Inflammatory airway responses by nasal inoculation of suspended particulate matter in NC/Nga mice
Abstract
To evaluate the allergic effect of airborne particulate matter (PM) on the airway, separated soluble supernatant (Sup) and insoluble precipitate (Pre) in suspended PM were inoculated into NC/Nga mice with a high sensitivity for mite allergens. Sup, Pre, or both Sup and Pre with or without pronase treatment were inoculated via the nasal route five times for sensitization and a challenge inoculation on the 11th day in NC/Nga mice. On the 14th day, mice were examined for airway hyperresponsiveness (AHR), bronchoalveolar lavage fluid (BALF) cell count, mRNA expression of Th1 and Th2 cytokines in the lung tissue, and histopathology. Synergistic effects of Sup and Pre were observed as increases in AHR and a histopathological change of Periodic acid-Schiff (PAS) staining. Increases in neutrophils, macrophages, and lymphocytes of BALF cells were dependent on Pre. The expression of IL-4 mRNA was increased by Sup, and those of IL-5 mRNA and Il-13 mRNA was increased by Sup and Pre. Augmented AHR, mRNA expression of IL-4, peribronchial inflammation, and PAS staining by Sup plus Pre were attenuated by treatment of Sup with pronase to digest proteins. These results suggest that some proteins of ambient PM may be important environmental factors for AHR and airway inflammation with the aid of insoluble particulates, although some soluble factors such as endotoxins cannot be ruled out. © 2012 Wiley Periodicals, Inc. Environ Toxicol 29: 642–654, 2014.
INTRODUCTION
Particles of various sizes exist in the atmosphere as particulate matter (PM). Associations between PM and the respiratory system have been shown in epidemiological studies (Dockery et al., 1993; Schwarz, 1994; Pope et al., 1995). Although specific components responsible for adverse effects have not been fully investigated, biological agents such as endotoxin, β-glucan, and mold spores (Schwarz et al., 2006), diesel exhaust particles (DEPs) (Fujimaki et al., 1997; Ormstad et al., 1998), metals (Pope, 1989; Costa and Dreher, 1997; Gavett et al., 2003), and ultrafine particles adhered to coarse particles (Oberdorster et al., 1994) in PM are considered to be constituents having inflammatory and toxic effects on the respiratory system. For respiratory allergic reactions in particular, DEP (Takano et al., 1997) and metals in residual oil fly ash (Gavett et al., 1997) appear to be involved. Moreover, ambient urban Baltimore (AUB) particulates induced allergic airway reaction in mice (Walters et al., 2001). However, among these allergy-related PM components, there was no evidence to demonstrate the contribution of soluble protein to PM-induced airway allergic reaction although almost all PM-related allergic reactions, except for the evidence from urban Baltimore, occurred with the aid of ovalbumin (OVA).
Airway inflammation, especially that involving recruited Th2 lymphocytes, is a prerequisite for asthma. Th2 cytokines such as IL-4, IL-5, and IL-13 play critical roles in allergic disorders (Barnes, 1999). Previously, we established a new experimental asthma model in NC/Nga mice with intranasal mite allergen exposure (Shibamori et al., 2006). Instead of the commonly used animal model of asthma induced by OVA, our mouse model showed increased eosinophils and high expression of IL-4, IL-5, and IL-13 detected in bronchoalveolar lavage fluid (BALF), and the lung tissue was considered to bear a closer resemblance to human asthma (Shibamori et al., 2006; Takemoto et al., 2007; Takahashi et al., 2010). Moreover, it has been shown that NC/Nga mice have high sensitivity to mite allergen not only in terms of airway inflammation but also atopic dermatitis (Suto et al., 1999; Sasakawa et al., 2001; Kubo et al., 2005). NC/Nga mice, originating from Japanese fancy mice (Nishiki-Nezumi) and established as an inbred strain by Kondo (Nagoya University, Nogoya, Japan), have certain characteristics such as high susceptibility to irradiation and to anaphylactic shock by OVA (Matsuda et al., 1997; Vestergaard et al., 1999).
In this study, we demonstrated for the first time the contribution of protein in PM to airway hyperresponsiveness (AHR) by PM in NC/Nga mice, which are potentially hypersensitive to mite allergens. Therefore, we evaluated the pathophysiological changes in the respiratory system by exposing mice to soluble and insoluble fractions, especially focusing on the effects of proteins in the soluble element of PM.
MATERIALS AND METHODS
Animals
Pathogen-free male (7-week-old) NC/Nga mice were obtained from Charles River Laboratories Japan (Yokohama, Japan). All mice were housed in a specific pathogen-free environment with a 12-h light and 12-h dark cycle. The mice were allowed to take water and food ad libitum. The care and handling of the animals were in accordance with the Guidelines for the Care and Use of Laboratory Animals at Okayama University and approved by an ethics committee.
Chemicals
Pronase was purchased from Roche Diagnostic GmbH (Mannheim, Germany). Oligonucleotide primers for reverse-transcription polymerase chain reaction (RT-PCR) were synthesized by TAKARA BIO (Otsu, Shiga, Japan). All other chemicals were of the highest quality that was commercially available.
Source of Air PM
Airborne PM of total suspended matter was collected with a high-volume sampler (HV-1000F, SHIBATA, Souka City, Japan) using a quartz filter (8 cm × 10 cm, 2500QAT-UP, Pallflex Products, Putnam, CT) at a flow rate of 1000 L/min continuously from March 2 to 7 and May 21 to 28, 2009 in Okayama city, Okayama Prefecture, Japan. The filters were stored in a freezer (−30°C) until pretreatment.
Separation of PM Fractions
Quartz fiber filters were cut and mixed with a vortex in 2 mM phosphate buffer (PB), pH 7.4, and left to stand for 20 min. Contaminated quartz fibers were sedimented naturally by gravity. Supernatant fractions were filtrated with a 41-μm nylon net filter (Millipore, Billerica, MA) and centrifuged at 5000 rpm for 10 min. After centrifugation, the supernatant (Sup) was concentrated by vacuum freeze drying. Water was added to the freeze-dried Sup, and then its protein concentration was adjusted. The weight of precipitated insoluble PM (Pre) was measured after vacuum drying, and it was then suspended in physiological saline solution or protein-containing Sup.
Measurement of Polynuclear Aromatic Hydrocarbons by GC-MS
Polynuclear aromatic hydrocarbons (PAHs) were eluted from insoluble PM using toluene (Wang et al., 2007). The determination of PAHs was carried out using a 7890A gas chromatograph equipped with a 5975C inert XL mass spectrometer (GC-MS; Agilent Technologies, Palo Alto, CA). The column was a 30 m × 0.25 mm ID DB-5 MS capillary column with 0.25-mm film thickness (Agilent Technologies). Helium was used as the carrier gas at a flow rate of 1.2 mL/min. The temperatures of the injection port and the transfer line were set at 300°C. The oven temperature was set at 75°C for 3 min and then increased to 250°C at a rate of 25°C/min and to 300°C at a rate of 5°C/min. Samples (1 mL) were injected in pulsed split mode (split ratio: 10:1, pulse pressure: 25 psi, and pulse time: 1 min). The mass spectrometer was operated in the electron impact mode at an electron energy of 70 eV. The ion source and quadrupole analyzer were maintained at 230 and 150°C, respectively. Data were obtained in scan mode with the scan ranging from m/z 40 to 450. EPA 610 Polynuclear Aromatic Hydrocarbons Mix (Supelco, Bellefonte, PA) was used for the standards for PAHs.
Measurement of Metals by ICPM
Metals of soluble or insoluble PM were digested in collision cell using Ethos 900 Milestone Digestor (Milestone General K.K., Kawasaki, Japan) in addition to nitric acid, hydrogen peroxide, and hydrofluoric acid, and digested metals were quantitatively or qualitatively measured using an Inductively Coupled Plasma Mass Spectrometer (ICPM-8500; Shimadzu, Tokyo, Japan).
Pronase Treatment of Supernatant
Protein digestion of Sup fraction was performed according to our previous study (Hitomi et al., 2007). Protein-containing Sup was digested with pronase (Sup protein: puronase protein = 1:0.75) in 0.1 M acetate buffer (pH 7.4) at 50°C for 14 h. After digestion, solution was filtered with Amicon ultra-free MC (Millipore) for 5 kDa.
Administration to Animals
Sample solution containing 200 μg of precipitate (Pre) in 25 μL of sterile PB (20 mM, pH 7.4) and 50-μg-protein-containing Sup solution or 200 μg of Pre suspended in 25 μL of 50-μg-protein-containing Sup solution (Sup plus Pre) was administered to NC/Nga mice by intranasal instillation on 5 consecutive days (experimental days 0–4) and on day 11 (single challenge) under anesthesia with pentobarbital, as shown in Figure 1 and as previously described (Shibamori et al., 2006). To determine the contribution of protein in Sup, 350 μg of Pre mixed with 100-μg-protein-containing Sup or 100-μg-protein-containing Sup digested with pronase was inoculated. Mice were killed on experimental day 14. For the control group, 25 μL of PB or 40 μg of quartz fiber in 25 μL of PB was administered because 40 μg of quartz fiber was found to be equivalent to 200 μg of Pre with the same experimental procedure using unused filter. Serum and whole lung tissue were collected for further analysis.
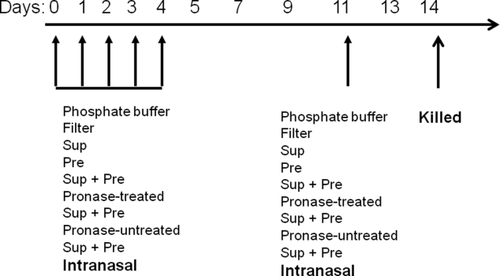
Schematic representation of the experiment. Twenty-five microliters of 20 mM PB pH 7.4 as buffer control, filter control (40 μg) in 25 μL of PB, supernatant fraction of PM (Sup) containing 100–200 μg of protein in 25 μL of PB, Pre (100–350 μg) in 25 μL of PB, or Sup plus Pre was administered intranasally for 5 consecutive days (days 0–4), and on day 11, a single challenge with the PM was administered intranasally to NC/Nga mice under anesthesia.
Analysis of Cells in the BALF
BALF was collected with 1.0 mL of saline after death using a tracheal cannula while gently massaging the thorax. After centrifugation, cell pellets were resuspended in 1 mL of phosphate-buffered saline (PBS) and total BALF cell counts were determined with a hemocytometer. BALF cytospins were prepared, slides were fixed in acetone, and then Wright–Giemsa staining was performed. A blinded observer determined the percentages of eosinophils, neutrophils, and mononuclear cells on each slide by counting a minimum of 200 cells in random high-power fields with a light microscope.
Measurement of AHR
On day 14, the degree of bronchoconstriction was measured according to the overflow method (Shibamori et al., 2006). Briefly, mice were anesthetized with pentobarbital (80 mg/kg) and connected to an artificial ventilator following surgical incision of the trachea. A pulmotor system was constructed with a rodent ventilator (Model 132; New England Medical Instrument, Medway, MA), a bronchospasm transducer (Model 7020; Ugo Basile, Comerio-Barese, Italy), and a data recorder (Omniace II data acquisition system, Model RA1300; NEC San-ei, Tokyo, Japan). Gallamine triethiodide (350 μg per mouse) was intravenously administered immediately to eliminate spontaneous respiration and followed by administrations of acetylcholine with stepwise increases in the concentration from 62.5 to 2000 μg/kg. Dose-response curves for acetylcholine in anesthetized, mechanically ventilated mice were obtained. Bronchoconstriction was expressed as the respiratory overflow volume provoked by acetylcholine as a percentage of the maximal overflow volume (100%) obtained by totally occluding the tracheal cannula (Nagai et al., 1993). Mice were suffocated by occluding the tracheal cannula.
RT-PCR for Cytokines in the Lung Tissue
Total RNA from lung tissue was isolated using ISOGEN with modifications of the manufacturer's instructions to strictly collect RNA. Briefly, the resultant RNA pellet was dissolved in ISOGEN again and washed with chloroform twice to remove contaminating DNA and proteins thoroughly. The RNA samples were stored at −80°C until the analyses. RT and PCR were performed using TaKaRa RNA PCR kit AMV ver. 3.0 (TAKARA BIO) with oligo(dT) primers according to the manufacturer's instructions. The sequences for the primers used in this study are listed in Table 1. PCR was performed using a Thermal Cycler MP (TAKARA BIO) with optimal thermal conditions and a cycle number specific for each primer set (indicated in Table 1). Electrophoresis was performed with a 2% agarose gel containing 0.5 μg/mL ethidium bromide. PCR bands were visualized using a UV transilluminator. The expression of each mRNA was analyzed using the Scion Image program (Scion, Frederick, MD). The results were compared as relative values using GAPDH as an internal reference.
| Target gene | Sense | Antisense | Denaturation | Amplification | Cycle | Elongation |
|---|---|---|---|---|---|---|
| IL-4 | CTAGTTGTCATCCTGCTCTTCTTT | CTTTAGGCTTTCCAGGAAGTCTTT | 94°C, 3 min | 94°C, 60 s 55°C, 75 s 72°C, 75 s | 40 | 72°C, 7 min |
| IL-5 | GTGAAAGAGACCTTGACACAGCTG | CACACCAAGGAACTCTGCAGGTA | 94°C, 3 min | 94°C, 60 s 55°C, 75 s 72°C, 75 s | 35 | 72°C, 7 min |
| IL-13 | CTCCCTCTGACCCTTAAGGA | GAAGGGGCCGTGGCGAAACAG | 94°C, 3 min | 94°C, 60 s 55°C, 75 s 72°C, 75 s | 35 | 72°C, 7 min |
| IFN-γ | TGTTACTGCCACGGCACAGTCATT | GTGGACCACTCGGATGAGCTCATT | 94°C, 3 min | 94°C, 60 s 55°C, 75 s 72°C, 75 s | 35 | 72°C, 5 min |
PM Protein-Specific IgG and IgE
After inoculation of PM or control, on experimental day 14, blood was collected at the end of the AHR experiment. Serum was separated by centrifugation and used for the assay of IgG or IgE against PM containing proteins. Immuno Microsorp 96-well microtiter plates (Nunc, Roskide, Denmark) were coated with 2 μg/100 μL/well of Sup overnight at 4°C. After blocking with PBS containing 5% fetal bovine serum (FBS) and washing with PBS containing 0.05% Tween 20, serum samples diluted in PBS containing 5% FBS were added to the plate and incubated overnight at 4°C. The bound IgG or IgE was quantitated colorimetrically using biotinylated anti-mouse IgG (Zymed, San Francisco, CA), HRP-streptavidin conjugate (Zymed), and 3,3′,5,5′-tetramethylbenzidine substrate solution (COSMOBIO, Tokyo, Japan). Results were expressed as absorbance at 450 nm after subtraction of the background.
Histological Experiments
After exsanguination, the lungs were cut and fixed using 10% neutral phosphate-buffered formalin and were embedded in paraffin. The fixed lung tissues were dehydrated, embedded in paraffin, and sectioned. Hematoxylin and eosin (H&E) staining was used to assess the degree of inflammation (Lee et al., 2006). Periodic acid-Schiff (PAS) stainings were also conducted to observe interstitial collagen deposition and goblet cell hyperplasia (Takemoto et al., 2007). Slides were evaluated as grades in a blind fashion using a reproducible scoring system (Curtis et al., 1991). The levels of inflammation in peribronchial and perivascular spaces of the lung were recorded on an ordinal scale ranging from 0 to 3, as described elsewhere (Tournoy et al., 2000). A value of 0 meant that no inflammation was detectable, a value of 1, occasional cuffing with inflammatory cells, a value of 2, most bronchi or vessels were surrounded by a thin layer (one to five cells thick) of inflammatory cells, and a value of 3, most bronchi or vessels were surrounded by a thick layer (more than five cells thick) of inflammatory cells.
Statistical Analysis
The results are presented as means ± SD. If the difference evaluated with one-way or two-way analysis of variance (ANOVA) was significant, Bonferroni post hoc tests or Dunnett's test was used for paired comparisons. A two-tailed unpaired t-test was used to compare with control values, using GraphPad Prism 5.0c for Mac (GraphPad Software, San Diego, CA). A P value less than 0.05 was considered statistically significant.
RESULTS
PM Characterization
Measurement of metals and PAHs in PM is shown in Table 2. Ambient PM contained high levels of zinc, lead, iron, manganese, and aluminum. As for organic components in PM shown in Table 2, high levels of fluoranthene, benzo(b, k)fluoranthracene, indeno(1,2,3-cd)pyrene, benzo(a)pyrene, chrysene, pyrene, and benzo(g, h, i)perlene were detected.
| Metal | Concentrations (μg/g) | Organic compound | Concentrations (ng/g) |
|---|---|---|---|
| Alminium | 7275.7 | Acenaphthene | 624.4 |
| Arsenic | 825.6 | Acenaphthylene | 654.2 |
| Berylium | 10.9 | Anthracene | 1698.1 |
| Cadmium | 208.9 | Benzo(a) anthracene | 15,005.5 |
| Chromium | 889.3 | Benzo(b,k) fluoranthene | 87,234.5 |
| Copper | 4419.8 | Benzo(g,h,I) perylene | 34,495.0 |
| Iron | 11,846.2 | Benzo(a)pyrene | 40,84.6 |
| Lead | 12,140.4 | Chyrsene | 34,968.2 |
| Manganase | 11,379.3 | Dibenzo(a,h) anthracence | 4986.2 |
| Nickel | 1277.4 | Fluorathene | 411,174.3 |
| Selenium | 494.8 | Fluorene | 1946.3 |
| Uranium | 17.7 | Indeno(1,2,3-cd)pyrene | 66,765.6 |
| Vanadium | 1517.4 | Naphthalene | 653.7 |
| Zinc | 18,731.2 | Pyrene | 34,667.8 |
Effect of PM on AHR
Bronchoconstriction values for AHR of the filter control, Sup, and Pre did not show any significant values compared with that of the buffer control at acetylcholine doses ranging from 62.5 to 4000 μg/kg; however, bronchoconstriction values of Sup plus Pre against 2000 and 4000 μg/kg acetylcholine were significantly higher than that of the buffer control [Fig. 2(A)]. Augmented AHR in the mice exposed to 100-μg-protein-containing Sup and 350 μg Pre was significantly reduced in the mice exposed to pronase-treated Sup and 350 μg Pre [Fig. 2(B)].
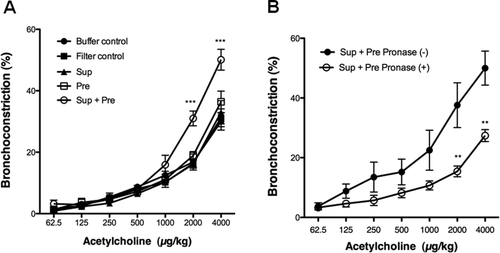
(A) AHR to acetylcholine after exposure to PM. Buffer control (20 mM PB, pH 7.4), filter control (40 μg/25 μL of PB), Sup (supernatant fraction containing 100 μg of protein), Pre (200 μg of Pre in 25 μL of PB), and Sup plus Pre were administered intranasally in NC/Nga mice. The bronchoconstriction (%) is expressed as mean ± SE of six to eight animals. Two-way ANOVA showed P < 0.0001 concerning responses to acetylcholine concentration and mouse groups. ***P < 0.001 represents a significant Dunnett's test result of the dose responses to acetylcholine in each mouse group vs. the mouse group of the buffer control. (B) Effect of pronase treatment in Sup on AHR to acetylcholine. Sup (200 μg of protein) plus Pre (350 μg) or pronase-treated Sup (200 μg of protein) plus Pre (350 μg) was administered. The bronchoconstriction (%) is expressed as mean ± SE of six to eight animals. Two-way ANOVA showed P < 0.0001 concerning responses to acetylcholine concentration and mouse groups. **P < 0.01 represents multiple-comparison test result of the dose responses to acetylcholine in the mouse group of Sup plus Pre vs. pronase-treated mouse group of Sup plus Pre.
Effect of PM on BALF Cell Analysis
Total cell numbers in BALF were significantly increased in mice inoculated with Pre or Sup plus Pre compared with those of mice exposed to buffer control, filter control, Sup, and Pre. The increase in total cell numbers in BALF in mice exposed to Pre or Sup plus Pre was dependent on macrophages and lymphocytes [Fig. 3(A)]. The increase in BALF cell numbers in mice exposed to 100-μg-protein-containing Sup and 350 μg Pre did not significantly change in mice exposed to pronase-treated Sup and 350 μg of Pre [Fig. 3(B)].
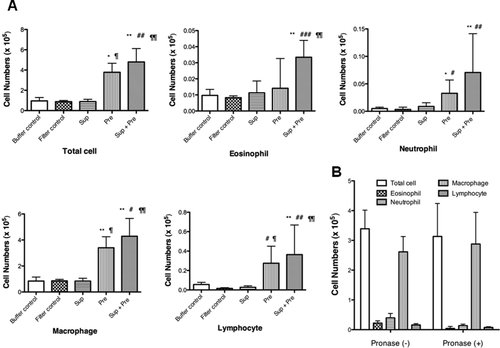
(A) BALF cell numbers after exposure to buffer control, filter control, supernatant (Sup) containing 100 μg of protein, 200 μg of Pre, and Sup plus Pre. (B) Effect of pronase treatment in Sup on BALF cell numbers. Sup (200 μg of protein) plus Pre (350 μg) or pronase-treated Sup (200 μg of protein) plus Pre (350 μg) was administered. The counts of total cells, eosinophils, neutrophils, macrophages, and lymphocytes in the BALF are expressed as mean ± SD of six to eight animals. Multiple-comparison test results among each group are shown by *P < 0.05 and**P < 0.01, which represents each group vs. buffer control group, by #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. filter control, and by ¶¶P < 0.01 vs. Sup group.
Effect of PM on Expression of mRNA for Cytokines in the Lung Tissue
The expression of mRNA of several cytokines was evaluated with PCR (Fig. 4). Prominent upregulation, ∼ 2.9 times higher than that of the buffer control, was observed for the mRNA of IL-4 in mice exposed to Sup plus Pre. Upregulation of Il-4 mRNA was also observed by Sup. Upregulation of mRNA in IL-5 and IL-13 was also detected in mice exposed to Sup, Pre, and Sup plus Pre. However, there was no difference in mRNA levels of IL-5 and IL-13 among mice exposed to Sup, Pre, and Sup plus Pre. Regarding interferon-gamma (IFN-γ), expression of this cytokine was significantly suppressed in mice exposed to Sup and Pre compared with those of buffer control and filter control. However, mixed inoculation of Sup and Pre reversed mRNA expression of IFN-γ to the control level.
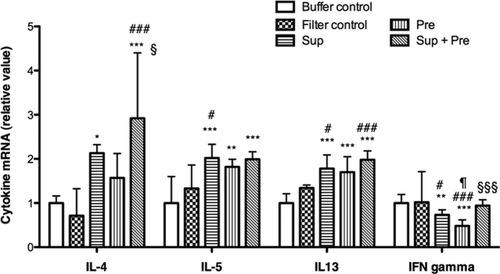
Changes in mRNA expression for cytokines in the lung tissue after exposure to PM. Mice were administered with buffer control, filter control, supernatant (Sup) containing 100 μg of protein, 200 μg of Pre, and Sup plus Pre. RT-PCR for the cytokines IL-4, IL-5, IL-12, IL-13, and IFN-γ was performed under optimized conditions. Relative expression was calculated with GAPDH as an internal standard. Data are expressed as the means ± SD from six to eight mice. Multiple-comparison test results among each group are shown by *P < 0.05, **P < 0.01, and ***P < 0.001 vs. buffer control group, by #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. filter control group, by ¶P < 0.05 vs. Sup group, and by §P < 0.05 and §§§P < 0.001 vs. Pre group.
Expressions of mRNA for IL-4, Il-5, IL-13, and IFN-γ were compared between two mouse groups exposed to 100-μg-protein-containing Sup plus 350 μg of Pre or to pronase-treated Sup plus 350 μg of Pre (Fig. 5). Expression of IL-4 mRNA was significantly suppressed by the treatment of Sup with pronase. However, mRNA levels for IL-5, IL-13, and IFN-γ were not changed by the treatment of Sup with pronase.
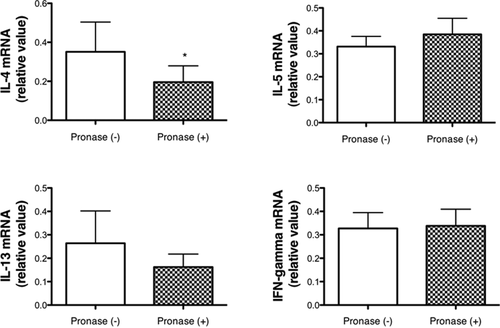
Changes in mRNA expression for cytokines in the lung tissue between mouse groups exposed to Sup (200 μg of protein) plus Pre (350 μg) and pronase-treated Sup (200 μg of protein) plus Pre (350 μg). Data are expressed as the mean ± SD from six to eight mice. Unpaired t-test or Mann–Whitney test was performed, and a significant result between two groups is shown by *P < 0.05.
PM Protein-Specific IgG and IgE
Serum levels of IgE and IgG for proteins of Sup in mice inoculated with buffer control, filter control, Sup, Pre, and Sup plus Pre are shown in Figure 6. Serum IgE against proteins in Sup of PM significantly increased in mice inoculated with Sup and Pre compared with mice inoculated with buffer control. Serum IgG for proteins in Sup of PM significantly increased in mice inoculated with Sup plus Pre compared with mice inoculated with buffer control and Pre.
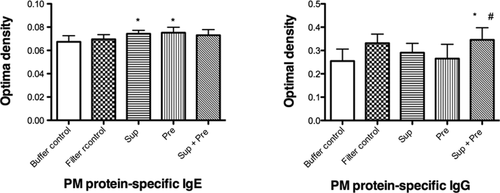
Changes in serum IgE and IgG specific for proteins in PM after exposure to PM. Mice were administered with buffer control, filter control, supernatant (Sup) containing 100 μg of protein, 200 μg of Pre, and Sup plus Pre. Data are expressed as the means ± SD from six to eight mice. Multiple-comparison test results among each group are shown by *P < 0.05 vs. buffer control group, by #P < 0.05 vs. Pre group.
Effect of PM on Histopathological Changes of the Lung
Peribronchial and perivascular inflammation as well as goblet cell hyperplasia were observed in the lungs of Sup plus Pre-inoculated mice [Fig. 7(E,J)]. In contrast, no inflammation [Fig. 7(A–D)] and few PAS-positive epithelial cells [Fig. 7(F–I)] were observed in the lungs of mice exposed to buffer control, filter control, Sup, and Pre. Inflammatory scores in peribronchial and perivascular spaces and PAS-positive goblet cells in the bronchial epithelium were significantly increased in the lungs of Sup plus Pre-inoculated mice [Fig. 7(K–M)]. The synergistic effects of Sup plus Pre were observed in PAS staining. Marked inflammation and increased number of PAS-positive goblet cells observed in 100-μg-protein-containing Sup plus 350 μg of Pre were reduced by the treatment of Sup with pronase [Fig. 8(A–D)]. This reduction was also indicated by scores of peribronchial inflammation and PAS staining [Fig. 8(E–G)].
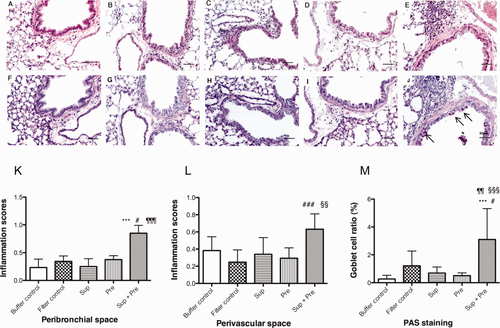
Histopathological findings of the lungs after exposure to PM. Paraffin-embedded sections were stained with H&E [A, buffer control; B, filter control; C, supernatant (Sup) containing 100 μg of protein; D, 200 μg of Pre; E, Sup plus Pre] and the PAS technique (F, buffer control; G, filter control; H, Sup; I, Pre; J, Sup plus Pre). Bars indicate 100 mm. Inflammatory scores of the lung in peribronchial space (K) and perivascular space (L) of the mice exposed to PM are presented. Goblet cell ratio of the mice exposed to PM is shown in M. Data are expressed as the mean ± SD from six to eight mice. Significant differences by multiple-comparison test among each group are presented as ***P < 0.001 vs. buffer control group, #P < 0.05, ###P < 0.001 vs. filter control group, ¶¶P < 0.01, ¶¶¶P < 0.001 vs. Sup group, and §§P < 0.01, §§§P < 0.001 vs. Pre group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
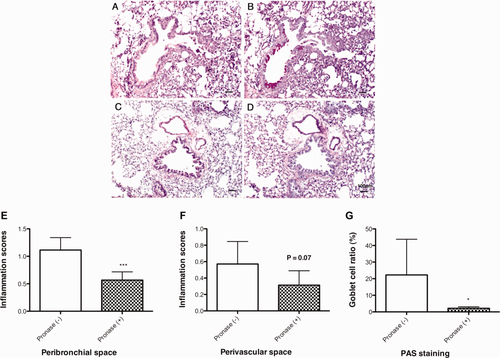
Histopathological changes of the lungs of mice exposed to pronase-treated or nontreated Sup plus Pre. Paraffin-embedded sections were stained with H&E (A, Sup containing 200 μg of protein plus 350 μg of Pre; C, pronase-treated Sup plus 350 μg of Pre) and the PAS technique (B, Sup containing 200 μg of protein plus 350 μg of Pre; D, pronase-treated Sup plus 350 μg of Pre). Inflammatory scores of the lung in peribronchial space (E) and perivascular space (F) of PM-exposed mice are shown. Goblet cell ratio is shown in G. Data are expressed as the mean ± SD from six to eight mice. Significant differences by unpaired t-test are presented as ***P < 0.001 and *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Ambient PM is a mixture of particles of various sizes from less than 0.001 μm to pollen and spores of between 2 and 50 μm (Salvaggio, 1994). The particle size associated with adverse respiratory function is said to be less than 10 μm (Choudhury et al., 1997). The terms PM10 (PM < 10 μm in mean aerodynamic diameter) and suspended PM (SPM) are often used to describe these particles. Moreover, the fine fraction (<2.5 μm in diameter), often referred to as PM2.5, has been focused on because of its penetration into the alveolar space. Therefore, in this study, inoculated PM filtrated with a 41-μm Millipore filter was consisted of suspended particles of PM2.5, PM10 and larger particles (Wilson et al., 2002).
Five hundred micrograms of intratracheal instillation of AUB particulate succeeded in inducing asthma in mice (Walters et al., 2001; Wang et al., 2008; Saunders et al., 2010). Intratracheal administration dose of DEPs was 125, 200, or 800 μg per mouse (Ichinose et al., 1995; Lim et al., 1998; Yanagisawa et al., 2003). Moreover, compared with intratracheal instillation, intranasal administered allergens might be not completely transferred to the airway, although it is not clear what percent is transferred to the esophagus of the GI tract; therefore, the 200 or 350 μg intranasal administration dose in this study was not high. Moreover, AHR stimulated by 1000 and 2000 μg/kg acetylcholine was augmented in mice inoculated with 350 μg Pre plus Sup containing 200 μg protein compared with mice inoculated with 200 μg Pre plus Sup containing 100 μg protein. From the analysis of BALF cells and the expressions of cytokines, Sup and Pre were effective together; therefore, although it is not clear which factor, Sup or Pre, contributed to the augmentation of AHR, a dosage of 350 μg Pre plus Sup containing 200 μg protein was selected in this study.
Concerning the exposure route, intranasal administration of PM, OVA, or allergens has been established in many mice asthma models (Kang et al., 2010; Li et al., 2010; Huang et al., 2011; Mori et al., 2011; Enomoto et al., 2012). Compared with intratracheal instillation, intranasal administration is an easy technique and suitable for successive administration, although, as mentioned above, a disadvantage of intranasal administration is that dose of the administered allergens into the airway is inaccurate.
We established experimental asthma by intranasal inoculation of extracts from Dermatophagoides farinae in NC/Nga mice (Shibamori et al., 2006). NC/Nga mice are known to easily show allergic reactions to mite allergens, which resulted in atopic-like dermatitis (Suto et al., 1999; Sasakawa et al., 2001; Kubo et al., 2005) and asthma (Shibamori et al., 2006; Takemoto et al., 2007; Takahashi et al., 2010). In our previous study using NC/Nga mice, OVA did not induce airway allergic reactions (Shibamori et al., 2006). Therefore, some aeroallergens may be involved in this airway allergic reaction because aeroallergens are known to be airborne allergens and usually consist of airborne particles, the sources of which are mite feces and pollen grains (Utell and Looney, 1995; Platts-Mills, 1997); on the surface of SPM, well-known allergens such as Fel d 1 (cat), Can f 1 (dog), and Bet v 1 (birch pollen) could attach, but Der p 1 could not (Ormstad, 2000), although we did not measure these allergens in soluble Sup and insoluble Pre, and it is not clear whether these allergens can induce an airway allergic reaction in an NC-Nga mouse model.
The direct eliciting effects of PM or airway allergic reactions in an animal model have been reported. Metal and sulfate composition of residual oil fly ash contributed to the development of airway hypersensitivity and lung injury in rats (Gavett et al., 1997) and AUB PM-induced AHR and inflammation in mice (Walters et al., 2001). The contribution of metals in PM-induced pulmonary injury was associated with an increase in eosinophils (Costa and Dreher, 1997). The mechanism of AUB PM-induced AHR in mice is unknown. However, a single aspiration of 0.5 mg of PM induced AHR at 6 h after exposure, which continued for at least 7 days, and a water-soluble fraction was not associated with AHR. In this study, high levels of transition metals such as iron, manganese, and copper were detected in PM compared with the levels in Baltimore PM because of the larger size of PM in this study. Transition metals have a specific chemical property of redox cycling (Aust et al., 1985), which may contribute to AHR and pulmonary injury through the production of reactive oxygen species by neutrophils, eosinophils, macrophages, and epithelial cells. Different from copper and manganese, zinc cannot show one electron cycling. However, high levels of zinc in residual oil fly ash are considered to contribute to AHR and neutrophil inflammation (Gavett et al., 1997). Our results showed high levels of zinc in PM. Pronase treatment of Sup is not related to the removal of metals. Therefore, although metal levels in pronase-treated Sup were not determined, it was suggested that the contribution of metals was not substantial in this study, and the mechanisms for AHR in this study may be different from those of our previous study.
PAHs in organic fractions of AUB have been considered to be associated with T-cell activation (Walters et al., 2001). High levels of PAHs were contained in water-insoluble Pre. Pre may contribute to increases in macrophages and lymphocytes in terms of BALF cell numbers and upregulation of mRNA of IL-5 and IL-13. However, regulation of mRNA for IL-5 and IL-13 was also increased by Sup in addition to Pre, and the degree of increase of mRNA of IL-5 and IL-13 was not large compared with that of IL-4. The regulator of IL-5 and IL-13 may be included in both Sup and Pre. Therefore, it is likely that PAHs are not main participants in the increase of IL-3 and IL-5.
In the results of AHR, mRNA of IL-4, histological inflammatory scores, and PAS staining, synergistic or synergistic-like action of Sup and Pre for Sup plus Pre was observed. This synergistic or synergistic-like action of Sup and Pre almost matched with the attenuated results of pronase-treated Sup plus Pre. Sup was treated with pronase at 50°C for 12 h and filtered with Amicon ultra-free MC for 5 kDa. By these procedures, proteins in Sup may be digested to amino acids and reserved Sup or pronase proteins may be denatured or be trapped by Amicon filter, although we did not determine the unchanged state of metals in pronase-treated Sup. Moreover, PM such as DEP is said to induce airway inflammation by a synergistic action with an endotoxin (Yanagisawa et al., 2003). Carbonaceous nuclei of DEP might contribute to this synergistic action. Therefore, some protein in Sup may be associated with AHR, inflammation, and glycogen synthesis via upregulation of IL-4 mRNA by synergistic action with Pre although some soluble factor such as endotoxins other than proteins cannot be ruled out. Serum levels of IgG and IgE specific for proteins in Sup were investigated in mice inoculated with PM. There is a significance in serum IgG and IgE levels among these inoculated mice groups. Concerning IgE, although the increased range was not so large, mice inoculated with Sup and Pre showed significantly increased levels compared with buffer control. This result implies that some common protein between Sup and Pre exists and is sensitized although PM protein-specific IgE might not be involved in the airway inflammation in this study. Moreover, PM-specific IgG increased in mice serum of Sup plus Pre compared with buffer control and Pre. This result cannot explain the completely synergistic effect of Sup and Pre. However, when we collected serum at later days in this experiment after the last challenge inoculation, higher titers of IgG may be detected in association with AHR and airway inflammation.
Upregulation of Th-2 cytokines and a downregulation of Th-1 cytokines are frequently observed in allergic reactions. Endotoxins induce upregulation of inflammatory cytokines such as IL-1β, IFN-γand TNF-α (Ulich et al., 1991; Johnston et al., 1998) and chemokines such as MIP-1 and MCP-1 (Speyer et al., 2004). DEP suppresses IFN-γ (Finkelman et al., 2004) and increases TNF-α (Saber et al., 2006). The suppression of IFN-γ by Sup or Pre in this study leads to speculation over the contribution of upregulated Th-2 cytokines and denies the involvement of endotoxins. However, to achieve a proper conclusion, further investigations of cytokines such as Il-1, IL-2, and TNF-α as well as chemokines such as MIP-1 and MCP-1 are needed.
Pronase treatment of Sup did not show a significant reduction in BALF cell numbers, although a reduction in inflammation was observed in the histopathological examination. Therefore, there seems to be a discrepancy, with no clear explanation. However, there was a decreasing tendency in eosinophil and neutrophil numbers because of treatment with pronase of Sup showing P = 0.057 and P = 0.076, although the P values of macrophages and lymphocytes are 0.812 and 0.183, respectively. A significant influx of eosinophils into BALF was observed soon after rIL-13 administration; however, pulmonary eosinophils were not observed at the time of expression of AHR (Wills-Karp et al., 1998). In this study, pronase treatment with Sup suppressed mRNA expressions of IL-4. An IL-4 signal is transferred via a common receptor with IL-13. Anti-IL-13 mAb partially suppressed eosinophil infiltration into the airway (50%) without affecting other cell types, albeit with an apparent suppression of histological inflammatory cell infiltration (Yang et al., 2004). A time lag between BALF cell infiltration, pulmonary cell infiltration, and cytokines such as IL-4 or IL-13 may be associated with this discrepancy.
In this study, we demonstrated for the first time that ambient PM could directly elicit AHR by synergistic action of soluble protein-like factor and insoluble particles in PM in NC/Nga mice. Our results suggest that some allergens in atmospheric environment may contribute to direct allergic airway reaction in genetically highly sensitive mice and may be associated with asthma attack. Therefore, the factors contributing to airway allergic reaction in soluble or insoluble PM should be investigated in the future.




