Modification of Starches with Different Amylose/Amylopectin-Ratios Using the Dual Approach with Hydroxypropylation and Subsequent Acid-Thinning—Impacts on Morphological and Molecular Characteristics
Abstract
Commercial starches with different amylose (AM)/amylopectin (AP)-ratios (waxy potato: WxPS, regular potato: PS, high AM corn: HACS) are hydroxypropylated (HP, pilot plant scale, two levels) and subsequently acid-thinned (AT, laboratory scale) to produce dual-modified samples. The gradation of the molar substitution (MS) obtained is significant for each starch basis. Investigation by means of scanning electron microscopy reveals a basically intact granular structure with surface defects. The molecular properties are comprehensively characterized using size exclusion chromatography-multi angle laser light scattering-differential refractive index detection (SEC-MALS-DRI) and corresponding conventional calibration (SEC-cal-DRI). The evaluation of the molecular data (MS and weight average molar mass, Mw) by means of statistical analysis (ANOVA) identifies statistically significant impacts with respect to the starch type, the substitution level, and the AT treatment.
1 Introduction
The natural polymeric carbohydrate starch is mainly composed of glycosidic linked anhydroglucose units (AGU) building the two main molecule structure fractions amylose (AM, essentially linear) and amylopectin (AP, highly branched). Most regular starches, based, for example, on potato, wheat, and corn, respectively, have an AM content of about 25 ± 5% w/w and a corresponding AP percentage of 75 ± 5% w/w. Besides, several genotypes with an AM/AP-ratio differing from that are commercially available. For example, some waxy starches consist of nearly 100% w/w AP, and the so-called high AM varieties contain up to about 75% w/w AM on the other hand.[1] The development of an AM-only variety (barley) was reported a few years ago.[2] The starch polymers are generally synthesized in the form of granules having a semi-crystalline structure with alternating crystalline (preferential crystallized AP branch chains sections) and amorphous (preferential AP branch section bearing the α-1,6-linkages) lamellae.[3, 4] Further structural levels contain the blocklets[5, 6] as well as alternating more crystalline and less crystalline shells (layers), which are also referred to as crystalline and amorphous shells, although neither is entirely one or the other.[7] Different starch types, that is, obtained from different plant sources or genotypes, can vary in terms of, for example, granule morphology and size,[8, 9] crystalline type and relative crystallinity,[10] and the molecular properties of both the AM and the AP fraction.[11-14] Moreover, several physicochemical and functional properties like, for example, swelling and gelatinization characteristics (differential scanning calorimetry,[15] rapid visco analyzer[16]), hot paste viscosity, light transmittance of the aqueous solution or the gelation capability depend strongly on the starch type and the AM/AP-ratio, respectively.[17]
Starch products are widely used for several industrial food applications (e.g., gelling agent). However, because of its limited applicability in the native form, a multiplicity of mostly chemical modifications is established. Each specific modification controls the techno-functionality of the modified starch and can potentially improve the respective characteristic of a food system in a noteworthy way. Hydroxypropylated (HP; etherified, reaction with propylene oxide, granular state) and acetylated (esterified, reaction with acetic anhydride, granular state) are so-called “stabilized starches,” since they have a reduced tendency to undergo retrogradation due to the incorporation of bulky substituent groups (spacer) along the polymer chains, that hinder (partially) intermolecular associations.[18] Within the AGU, the preferential molar substitution (MS) of the C-2 was proved earlier for different HP starches (wheat, corn, waxy corn, high AM corn).[19] For samples with MS values between 0.08 and 0.32, a substitution of 90–97% was found on C-2, and on the primary hydroxyl group on C-6 position of 10–3%. However, the relative substitution of C-3 was negligible. Experiments based on potato and corn starch revealed that AM is modified to a greater extent than AP.[20, 21] The modification of the AP occurs close to branch points, most probably because the higher accessibility of the amorphous regions to the modifying reagent (higher levels of substituents present in the amorphous regions and periphery of clusters).[22] Accordingly, the hydroxypropyl substituents are located in the amorphous lamellae (branched zone) and amorphous regions between the clusters of the AP side chains in the crystalline lamellae. Substituted branching points of the AP make them (partially) resistant to degradation by pullulanase. It seems evident that AM, likewise, is not uniformly modified.[20] A molecular degradation due to the chemical reaction of the starch with propylene oxide per se is not accepted, but the processing after modification (e.g., different drying technology) can impact the molecular properties of the final HP starch.[23] In any case, in contrast to the native starch, the incorporation of the substituents (hydroxyl groups) and reduction of the intermolecular interaction, explain the reduced capability to gel in the same manner, and the enhanced light transmittance of an aqueous starch solution, increased freeze-thaw-stability[24] as well as increased enzymatic digestibility (granular state).[25] In contrast to stabilized products, acid-thinned (AT) starches, which underwent a partial molecular degradation by specific hydrolysis in the granular state (mineral acids), are known to have improved gelation characteristics and the ability to form mechanically stable gels on the one hand, and a (drastically) reduced hot paste viscosity on the other hand.[26, 27] However, the necessity of a specific molar mass (MM) range of the AT starch to implement the capability to develop a 3D polysaccharide network consisting mostly of retrograded AM[28] and embedded AP[29] was demonstrated recently for a normal potato starch (PS).[30, 31] The character of the specific acid modification seems to be a compromise between size reduction of the AP (desired) by the chain cleavage and simultaneously occurring molecular degradation of the AM fraction (non-desirable). The general approach of a multiple starch modification, that is, two or more modifications consecutively, is established and known from industrial products (e.g., E 1451) at the same time and the focus of current scientific research.[32-40]
The present study deals with the systematic modification of starches having different AM/AP-ratios (waxy, normal, and high AM) using the dual approach. The starches were modified using HP first and subsequently acid hydrolyzed (AT) aiming in particular at improving of the application properties as a gelling agent. It is hypothesized, that the advantages of both the enhanced clarity (HP) and the improved gelation acquirement (AT) can be implemented in the starch synergistic. Additionally, the effect of the inherent characteristics of the initial starch on the functionality, which is profoundly controlled by the AM/AP-ratio (starch type), is of great interest. This is the first of a series of publications and focused on the impact of the modification on the granules’ morphology and the molecular properties of the starch polysaccharides.
2 Experimental Section
2.1 Native Starch Samples
Commercial native waxy potato starch (WxPS; Empure AKS 100), commercial native regular potato starch (PS; Superior), and commercial native high AM corn starch (HACS; Hylon VII) were used as initial starch material for the experiments. The potato based starches were provided by Emsland-Stärke GmbH (Emlichheim, Germany) and the corn starch genotype by Ingredion Germany GmbH (Hamburg, Germany). The AM contents were 0% w/w (WxPS, supplier information), 24.3 ± 0.2% w/w (PS, determined elsewhere[41]), and 73.8% w/w (HACS, supplier information). The dry matter content (DM) was determined using a moisture analyzer (MA 30, Sartorius, Göttingen, Germany), and the protein as well as lipid content were supplier information (WxPS: 80.75% w/w DM, 0.1% w/w protein, 0.1% w/w lipid; PS: 81.24% w/w DM, 0.1% w/w protein, 0.1% w/w lipid; HACS: 88.37% w/w DM, 0.5% w/w protein).
2.2 Modification
Based on the native starch samples (WxPS, PS, and HACS) with significantly different AM/AP ratios the modification was executed in two steps. Initially the starches were hydroxypropylated (HP) and the acid-thinned (AT), respectively (single modification). The HP samples were subsequently AT (dual modification). The procedure is shown in Figure 1. All samples were stored in closed containers at 20 ± 2 °C.
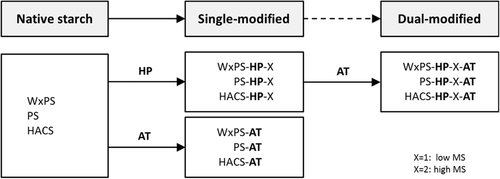
2.2.1 Single Modification
The single modified starch samples were prepared using either the HP at two different levels (pilot plant scale) or the AT process (laboratory scale).
Hydroxypropylation
The native starches (WxPS, PS, and HACS) were hydroxypropylated in the pilot plant scale (Emsland-Stärke GmbH, Emlichheim, Germany). The samples were prepared by reaction in suspension (40% w/w; temperature: 35 °C; reaction time: 24 h) with propylene oxide to two different levels of molar substitution (MS) by grading the amount of propylene oxide added. Na2SO4 was added before adding propylene oxide to inhibit swelling of the starches. The pH-value was adjusted by means of NaOH and H2SO4, respectively. The denotations were -HP-1 and -HP-2 referring to low and high MS, respectively. The MS values are summarized in Table 1.
| Native | HPa) | |||
|---|---|---|---|---|
| DMb) [%] | DM [%] | MSc) | ||
| WxPS | 80.75 | WxPS-HP-1 | 81.45 | 0.08 |
| WxPS-HP-2 | 84.45 | 0.19 | ||
| PS | 81.24 | PS-HP-1 | 83.30 | 0.10 |
| PS-HP-2 | 83.41 | 0.17 | ||
| HACS | 88.37 | HACS-HP-1 | 87.35 | 0.04 |
| HACS-HP-2 | 84.84 | 0.13 | ||
| ATd) | HP-ATe) | |||
| WxPS-AT | 92.28 | WxPS-HP-1-AT | 92.55 | 0.07 |
| WxPS-HP-2-AT | 89.37 | 0.16 | ||
| PS-AT | 92.00 | PS-HP-1-AT | 92.71 | 0.08 |
| PS-HP-2-AT | 90.85 | 0.13 | ||
| HACS-AT | 92.58 | HACS-HP-1-AT | 92.62 | 0.04 |
| HACS-HP-2-AT | 89.54 | 0.09 | ||
- a) Hydroxypropylated starch samples; single modification;
- b) Dry matter content;
- c) Molar substitution;
- d) Acid-thinned starch samples; single modification;
- e) Acid-thinned hydroxypropylated starch samples; dual modification.
Acid Thinning
AT modification was performed in slurry according to the description elsewhere with modifications.[42] An acid concentration of 0.6 m was adjusted in the continuous phase of the slurry by adding a volume of 144 mL of 1 m HCl to reach a total mass of 400 g suspension (40% w/w). The hydrolysis was performed for 24 h at 30 °C and with continuous stirring (250 min−1). The reaction was stopped by adding a volume of 144 mL of 1 m NaOH (pH: 5.5–6.0). The denotation of the samples was -AT.
2.2.2 Dual Modification
The HP starches (single modification) were the basis for the preparation of the dual-modified samples. The HP samples were acid-thinned according to the description in 2.2.1.
The denotations and the DM contents of the modified starches are summarized in Table 1.
2.3 Morphological Characterization—Scanning Electron Microscopy
The SEM micrographs were taken with a ZEISS Gemini SEM500 NanoVP microscope (Carl Zeiss AG, Oberkochen, Germany) with a wolfram cathode. The measuring distance was 9.9 mm and the accelerating voltage was adjusted to 14 kV. The micrographs were taken at a magnification of 1200 (WxPS and PS) and 1500 (HACS), respectively. The samples were affixed with double-faced adhesive tape on the sample carrier and sputtered with gold (Au) with a layer thickness of about 3 nm (SCD 030, Balzers Union, Balzers, Liechtenstein).
2.4 Molecular Characterization
2.4.1 Determination of the Molar Substitution
The molar substitution (MS) of the HP and HP-AT samples was determined according to the description of Lawal[25] with modifications. HP starch samples (0.300 g) were weighed and 0.5 m H2SO4 (25 mL) was added. The mixture was placed in a boiling water bath until a clear solution was obtained (WxPS and PS: 15 min; HACS based samples: 60 min). The solution was cooled and filled up to 100 mL with water. An aliquot (1 mL) was pipetted into 25 mL graduated test tubes (with ground-in glass stoppers), immersed in cold water, and 8 mL of concentrated H2SO4 was added drop wise to the tube. After thorough shaking, the tubes were placed in a boiling water bath for 20 min and subsequently cooled in an ice bath. Ninhydrin reagent (3 g 100 mL−1 ninhydrin in 5 g 100 mL−1 Na2S2O5, 0.6 mL) was added and the tubes were placed in a water bath (25 °C) for 1 h. The solutions were filled up to 25 mL with concentrated H2SO4 and thoroughly mixed. After 10 min, the absorbance was measured at λ = 590 nm. The starch blank (native) was used as reference. A calibration curve was prepared with an aliquot (1 mL) of standard aqueous solutions, containing 10, 20, 30, 40, and 50 mg mL−1 propylene glycol. The propylene glycol concentration in the starch was calculated from the standard curve and converted to equivalent hydroxypropyl groups from each molar solution. The MS was ascertained in triplicate determination.
2.4.2 Characterization by Means of Size Exclusion Chromatography Techniques
Preparation of Starch Solutions
Starch solutions for the molecular characterization were prepared by heating aqueous dispersions of 2.5% w/w in an autoclave (Model I, Carl Roth GmbH & Co. KG, Karlsruhe, Germany) to 145 °C under continuous stirring (300 min−1) for 30 min and subsequent high-shear-treatment using an Ultra-Turrax T25 (IKA-Werke GmbH & Co. KG, Staufen, Germany) at 24 000 min−1 for 2 min at about 80 ± 5 °C. The pastes were diluted 1:10 v/v in preheated DMSO to a concentration of about 2.5 mg mL−1. The stabilized solutions were passed through 5 µm PTFE filters (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) before analysis (SEC-MALS-DRI, starch total, no enzymatic treatment).
Additionally, enzymatic debranching of the starch polysaccharides was performed using pullulanase (PromozymeD2, Novozymes A/S, Bagsvaerd, Denmark) as described elsewhere.[43] The enzyme solution contained potassium sorbate and sodium benzoate as preservatives and sucrose as well as glucose as stabilizers (biological origin: microorganism). Before dilution of the aqueous solutions in warm DMSO, a volume of the enzyme formulation was added to an aliquot of 10 mL and the solution kept at 40 °C for 20 min under continuous stirring (400 min−1). The specific concentration of the enzyme was 600 U g−1 starch (748 µL) for the WxPS samples and 150 U g−1 starch (187 µL) for the PS and HACS samples. The hydrolysis was terminated by keeping the dispersion at 90 °C for 20 min. Before analysis, the solutions of the enzymatically treated samples underwent a stabilization and filtration process according to the earlier description.
Chromatographic System and Data Processing
The molecular characterization of the polydisperse solutions was carried out by means of SEC-MALS-DRI. The separation was executed with an SEC-3010 module (WGE Dr. Bures GmbH & Co. KG, Dallgow-Doeberitz, Germany) including degasser, pump, and auto sampler connected to a MALS detector and a differential refractive index detector (DRI). The MALS detector was a Bi-MwA (Brookhaven Instruments Corporation, Holtsville, NY, USA) fitted with a diode laser operating at λ = 635 nm and equipped with seven detectors at angles ranging from 35° to 145°. The DRI was a SEC-3010 RI detector operating at λ = 620 nm. Three columns in a row were used: AppliChrom ABOA DMSO-Phil-P-100 (100–2500 Da), P-350 (5–1500 kDa), and P-600 (20 to > 20 000 kDa) (Applichrom, Oranienburg, Germany). The samples were eluted with degassed DMSO (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) containing 0.1 m NaNO3 at a flow rate of 0.5 mL min−1 and a temperature of 70 °C. During the sample run on the SEC-MALS-DRI system (single determination), the data from the MALS and DRI detectors were collected and processed using ParSEC Enhanced V5.61 chromatography software to give the concentration of the eluted solution and MM at each retention volume (Mi). The basis for the molecular characterization by means of SEC-MALS-DRI has been described elsewhere.[44, 45]
The separation system was additionally calibrated (SEC-cal-DRI) using a set of ten pullulan standards with a MM range between 342 and 805 000 g mol−1 (PSS Polymer Standards Service GmbH, Mainz, Germany). The standards were dissolved in DMSO (2.5 mg mL−1 w/v) and gently stirred for 24 h at 80 °C. The standard solutions were measured and the elution volume at the position of the peak maximum was used as the reference for the particular Mi and the calculation of the calibration curve. The calibration related to the degree of polymerization (DP) was calculated from the Mi divided by 162. The weight average DP (DPw) was calculated from the Mw divided by 162.
The SEC-chromatograms of the enzymatically treated samples were recorded (all starch samples). Additionally, the SEC-chromatograms of the native and AT samples (without HP, completely debranched) were advanced analyzed using peak separation and analysis software PeakFit Version 4.12 as described elsewhere.[46] Based on the fitted SEC-chromatograms, single peaks representing the AM fraction (1; fraction I), the AP branch chains fraction (2; fraction II) and the enzyme (3) were calculated. The Mw of the AM fraction (Mw AM) was calculated by means of the correspondent separated chromatogram (1) and the MM curve (fit) from the MALS-detector (SEC-MALS-DRI). The Mw of the AP branch chain fraction (Mw AP branch chains) was determined based on the relevant chromatogram (2) and the standard calibration curve (SEC-cal-DRI) according to the description elsewhere.[14] The chromatogram relating to the enzyme solution was not further utilized.
2.5 Statistical Evaluation of the Data
The impact (singe and in combination) of the different modification parameters (starch type, degree of HP, AT treatment) on the MS and the Mw of the starch (total) was investigated based on an experimental design (n = 18) using Statgraphics Plus 5.0 software. The values for the probability of error (p-value) are listed in the ANOVA table (analysis of variance; Table 2). With a p-value less than 0.05, the factor investigated had a statistically significant effect with a 95.0% level of significance (boldface type in the ANOVA table). Additionally, the results from the statistical evaluation were summarized in mean and interaction diagrams showing the calculated means of each category and the confidence interval with a 95.0% confidence level. With no overlapping of the confidence intervals in the mean diagrams, there was a statistically significant difference in the observed categories with a 95.0% level of confidence.
| Impact | p-value | ||
|---|---|---|---|
| MS | Mw | ||
| Single | AM contenta) | 0.0005 | 0.0015 |
| Degree HPb) | 0.0000 | 0.3769 | |
| Acid-thinning | 0.0022 | 0.0005 | |
| Interaction | AM content-Degree HP | 0.0026 | 0.3698 |
| AM content-Acid-thinning | 0.4444 | 0.0062 | |
| Degree HP-Acid-thinning | 0.0058 | 0.4987 | |
- a) Starch type with different AM contents (WxPS, PS, and HACS);
- b) Hydroxypropylation to different degrees (HP-1 and HP-2).
3 Results and Discussion
3.1 Dry Matter Content
Table 1 summarizes the DM contents of all starch samples investigated. The native potato based starches had a DM content of about 81 ± 0.25% w/w, and corn based starch of about 88.50% w/w, which is typical. The HP procedure (slurry, pilot plant scale) including subsequent drying of the granular starch led to slightly enhanced DM contents of the modified WxPS as well as the PS samples (0.7–3.7% w/w) and slightly reduced DM contents in the case of the etherified HACS samples (1-3.5% w/w). The AT process (slurry, laboratory scale) effectuated significantly enhanced DM contents of about 92.0–92.5% w/w. Based on that, the remarkably higher DM of the dual-modified starch samples compared to the respective initial material is explained by the AT modification.[42]
3.2 Granule's Surface Characteristics
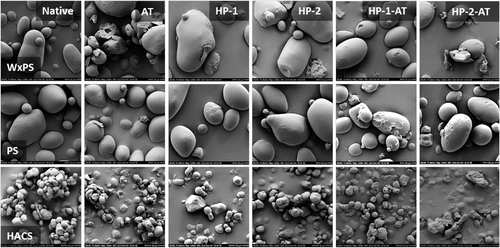
Figure 2 shows the SEM micrographs of the native (WxPS, PS, and HACS) and modified starch samples (AT, HP, and HP-AT). The appearance of the native granules was characteristic and typical without appreciable damages on the surface.[9, 14, 42, 47] Compared to the potato starches, the HACS granule population possesses a remarkably smaller average diameter and appears largely as agglomerates. However, AT (single modification) provoked a certain chipping, some cracks and visible changes of the surface in general.[14, 43] The HP (single modification) also caused changes on the surface, but basically no difference was found in dependence on the level of modification (HP-1 and HP-2). An altered granule morphology due to HP was also reported elsewhere.[25, 48] Moreover, Liu et al.[49] reported increasing changes of the granules with increasing MS (HACS). The phenomenon of a rougher surface and many tiny bubbles was explained by the (limited) granular swelling during HP, which corresponds to the observation within the present study. The subsequent acid hydrolysis of the HP starch samples (dual modification, HP-1-AT, and HP-2-AT) resulted in noticeable damage, which actually represents the sum of both HP and AT. Both single and dual modification basically did not change the shape and size of the granules remarkably. For potato starch, Kim et al.[50] reported evidence that potato starch granules are mainly hydroxypropylated in their central region (staining techniques and light microscopy). Indeed, information regarding the distribution of substituents within the granule cannot be provided by SEM micrographs, but indications for a somewhat heterogeneous HP within the granule population are revealed.[7] Some granules seem to be affected to a larger extent than others, which is evident from the fact that there are single granules without any visible surface damage.
3.3 Molecular Properties
3.3.1 Degree of Hydroxypropylation—MS
Table 1 summarizes the values of the MS of the HP (single-modified) and the HP-AT samples (dual-modified). The HP of the starches resulted in MS differing between 0.04 and 0.10 (low degree: -HP-1) and between 0.09 and 0.19 (high degree: -HP-2), respectively. The MS varied markedly in dependence on the starch basis modified. However, a significant gradation of the MS (HP-1 and HP-2) was achieved. The detailed investigation by means of statistical data evaluation (ANOVA, mean and interaction plots) is presented in Section 3.3.2.
3.3.2 Statistical Evaluation of MS and Mw
All starch samples were characterized by means of SEC-MALS-DRI and Mw was calculated. Additionally the MS of all HP as well as HP-AT starch samples was determined. Since the native and the AT starches were not modified by HP, the MS was taken as zero in each case. Therefore a complete statistical design (n = 18) was evaluated in terms MS and Mw (starch) by means of the ANOVA and corresponding mean and interaction plots, respectively.
Table 2 shows the ANOVA from the statistical analysis of the factors varied in the study for both MS and Mw. Since the p-values are lower than 0.05, the starch type and the AM/AP-ratio, respectively, degree of HP and the AT treatment as well as two combinations of the faction have a statistically significant impact on the level of MS. In contrast, Mw was impacted in a statistically significant manner, by the starch type (single) and the AT treatment (single) and their combined impact (interaction) exclusively.
Figure 3 shows the mean diagrams from the statistical evaluation. The MS of the HACS samples was found to be significantly lower compared to the potato based starches (Figure 3A.1), probably due to restricted accessibility of the propylene oxide to the HACS. The MS achieved increased significantly due to HP targeted on different levels (Figure 3B.1), which meets the expectation, but surprisingly, the (subsequent) AT of the HP starch effectuated a reduction of the MS (Figure 3C.1). The interaction plots in Figure 4 allow a more differentiated consideration of the varied impacts on the starch properties. The higher the level of HP (HP-2), the more pronounced decreased MS with increasing AM content of the starch was evident (Figure 4A.1). The decrease in MS after AT was found for all starch types by trend, but significant for the (regular) PS (Figure 4B.1). Reasons are probably leaching effects during the acid modification in slurry changing the molecular composition accessorily, whereas the molecular degradation due to hydrolysis is of course beyond question. This suggestion is supported by the fact of the increasing difference of MS due to AT with an increasing degree of HP (Figure 4C.1). The higher level of HP effectuates a higher degree of granule weakening and hence, most likely, higher accessibility of the granular structure for water during the hydrolysis procedure accompanied by a higher swelling state and carbohydrate solubilization by leaching certain polymer fractions.
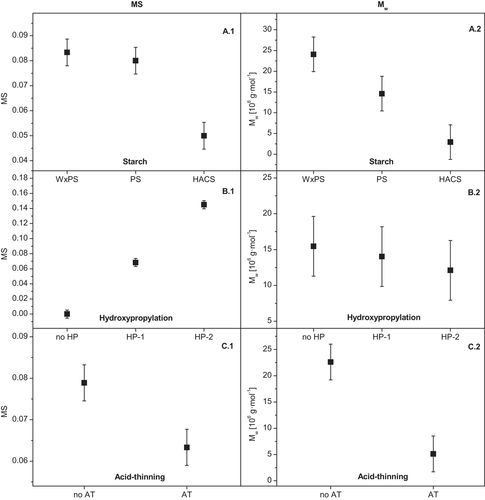
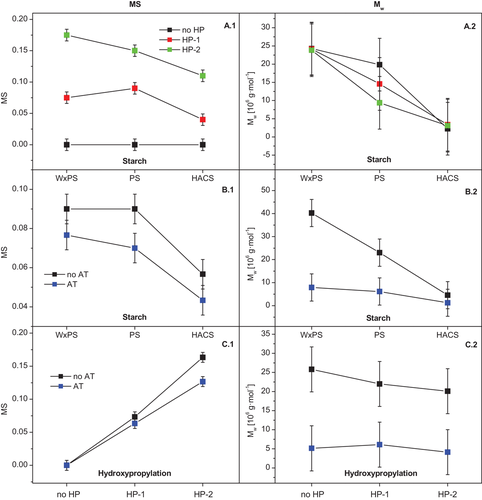
A statistically significant decrease of Mw is evident with increasing AM content on the samples (Figure 3A.2) on the one hand, and due to AT on the other hand (Figure 3C.2), which basically meets the expectation. However, increased HP level reduced the Mw marginally (not statistically significant; Figure 3B.2), whereupon this trend was predominantly effectuated by the (regular) PS (Figure 4A.2) and samples without an additional AT (Figure 4C.2), respectively. A remarkable relationship is obvious from the interaction plot in Figure 4B.2. The specificity of the reduction of Mw due to acid hydrolysis decreased systematically with increasing AM content of the starch samples and was not statistically significant within the group of the HACS samples (Figure 3B.2). Both specific characteristics of the starch granule structure (supramolecular level) as well as the molecular composition (incremental AM/AP-ratio) are suggested to be potential reasons.
3.3.3 Impact of AT on Molecular Properties
Figure 5 shows the chromatograms of the native and the corresponding AT samples (A.1: WxPS, B.1: PS and C.1: HACS). The chromatograms of the native starches are characteristic and typical, and were basically as expected. The WxPS (Figure 5A.1), without a remarkable AM content (0–5% w/w,[51] has a distinct peak between 14.5 and 17.5 mL elution volume, which is related mostly to the highly branched AP, the main structure fraction of a waxy starch. The AT modification of the WxPS reduced the Mw drastically from 42.90 × 106 g mol−1 (native) to about 8 × 106 g mol−1. Since the molecular degradation of the AP generates branched dextrins with supposed, considerably reduced hydrodynamic volume of the molecules, the chromatogram was shifted to a higher elution volume and changed from a monomodal to a bimodal distribution. A comparatively strong reduction of the MM by means of AT was reported elsewhere for a waxy corn starch,[14] which basically confirms our results. Due to the regular AM/AP-ratio of the PS (about 25:75 w/w), the Mw of the native PS was significantly lower than that of the waxy variety. The shoulder of the chromatogram in the range between 17 and 21 mL corresponds to the AM fraction (Figure 5B.1). The reduction of Mw due to the hydrolysis step from 33.20 × 106 g mol−1 (native) to 6.50 × 106 g mol−1 (PS-AT) was most probably a result of chain cleavage within both the AP as well as the AM fraction.[30] The HACS with an AM content of about 70% w/w has a distinct and higher relative chromatogram area between 18 and 23 mL elution volume, which is assigned to the AM fraction (Figure 5C.1). The AT provoked a reduction of Mw from about 3.70 × 106 to 870 000 g mol−1, accompanied by a slight shift of the chromatogram.
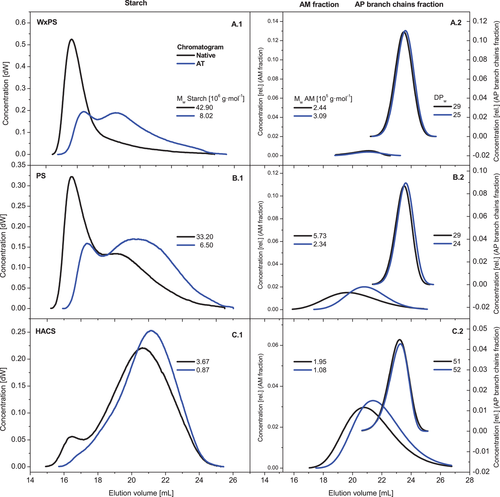
Figure 5 also includes the chromatograms of the AM as well as the AP branch chain fraction of the native and DK samples obtained by means of peak separation procedure based on the chromatogram of the completely debranched samples. A visible shift of the chromatogram of the AM was found for the PS (Figure 5B.2) and HACS sample (Figure 5C.2) due to the acid-induced molecular degradation, and the corresponding Mw was reduced significantly (PS: reduction from 5.73 to 2.34 × 105 g mol−1; HACS: reduction from 1.95 to 1.08 × 105 g mol−1). This is mainly attributed to the polymer chain cleavage occurring in both the AM as well as the AP fraction, but a certain change of the molecular composition due to leaching effects has to be included in the interpretation. In contrast to HACS and PS, the chromatogram and the corresponding Mw of the AM fraction of the WxPS were just marginally affected by the AT (Figure 5A.2). The reasons suggested is the very small amount of AM in the waxy variety, which hampers the analysis on the one hand. On the other hand, leaching effects due to annealing and vigorous stirring during hydrolysis (AT) could basically change the molecular composition of the AM fraction also. Figure 5 also contains the chromatograms of the AP branch chains fraction of the native and DK samples. Changes due to the AT modification are visible. The AP branch chain fraction from the HACS has a DPw of about 51, which is within the typical range for a high AM corn starch genotype. The DPw remained basically unchanged after AT (DPw 52), which strongly indicates a degradation of the AP fraction having a debranching character (Figure 5C.2). On the one hand, the cleavage of predominantly α-1,6-glycosidic bonds during the acid-induced hydrolysis reduces the molecular size of the AP and the branched dextrins fraction, respectively, but on the other hand, the average molecular size of the AP branch chains remains constant. In contrast to the HACS, the AP branch chains of the WxPS and the PS were perceptibly shortened on average by the AT, which is indicated by the shift of the respective chromatograms (Figure 5A.2,B.2) to higher elution volume. The DPw was reduced from 29 to 25 (WxPS) and 24 (PS), respectively. Similar values for the average branch chain length of a native WxPS[52] and a native PS[41] were reported elsewhere. Anyway, the slight reduction indicates a cleavage of the AP not exclusively at the branches (α-1,6-glycosidic bonds).
3.3.4 Impact of HP and the Combination with AT on Molecular Properties
The impact of the HP on the Mw was discussed earlier in this study based on the ANOVA (Table 2) and the mean diagram (Figure 3B.2) as well as the interaction plot (Figure 4C.2). The slight decrease of Mw with increasing degree of HP was evident, which is mainly attributed to the samples without additional AT, whereas the combination with AT did not change the Mw (Figure 4C.2). Moreover, particularly in the case of the regular PS, the HP was found to induce a (systematical) reduction of the Mw by trend (Figure 4A.2).
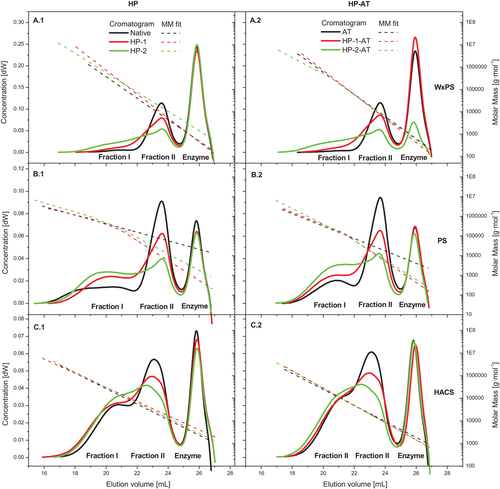
The specific changes of the molecular properties, in particular due to the HP step, were strongly controlled by the starch type. This is illustrated in detail by the chromatograms and the respective MM fits in Figure 6. The chromatograms and the MM fits of the WxPS after HP were slightly different to that of the native sample (Figure 6A.1). Specific reasons were suggested to be the slightly changed solubility of the starch polymers 1) and changed behavior in solution 2). Since the Mw remained nearly constant (42.9–39.5 × 106 g mol−1), a remarkable molecular degradation of the WxPS due to the HP was not proved. A basically similar situation was evident for the corresponding HP-AT samples of the WxPS (Figure 6A.2; Mw between 7.0 and 8.8 × 106 g mol−1). No remarkable effect of the HP on the molecular composition of the starch samples was found.

The regular PS was degraded after HP, which is indicated by the successive shift of the chromatograms (PS-HP-1 and PS-HP-2) to higher elution volume on the one hand and the slight decrease of the MM fit concomitantly on the other hand (Figure 6B.1). The Mw was reduced systematically from 33.2 × 106 g mol−1 (native PS) to 21.2 × 106 g mol−1 (PS-HP-1) and 14.7 × 106 g mol−1 (PS-HP-2), respectively, which corresponds to a reduction of about 55%. That incremental molecular degradation due to HP was most likely a feature of the corresponding dual-modified samples (Figure 6B.2), too. Indeed, the Mw of the AT samples was slightly lower than that of the HP-1-AT sample, which is probably attributed to different solubility and achievable solution state of the polymers, respectively, due to the chemical modification in the granular state. On the other hand, the introduction of the hydroxypropyl groups could simply enhance the molecule dimension in solution. However, a remarkable MM reduction at higher degree of HP was obvious (reduction about 50%). Independent on the AT modification, the MM of the HACS-HP samples increased visibly compared to the native and the AT sample, respectively. That was evident from the shift of the chromatograms to lower elution volume, the slightly higher level of the MM fit and finally by the respective values for Mw. The Mw of the HP samples was increased about 35% (single modification) and 67% (HP-AT samples, dual modification), respectively. Accordingly to the explanation for dual-modified PS samples, that systematical change of the molecular characteristics could be ascribed to enlarged molecule dimensions as a consequence of the partial substitution of the hydroxyl groups within the starch polysaccharides. Additionally, an impact due to certainly improved solubility owing to the chemical modification in the granular state should be included in the interpretation, since the HACS variety is known to have restricted swelling properties at least in the native granular state. Comparatively harsh disintegration process conditions (e.g., pressure cooking and subsequent high shear treatment) are usually necessary to dissolve the supramolecular structure and to prepare an aqueous polydisperse solution. Moreover, the partial introduction of the hydroxypropyl groups within the polysaccharide structure acting as a spacer most probably hampered a rapid retrogradation and crystallization of the predominantly unbranched AM chains. Based on that, an improvement of the granular disintegration (1), an advanced solubility of molecules with high MM (2), and an improvement of the solution state (3) as well as the stability of the aqueous system (4) due to the HP are hypothesized.
The analysis of the AM as well as the AP branch chain fraction of all non-HP starch samples (native and AT) was presented comprehensively earlier in the study. Since the complete debranching of the starch polymers after modification by means of HP is hampered, the chromatograms of the HP samples (HP and HP-AT) differ strongly from the respective initial starch (Figure 7). Without HP (native and AT), the fractions I and II are clearly ascribed to the AM fraction (higher MM) and the AP branch chains fraction (lower MM), respectively. The chromatogram peak between 25 and 27 mL (glucose) originates from the enzyme solution and is without relevance for the starch polymer characterization. Fraction II originates from the chain cleavage within the AP structure (debranching) and represents chains with a DPw of about 30 (WxPS and PS) and 50 (HACS), respectively. Due to incomplete debranching of the HP starches (HP and HP-AT) by pullulanase digestion,[22] the relative concentration of this fraction decreased and that of fraction I consequently increased. This change was found to be incremental in dependence on the degree of HP (MS). Moreover, the specificity of the relative decrease of fraction II varied slightly in dependence on the starch type and the AM content, respectively. To estimate the drop of the relative portion of fraction II due to HP, the specific chromatogram areas between 22 and 24.7 mL elution volume were related to the corresponding one of the initial starch (native: Figure 7A.1,B.1,C.1; AT: Figure 7A.2,B.2,C.2). The decrease was found to be slightly higher for the dual-modified starches compared to the corresponding HP starch (single modification) in most cases. The reduction was calculated to be within the range 5–15% for the low degree of HP (HP-1 and HP-1-AT) and between about 15% and 40% for the high one (HP-2 and HP-2-AT). Based on that, it is concluded that a remarkable portion of the AP fraction was etherified, leading to partial resistance against enzymatic debranching. These branched dextrins, released from the AP and remaining after debranching, were eluted concomitantly with the AM fraction. The branched character is additionally confirmed and basically evidenced by the different positions of the MM fits. The HP- and HP-AT-samples have MM fits which are slightly shifted to a higher MM compared to the corresponding non-HP starch sample (Figure 7). However, the specific impact due to partial molecular degradation during the HP could not be a part of interpreting the debranching experiments. Zhao et al.[22] reported the substitution occurring mainly in the amorphous lamellae and the amorphous regions between clusters of side chains in the crystalline lamellae. Because the amorphous areas are accessible for chemical agents, propylene oxide in this case, some branching points of the AP are substituted and hence resist cleavage by pullulanase. An incomplete debranching of HP potato starch by isoamylase was reported elsewhere.[20] A higher relative substitution of the AM fraction of HP starches was reported by Shi and BeMiller[21] for common corn starch and Kavitha and BeMiller[20] for potato starch (fractionation, 1H NMR experiments).
4 Conclusions
The single and dual modification of starches having different AM/AP-ratios with HP and AT consecutively was successful. An incremental MS by gradation of the propylene oxide dosage was achieved for all starches, but presumably, the unique granular integrity of the high AM corn starch variety reduced the level of substitution compared to the way potato starches reacted to the same conditions. The granular structure remained intact and smaller defects can be assigned to both HP and AT. Retained starch granule integrity is an advantage in terms of the removability of undesired by-products by filtering and washing the finished starch product. The relative modification within the granule population was hypothesized to be apparently heterogeneous. The disintegration of the starch samples by means of pressure cooking, a subsequent high shear treatment and stabilization in DMSO provided molecular dispersed solutions with good solution states. SEC-MALS-DRI was an appropriate technique for the comprehensive molecular characterization of the modified starches. The initial AM/AP-ratio controlled the effect of the HP on the MM. The regular starch was remarkably molecularly degraded due to the modification procedure. In contrast, the waxy and the high AM variety had just marginally decreased and increased Mw, respectively, after HP. The same systematic was proved for the corresponding dual-modified samples. An increased resistance to digestibility by pullulanase was evidently shown for both HP and dual-modified samples, independent of the starch type. Hence, the complete debranching as an additional analytical tool for the molecular characterization is not suitable for HP starches. Based on the combination of both the specific molecular degradation (AT) and the HP to comparatively low levels (sustainable for use in foods), the dual-modified starches produced could be interesting alternatives for gelled sweets.
Acknowledgements
This research was financially supported by the German Federal Ministry for Economic Affairs and Energy (BMWi) within the scope of the Industrial Collective Research (IGF) of the German Federation of Industrial Research Associations (AiF; research project 20248 N). The authors would like to thank Emsland-Stärke GmbH for preparing the HP starches. The authors also thank Ewgenia Kuhl and Sakurako Yagami for their assistance in performing numerous experiments and Christoph Fahrenson (ZELMI, TU Berlin) and Fenglei Hei for preparing the SEM micrographs. The authors thank Donna Hastings for checking the manuscript linguistically.
Conflict of Interest
The authors declare no conflict of interest.




