrose cushion buffer (1 M sucrose, 1 mM Na3VO4, 1 mM PMSF, 50 mM NaF, and protease cocktail inhibitor) and centrifuged at 2,700 × g for 10 min at 4°C. The nuclei pellet was washed with washed buffer to remove residue sucrose. The nuclei were lysed by incubation with nuclear lysis buffer (20 mM HEPES, pH 7.9, 300 mM NaCl, 1 mM β-glycerophosphate,1.5 mM MgCl2, 0.2 mM EDTA, 1 mM Na3VO4, 1 mM PMSF, 50 mM NaF, and protease cocktail inhibitor) on ice for 30 min. The nuclear lysates were centrifuged at 16,000 × g for 15 min at 4°C. The samples were stored at −80°C until use. Levels of nuclear and cytosolic β-catenin were determined by immunoblot analysis.
Statistical Analysis
At least three independent experiments were completed for each treatment. Data are presented as mean ± SD. Statistical analysis was performed by Student's T-test, and statistical difference was accepted when p < 0.05.
RESULTS
Arsenite Induced VEGF Expression in Human Neuroblastoma SH-SY5Y Cells
Treatment of sodium arsenite for 24 h in human neuroblastoma SH-SY5Y cells caused significant increase in VEGF in a dose-dependent manner [Fig. 1(A)]. The level of VEGF peaked at 10 μM sodium arsenite. The induction of VEGF by sodium arsenite was confirmed by measurement of VEGF mRNA using RT-PCR. The results revealed that sodium arsenite increased VEGF mRNA following 6 and 24 h treatment with sodium arsenite [Fig. 1(B)] indicating that sodium arsenite induced VEGF expression at transcriptional level in a dose dependent fashion.
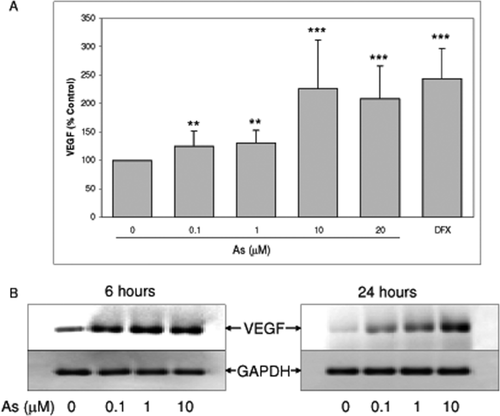
Sodium arsenite induced VEGF expression in SH-SY5Y cells. A: SH-SY5Y cells were treated with sodium arsenite in serum free medium for 24 h. Levels of VEGF in the medium were measured by ELISA. Data represented mean ± SD from at least five independent experiments. **, *** represent statistical difference from control at p < 0.01, and < 0.001, respectively; 200 μM deferoxamine (DFX, 200 μM) was used as a positive control for VEGF induction. B: Cells were treated with sodium arsenite for 6 or 24 h, and total RNA was isolated. VEGF expression was determined by RT-PCR.
Arsenite Treatment Did Not Increase HIF-1α in SH-SY5Y Cells
HIF-1α is a prominent transcriptional activator for VEGF (Takahashi and Shibuya, 2005), therefore we tested whether arsenite-induced VEGF in SH-SY5Y involved HIF-1α. Cells were treated with various doses of sodium arsenite, and level of HIF-1α was determined by immunoblot analysis. The result demonstrated that sodium arsenite did not cause significant increase in the level or nuclear accumulation of HIF-1α in this cell line [Fig. 2(A,B)]. While deferoxamine, which is an iron chelator mimicking hypoxia, caused significant increase in HIF-1α, a transcription factor well recognized for its role in VEGF expression upon hypoxia [Fig. 2(A)]. Furthermore, HIF-1 α nuclear accumulation was clearly evidenced in deferoxamine treated sample [Fig. 2(B)]. Moreover, VEGF expression was significantly elevated upon deferoxamine treatment [Fig. 1(A)]. These results suggested that HIF-1α-mediated VEGF expression signal is still intact in SH-SY5Y cells. Thus, the observation that HIF-1α was not activated by arsenite in SH-SY5Y cells suggested that HIF-1α may not be required for arsenite-induced VEGF in SH-SY5Y cells.
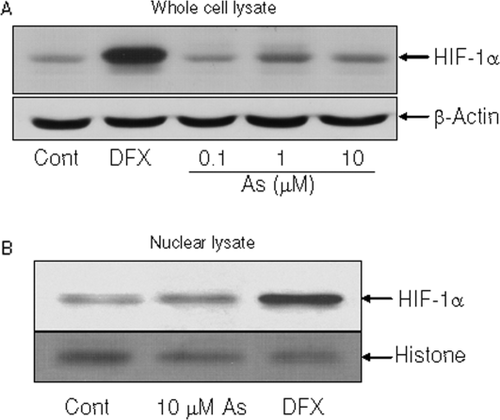
Sodium arsenite did not cause increase in HIF-1α in SH-SY5Y cells. SH-SY5Y cells were treated with sodium arsenite for 24 h, and level of HIF-1α in total cell lysates (A) and nuclear lysates (B) were analyzed by western blotting. Treatment of deferoxamine (DFX) was used as a positive control for HIF-1α induction.
Arsenite Caused β-Catenin Accumulation
It has been demonstrated that β-catenin can mediate VEGF expression (Easwaran et al., 2003; Skurk et al., 2005; Silva et al., 2007; Thirunavukkarasu et al., 2008), therefore we examined whether arsenite modulated the level of β-catenin in SH-SY5Y cells. Cells were treated with sodium arsenite for 24 h and levels of cytosolic and nuclear β-catenin were measured by immunoblot analysis. Elevation of β-catenin was observed in both the cytosol and nucleus following sodium arsenite treatment (Fig. 3). This indicated that arsenite caused β-catenin accumulation and translocation to the nucleus where it functions as a transcription factor.
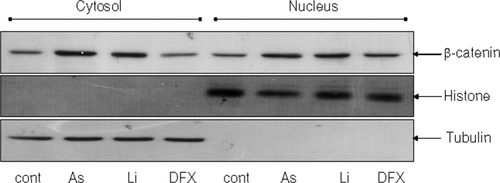
Sodium arsenite caused increase in β-catenin accumulation in SH-SY5Y cells. SH-SY5Y cells were treated with 10 μM sodium arsenite for 24 h, and nuclear and cytosolic fractions were isolated. Levels of β-catenin in the nucleus and the cytosol were detected by immunoblot analysis. Lithium (Li, 20 mM), an inhibitor of GSK3, which phosphorylates and targets β-catenin to ubiquitin dependent proteasome degradation was used as a positive control for β-catenin accumulation.
Arsenite Inhibited GSK3
It is well established that β-catenin phosphorylation by GSK3 plays a major role in its degradation (Kimelman and Xu, 2006; MacDonald et al., 2009) so we examined whether arsenite affected activity of GSK3 in SH-SY5Y cells. To determine GSK3 activity, level of inactive phosphorylation GSK3 (serine 21 for GSK3α and serine 9 for GSK3β) was detected by western blotting using antibody specifically recognized phosphorylation at serine 21/9 of GSK3α/β. The results showed that sodium arsenite treatment caused increase in phospho-serine 21/9 GSK3α/β (inactive form) suggesting reduction of GSK3 activity by arsenite in SH-SY5Y cells (Fig. 4). Interestingly, arsenite did not cause activation of Akt which is a kinase that phosphorylates and inhibits GSK3 activity (Fig. 4) suggesting that Akt may not be involved in arsenite-mediated GSK3 inactivation.
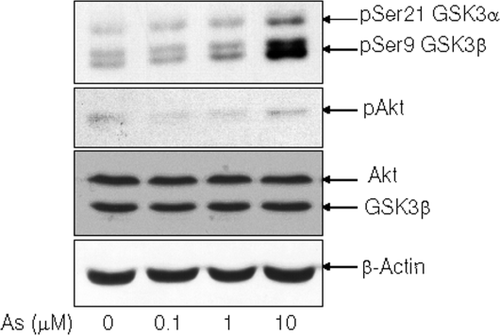
Sodium arsenite inhibited GSK3 in SH-SY5Y cells. SH-SY5Y cells were treated with sodium arsenite for 24 h, and levels of phospho-GSK3α/β (Ser 21/9) (inactive) and phospho-Akt (Ser 437) (active) were determined by immunoblot analysis.
Reduction of β-Catenin by GSK3 Activation Blocked Arsenite-Induced VEGF Expression
Since we found β-catenin nuclear accumulation by arsenite, we investigated its contribution in arsenite-induced VEGF expression in SH-SY5Y cells. To modulate β-catenin level, its negative regulatory partner, GSK3 was activated by inhibition of Akt signal using LY294002, an inhibitor of PI3K which is an upstream kinase responsible for phosphorylation and activation of Akt. Cells were pretreated with LY294002 followed by 24 h of sodium arsenite treatment. Measurement of VEGF by ELISA revealed that inhibition of PI3K/Akt by LY294002 abolished arsenite-induced VEGF [Fig. 5(A)]. Additionally, induction of VEGF mRNA level by arsenite was also attenuated with LY294002 pretreatment [Fig. 5(B)]. Furthermore, these reductions of VEGF protein expression and mRNA caused by arsenite were well correlated with the decrease of β-catenin nuclear accumulation following sodium arsenite when PI3K/Akt was inhibited [Fig. 5(C)]. These results suggested that β-catenin may contribute to arsenite-mediated VEGF expression in SH-SY5Y cells.
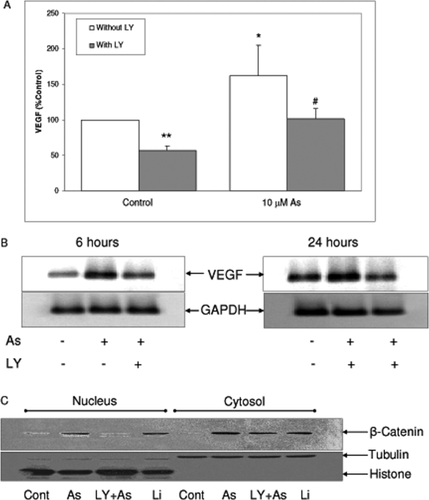
LY294002 attenuated sodium arsenite-induced VEGF expression and β-catenin nuclear accumulation. A: SH-SY5Y cells were treated with 10 μM sodium arsenite with or without 30 min pretreatment of 20 μM LY294002 in serum free medium for 24 h. Levels of VEGF in the medium were measured by ELISA. Data represent mean ± SD from four independent experiments. *, ** represent statistical difference from control without LY294002 at p < 0.05, and < 0.01, respectively. # represents statistical difference from sodium arsenite alone at p < 0.05. B: Cells were treated with 10 μM sodium arsenite with or without 30 min pretreatment of LY294002 (20 μM) for 24 h, and total RNA was isolated. VEGF expression was determined by RT-PCR. (C) SH-SY5Y cells were treated with 10 μM sodium arsenite with or without 30 min pretreatment of LY294002 (20 μM) for 24 h. Levels of β-catenin in the nucleus and the cytosol were detected by immunoblot analysis. Lithium was used as a positive control for β-catenin accumulation.
DISCUSSION
Arsenic, a natural metalloid element commonly found in environment, was reported to induce VEGF expression and angiogenesis (Duyndam et al., 2003; Kao et al., 2003; Soucy et al., 2003, 2004, 2005; Gao et al., 2004; Kamat et al., 2005; Liu et al., 2011; Watcharasit et al., 2012). Previously, arsenic-induced VEGF has been reported to mediate via a mechanism involving HIF-1α (Kamat et al., 2005; Liu et al., 2011), and controversially, via HIF-1α independent manner (Duyndam et al., 2003; Soucy et al., 2004). The results of the present study demonstrate that HIF-1α is not necessary for arsenic-induced VEGF expression in human neuroblastoma SH-SY5Y cells. The present investigation adds to the previous reports that arsenic mediated VEGF induction in HIF-1 independent manner through a mechanism involving β-catenin, and that arsenic causes GSK3 inhibition without activation of Akt, a primary upstream kinase from GSK3 hence resulting in β-catenin activation.
VEGF is a growth factor that is absolutely crucial for angiogenesis (Ferrara, 2009). It is well recognized that exposure to low levels of arsenic leads to induction of VEGF expression in multiple cell types and angiogenesis. The most prominent transcription factor regulates VEGF expression is HIF-1α; however, the role of HIF-1α in arsenic-induced VEGF remains controversial. The present study demonstrates that in neuroblastoma SH-SY5Y cells, arsenic-induced VEGF expression is not required for HIF-1α activation as arsenic treatment did not cause significant increase in HIF-1α level or its nuclear accumulation despite having intact HIF-1α signaling. Since HIF-1α is a labile protein that is degraded by proteasome dependent pathway, and its nuclear accumulation is observed parallel with its activation (Takahashi and Shibuya, 2005), our observation that HIF-1α accumulation did not occur following arsenic treatment in SH-SY5Y cells suggests it is unlikely that HIF-1α is involved in transcriptional control of VEGF expression induced by arsenic. We conclude that HIF-1α is not necessary for arsenic-induced VEGF in SH-SY5Y cells. This is in agreement with finding previously reported by Duyndam et al. (2003) and Soucy et al. (2003), which also found VEGF induction by arsenic in HIF-1α independent manner in OVCAR-3 human ovarian cancer cells and porcine primary vascular smooth muscle cells, respectively. It is interesting to note that the finding that HIF-1α is not essential for arsenic-induced VEGF (including in the present study) was observed in cells which are not vascular endothelial cells. However, HIF-1α has been reported to involve in arsenic-induced angiogenesis, a process in which VEGF is a critical activator, in A549 lung adenocarcinoma cells. Thus, the involvement of HIF-1α in arsenic induced VEGF is likely to be cellular content specific.
Since it has been reported that β-catenin regulates transcription of VEGF (Easwaran et al., 2003; Skurk et al., 2005; Silva et al., 2007; Thirunavukkarasu et al., 2008), we further investigated whether β-catenin was involved in arsenic-induced VEGF in SH-SY5Y cells. Our finding that β-catenin accumulation in the nucleus following arsenic treatment is in line with results previously reported by Patterson and Rice (2003). We further extend the action of β-catenin mediated by arsenic showing inhibition of PI3K/Akt by LY294002 caused decrease in arsenic-induced β-catenin and VEGF expression suggesting β-catenin contribution in VEGF expression upon arsenic exposure. Liu et al. (2003) have reported in lung adenocarcinoma A549 and human immortalized lung epithelial BEAS-2B cells that LY294002 reduced arsenic-induced angiogenesis, the process in which VEGF plays an essential role; however the role of β-catenin was not investigated. We postulate that β-catenin may be reduced in their experimental condition, since Akt phosphorylates GSK3 (at serine 21 for GSK3α and serine 9 for GSK3β) and inhibits its activity, as a result of Akt inhibition by LY294002, GSK3 activity increases leading to increase in β-catenin degradation and reduction of β-catenin level consequently attenuating VEGF induction. Hence, involvement of β-catenin in arsenic-induced VEGF expression may also occur in other cell systems, and this remains to be explored.
It is well established that β-catenin degradation is primarily regulated by GSK3 via phosphorylation mediated ubiquitin dependent proteasome degradation (Kimelman and Xu, 2006; MacDonald et al., 2009). The results from this study demonstrate that arsenic caused inactivation of GSK3 evidenced by the increase in its inactive phosphorylation form. The reduction of GSK3 activity by arsenic leads to decrease in β-catenin phosphorylation and degradation allowing it to accumulate and translocate to the nucleus where it binds to VEGF promoter to drive VEGF gene transcription. Surprisingly, in our experimental condition, the inhibition of GSK3 by arsenic was not accompanied with activation of Akt, a primary kinase that phosphorylates and negatively regulates GSK3 activity (Cross et al., 1995). Apart from Akt, inactivation phosphorylation of GSK3 can be mediated by a number of kinases including PKA (Fang et al., 2000), PKC (Fang et al., 2002), and CAMKII (Song et al., 2010), and activities of these kinases can be triggered by an increase in intracellular calcium. In fact, arsenic has been shown to increase intracellular calcium level (Goytia-Acevedo et al., 2003; Florea et al., 2007; Suriyo et al., 2012) hence this may lead to activation of PKA, PKC, and CAMKII and consequently increases GSK3 inactive phosphorylation.
In summary, we showed that arsenic induced VEGF expression in neuroblastoma SH-SY5Y cells through a mechanism involved β-catenin accumulation but not HIF-1α, and that β-catenin accumulation by arsenic occurred concomitantly with GSK3 inhibition in an Akt independent manner.




