Use of Bentonite and Organic Binders in the Briquetting of Particulate Residues from the Midrex Process for Improving the Thermal Stability and Reducibility of the Briquettes
Abstract
With the aim of achieving sufficient reducibility and thermal stability of briquettes for reuse as feedstock in the Midrex direct reduction process, a defined mixture of residues from the Midrex process is briquetted with an organic binder and bentonite. In the first step, briquetting tests are conducted to investigate the influence of water and binder content on the mechanical properties of the briquettes. Suitable briquettes are then used for reduction tests, where mechanical properties under Midrex-relevant conditions are considered. The tests show that briquettes with an organic binder (with and without bentonite) are not stable under Midrex-relevant conditions, whereas briquettes with a mixture composition with 5 wt% bentonite and 5 wt% water are. This can be justified on the basis of microscopic images. Bentonite favors the sintering mechanisms occurring between the particles. The organic binders decompose thermally and carbon remains, which prevents sintering. The reduction tests show that oxygen release from the briquettes occurs more slowly than for iron oxide pellets. However, the residual mixture already contains reduced material (Femet of briquettes with bentonite 28.1 wt%), so less oxygen has to be removed. Furthermore, a small briquette format (volume 10 cm3) shows better reduction behavior compared to bigger briquettes.
1 Introduction
The Midrex process is state of the art for the direct reduction of iron ore pellets to metallic iron. The metallic iron is referred to as direct reduced iron (DRI). In the Midrex shaft, the moving bed of pellets is penetrated in a counter-current mode by the reducing gas at temperatures of up to 1173 K. At the bottom exit of the Midrex process, the porous DRI is compacted to hot briquetted iron (HBI) to decrease its oxidation tendency when exposed to air and to prepare it for transportation to and further processing in steel plants electric arc furnace (EAF).[ 1 ] A sketch of the Midrex process with a roller press for hot briquetting and without an attached EAF and a detailed description is provided in Lohmeier et al.[ 2 ]
Not all the iron that is supplied as iron ore to the Midrex plant leaves it as HBI. Iron-containing material is removed from the process at several sites. Iron ore pellets that permeate a 6.3 mm sieve are removed from the solid feed to ensure the gas permeability of the moving bed. Some fines, which emerge due to friction, compression, and crush between the pellets inside the moving bed, are entrained with the reducing gas overhead. They are separated as sludge in the off-gas treatment process. Small particles and dust also result from the screening of the HBI. During the start-up and shut-down of the Midrex process, not completely reduced material accrues in the form of so-called remet fines. We refer to all the iron-containing material that does not leave the Midrex process as screened HBI as “residues.” Table 1 summarizes the chemical composition of the residues depending on where in the Midrex process they accrue.[ 2-5 ]
| Component | Amount in Fe-carrier mixture [wt%] | Fetot [wt%] | Fe2O3 [wt%] | Femet [wt%] | Water [wt%] | d m [mm] |
|---|---|---|---|---|---|---|
| Screened oxide fines | 30 | 67.20 | 96.10 | 0.00 | 1.0 | 0.764 |
| Dried sludge | 40 | 74.00 | 63.60 | 29.53 | 1.3 | 0.140 |
| Process classifier fines | 5 | 70.08 | 79.40 | 14.51 | 8.5 | 1.122 |
| HBI classifier dust | 5 | 83.94 | 34.51 | 58.17 | 10.8 | 1.416 |
| HBI screened fines | 15 | 88.71 | 17.79 | 74.92 | 0.41 | 1.865 |
| Remet screened fines | 5 | 84.87 | 30.94 | 59.81 | 5.4 | 1.401 |
Based on practical experience, the weight fraction of residue is about 3–6 wt% of the supplied material. The residue is composed of about 40 wt% dried sludge, 30 wt% oxide screened fines, 15 wt% HBI screened fines, 5 wt% remet fines, 5 wt% HBI classifier fines, and 5 wt% process classifier fines.[ 2-4, 6 ] Returning these ferrous residues to the Midrex process means improving plant efficiency. As the residual briquettes already contain metallic iron, less reduction gas is required and less oxygen has to be removed, which means that more DRI can be produced for the same input quantity. Furthermore, the waste to be disposed of is reduced. According to Midrex, ≈1.45 t of iron ore pellets are required to produce 1 t of DRI, taking into account material losses as well as chemical changes (oxygen release, carburization, etc.).[ 4 ] The loss of residual material can be assumed to be about 5 wt% of the feedstock (equivalent to 0.0725 t). If this material is briquetted and recycled, it can therefore be assumed that ≈0.0725 t are recycled. If these briquettes (assumption Fetot = 71.1 wt%) are used together with 1.3775 t iron oxide pellets (assumption Fetot = 67 wt%) as feedstock and reduced to DRI with Fetot = 91 wt%, the amount of DRI produced increases. This results in a rough estimate of an increase in production volume of 3.5 kg t−1 DRI. For a plant producing 3 million tons of DRI per year, this results in an increase in production volume of about 10 000 tons, with a decrease in the demand for iron oxide pellets. Another possibility for increasing productivity when considering the complete unit consisting of the Midrex process and EAF is to separate the residual components into a fraction with a high degree of metallization and one with a low degree of metallization and to use the fraction with a high degree of metallization directly in the EAF. The advantage of this variant would further be that a smaller quantity flow of the residual material mixture has to be briquetted and thus less binder is required. In this article, however, only the briquetting of the entire residual material mixture is considered. Before the residues can be reintroduced to the Midrex process, they have to be conditioned to qualify them for the reduction in the hot moving bed. To prevent their entrainment with the reducing gas and to ensure gas permeability through the moving bed, the residues must be agglomerated. These agglomerates should be as stable as not to disintegrate under the harsh conditions in the hot moving bed into fine particles. Furthermore, the iron compounds in these agglomerates should be reducible to metallic iron.[ 6, 7 ] Agglomerates of pure residues do not meet the aforementioned demands.[ 4, 6, 8 ]
The briquetting of residues from the Midrex process was reported by Pietsch[ 9 ] as early as 1978. There, cold DRI was still produced instead of HBI. The cold DRI fine material smaller than 3 mm was briquetted with a binder combination of powdered coal tar pitch, slaked lime (solid), and sodium silicate (liquid).[ 9, 10 ] The resulting briquettes were successfully used directly in the electric arc furnace.[ 9 ] However, only the metallic material was briquetted. Furthermore, fine material in the form of screened iron oxide pellets and sludge from the purification of the process gas and moist mill scale was also produced at that time. According to Pietsch,[ 9 ] there were efforts by Midrex to briquette this material as well and to return it to the Midrex process as feed material.[ 9, 11 ] A mixture of sodium silicate, slaked lime, and water was used as a binder.[ 12 ] To achieve sufficient briquette strength, the green briquettes were directly heated by gas flames for 1 min on the steel mesh belt of a tunnel kiln. Alternatively, drying the briquettes at 300 °C for 15 min also yielded sufficient briquette strength. A briquetting plant with a throughput of 20–25 t h−1 was installed at Georgetown, and this plant was also used to mine a fine ore dump of over 100 000 t that had accumulated over the years.[ 13 ] However, it is not known from the literature whether this process has gained acceptance. In particular, the posttreatment of the briquettes appears to be disadvantageous. Brunner[ 6 ] also shows a way to briquette residues from the Midrex process. The residues (sludge from process gas scrubbers, screened oxide fines, mill scale, DRI fines) are briquetted with an unnamed inorganic binder. Promising results from preliminary investigations are shown and a plant concept for briquetting 220 000 t per year of residuals is presented.[ 6 ] For the study presented here, briquetting of residual materials with binders was taken up, also using an inorganic binder, in this case bentonite, and two organic binders. The results to date have been published in Lohmeier et al.[ 3 ] The binder bentonite provided agglomerates that had sufficient mechanical properties even after reduction at Midrex-relevant temperatures but with insufficient reducing properties. Due to the low porosity of the bentonite-containing briquettes, it was hard for the reducing gas to penetrate the center of the agglomerates. Organic binders, such as starch and cellulose, provided agglomerates with insufficient mechanical properties after reduction at Midrex-relevant temperatures. But it is believed that the reducibility can be improved by using organic binders. At Midrex-relevant temperatures, the carbon compounds of the organic binders, which are distributed over the entire volume of the briquette, positively influence the reduction. The organic binders decompose thermally. Initially, dehydration of absorbed water occurs and, from 573 K, chemical dehydration and thermal decomposition occur (thermal condensation between hydroxyl groups to form ether segments). Amorphous carbon remains as a residue, which begins to form at 773 K.[ 14 ] The gaseous compounds exit the agglomerates and leave void spaces, which enable the reducing gas to penetrate the briquette to further reduce the iron compounds.[ 8, 15-21 ] The aim of the study presented here is the production of briquettes with sufficient mechanical stability and reducibility by using a mixture of bentonite and starch and/or cellulose as binder. The ratio of binder/residue and the binder composition were varied based on a statistical test plan. The reducibility properties of the briquettes were analyzed at Midrex-relevant conditions (high temperature and reducing gas atmosphere) only for those mixtures of binder and residue that showed acceptable mechanical properties at ambient temperature (cold condition). The abrasion resistance of the reduced briquettes was analyzed after they had been removed from the reducing oven and conditioned to ambient temperature. To derive from the mechanical properties measured at cold conditions at least qualitative mechanical properties at hot conditions, we present some bending stress measurements of long briquettes of a residue/binder mixture at Midrex-relevant conditions.
2 Experimental Section
2.1 Materials and Briquetting
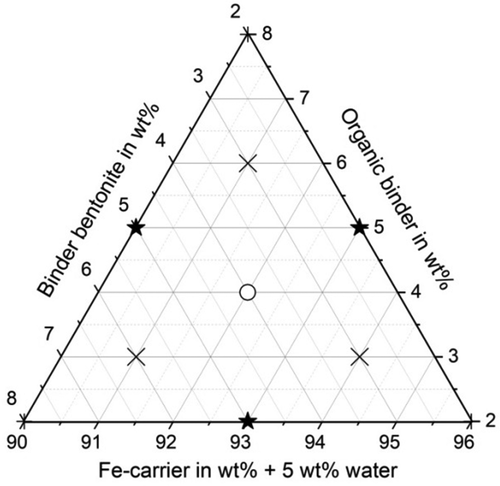
Figure 1 shows a ternary diagram of the mixtures of residue, bentonite (inorganic binder), and organic binder that were briquetted. The input material was a mixture of residues of various origins from the Midrex process as specified in Table 1. A more detailed chemical analysis (ISO 9516-1, ISO 16878) as well as the particle size distributions can be found in Lohmeier et al.[ 3 ] The chemical composition of the inorganic binder “bentonite” clay is shown in Table 2 . Two organic binders, wheat starch and cellulose glue, were used. Water was used when the moisture of the residue/binder mixture had to be increased to 5 wt%. The weight fraction of water is considered on the horizontal axis in Figure 1.
| Component | CO2 | Na2O | MgO | Al2O3 | SiO2 | SO3 | K2O | CaO | TiO2 | Fe2O3 | BaO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 0.35 | 4.7 | 1.89 | 27.18 | 60.33 | 0.54 | 0.51 | 1.11 | 1.18 | 1.67 | 0,54 |
The reducing gas hydrogen, the inert gas argon, and the inert gas nitrogen were purchased from Nippon Gases. Iron ore pellets, which were delivered by voestalpine Stahl GmbH, were used as a reference in the reduction tests. These iron ore pellets had a mean iron content of 66.1 wt%, with the iron being in the form of Fe2O3. The grain size of the pellets was between 12.5 and 14 mm. Their apparent density is 3.443 × g cm−3.
The residue/binder mixture was homogenized in an Eirich mixer for 10 min. The homogenized mixture was heated to 333 K and then briquetted with a hydraulic stamp press (Raster Zeulenroda, PYXE 250 F) conditioned to 333 K, too. Briquettes of different dimensions but with identical maximum compression pressure of p max = 140 MPa were produced. The hold time at maximum compression pressure was 3 s.
For the analysis of the mechanical properties of the not-reduced briquettes at cold conditions, cylindrical briquettes with a diameter of 5 cm, a height of ≈2 cm and a weight of 150 × g were produced. The constant briquette format was necessary here because the strength values of briquettes of different formats are not directly comparable and target values and methods of determination can only be transferred to a different briquette format to a limited extent.[ 8, 22 ] As all previous briquetting tests[ 3 ] were conducted with this briquette format, it should also be retained for the assessment of the mechanical strengths.
For the analysis of the reducing properties of the briquettes and the abrasion resistance of the already reduced briquettes, briquettes with a diameter of 3 cm, a height of ≈1.5 cm, and a weight of 40 × g were produced. The smaller briquette format was chosen to produce briquettes with better reduction properties. The applied briquette volume reduction is still feasible on industrial scale. For the analysis of the bending stress resistance at hot conditions, long noncylindrical briquettes with dimensions of 10 cm × 4 cm × 1 cm were required, resulting in a weight of ≈150 × g per briquette. The briquettes were stored for 1 day at ambient conditions before further examination. The elongated briquette format was necessary here to be able to determine the bending strength of the briquettes.
The results for the mechanical properties of the briquettes are comparable as far as trends are concerned despite the different briquette formats, because the same apparent density was ensured. For the metallurgical properties (especially reducibility), in contrast, the briquette format has a major influence, which is why briquettes with likewise small briquette volumes of ≈10 cm3 were targeted for large-scale implementation.
2.2 Mechanical Properties of Briquettes
The compressive strength σ P of the briquettes was determined according to TGL 9491:1976 with the universal testing machine UH-500kNA made by the Shimadzu Corporation. The compressive strength of five briquettes was determined and averaged. It is believed that briquettes with a compressive strength exceeding 30 MPa are suitable for the Midrex process.
It is believed that briquettes with an abrasion resistance exceeding 85% are suitable for the Midrex process.
It is believed that briquettes with a shatter strength exceeding 85% are suitable for the Midrex process.
The apparent density ρ app of the briquettes was determined by measuring the briquette dimensions with a caliper gauge and additionally weighing it. The apparent density is the ratio of the mass of the briquette and its volume.
2.3 Metallurgical Properties of Briquettes
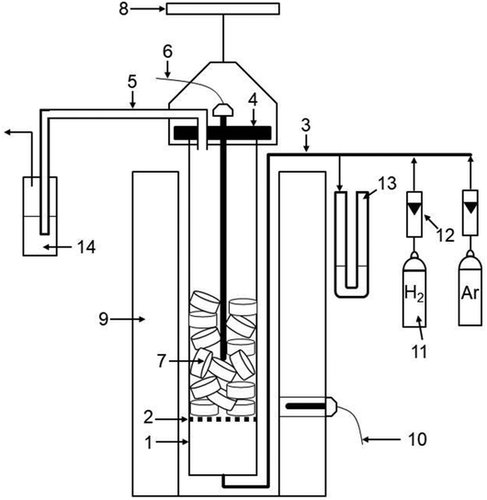
The setup used for the reduction tests in accordance with ISO 4695 is shown in Figure 2 . A retort with 110 mm internal diameter was filled with 53 briquettes. These 53 briquettes before the reduction process had a mass of ≈2100 g. The bed of those briquettes inside the retort had a height of ≈110 mm. First, the briquette-filled retort was purged with 1.5–2 L min−1 of argon and the furnace surrounding the retort was heated to 773 K at 12 K min−1. It should be noted that the thermocouple for the furnace temperature and thus also the temperature control of the furnace was unfavorably located on the insulation of the furnace and not on the heating spindles. This means that the actual temperature of the furnace was always higher than the specified and measured temperature of the furnace. When the thermometer penetrating the bed of briquettes also measured the set 773 K, the furnace temperature was set to the intended 1073 K for reduction with a temperature increase rate of 12 K min−1. When the set reduction temperature of 1073 K was reached in the briquette bed, the flow of the reduction gas hydrogen was started with 12 L min−1. At the same time the flow of argon was stopped. Because the actual furnace temperature at the heating spindles is higher than the specified furnace temperature of 1073 K, the temperature in the retort and thus the reduction temperature increases to ≈1093 K. During the reduction, the mass of the bed was continuously recorded with a weighing machine. The reduction experiment was stopped 3 h after initialization of the hydrogen gas flow by switching back the gas flow to argon and switching off the heating. The reduction experiments were conducted in replication. It should be noted that conducting the reduction tests with pure hydrogen is not standard practice, nor do they correspond to the conditions in the Midrex shaft. Typical standard tests to determine reducibility are based on a mixture of H2 and CO and sometimes small amounts of N2 and CO2. An overview of standard tests for the evaluation of iron carriers for direct reduction with appropriately applied gas composition is given in previous studies,[ 23, 24 ] where a distinction can be made between a variety of static tests (HYLSA batch reducibility test, verein deutscher eisenhüttenleute (VDE) reducibility test, Japanese Industrial Standard reducibility test, Gakushin reducibility test, ISO relative reducibility test, I.S.I test, HYL-III test) and the dynamic Midrex-Linder reducibility test. It is known that a shorter reduction time is required for the complete reduction of iron oxides when H2 is used as compared to CO. This is due to thermodynamic reasons and can also be attributed to the fact that H2 molecules are smaller than CO molecules, thus favoring pore diffusion. The influence of the reaction gas composition on the mechanical stability is small.[ 25-27 ] Nevertheless, to be able to interpret the results of the reduction experiments with pure H2, a reference experiment was conducted with standard iron ore pellets. Furthermore, it must be taken into account that CO2 is also present in the reduction gas in the real Midrex process. This can lead to a reoxidation of the already reduced iron in the residual briquettes, which can counteract a sufficient reduction, but is not taken into account in the reduction experiments with pure H2. If reoxidation of the metallized phase occurs during reduction in the Midrex process, direct use of the highly metallized residual components in the EAF would be preferable to avoid this problem.
The reduced and cooled briquettes from the reduction tests were tested for abrasion resistance in the abrasion drum described previously to assess their thermal stability. Due to the smaller briquette format, ten instead of five briquettes were tested. The mass fraction of briquettes larger than 30 mm and larger than 5 mm after 50, 100, and 200 revolutions was considered.
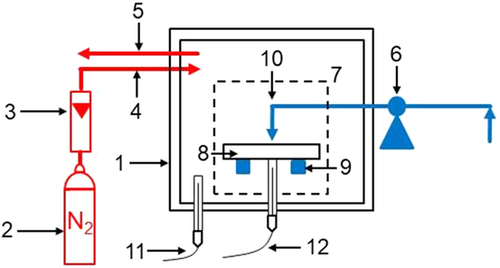
To explain the abrasion resistance of the briquettes after reduction, previous tests in the so-called Werner oven were used to compare the mechanical stability of briquettes at elevated temperatures without binder, with bentonite, and with organic binder. As the state of knowledge at the time of the tests had not progressed so far, these tests were conducted with a different organic binder and a different composition of the residual material mixture. Nevertheless, these tests should be listed here since they can well describe the sintering behavior of the briquettes with bentonite and thus the good abrasion resistance. An in-house-engineered oven, which is referred to as “Werner oven,” offered the possibility to determine the bending strength of briquettes at elevated temperatures up to maximum 1173 K.[ 28 ] The Werner oven consists of a chamber heated by means of electric heating rods and lined with a refractory corundum ramming mass. The briquette strength was determined inside the furnace by a three-point bending test, sketched in Figure 3 . For this purpose, a lever device was installed, which was operated from the outside and introduced the test force onto the briquette inside the oven.[ 28 ] The maximum possible heating regime was used for the tests (373–973 K: 20 K min−1; 973–1073 K: 15 K min−1; 1073–1173 K: 10 K min−1). The furnace was heated up to different test temperatures between room temperature and 1173 K and the briquette was then loaded with increasing force until breakage. The furnace was purged with nitrogen during the tests to prevent oxidation. A reducing atmosphere was not possible as the furnace was not completely sealed due to the lever device. Conducting the tests with nitrogen is not ideal, but prevents oxidation. Thus, the tests can only show tendencies and, in particular, explain the effect of the binder on the briquette strength as a function of temperature. Furthermore, the volume expansion due to the reduction of hematite to porous magnetite, which also leads to cracks and thus influences the briquette strength, cannot be considered.[ 29 ] For the briquettes used for bending strength testing, a deviating Fe-carrier mixture consisting of 60 wt% dried sludge, 30 wt% screened oxide fines, and 10% HBI screened fines was used. The water content of the briquetting mixtures was 5 wt% and the binder content was 5 wt%. The organic binder used was lignosulfonate.
3 Results and Discussion
3.1 Mechanical Properties of Briquettes
Figure 4 shows the compression strengths of the produced briquettes for either cellulose or starch as organic binder and bentonite as inorganic addition. The iso-compression-strength curves result from interpolation between the compression strengths measured at the points marked in Figure 1 for various residue mixture compositions. When cellulose is used as organic binder, the required minimum compression strength of 30 MPa is exceeded for all mixture compositions. The maximum compression strength is reached at 5 wt% bentonite, 2 wt% cellulose, 88 wt% Fe carrier, and 5 wt% water. When starch is used as organic binder, the required minimum compression strength of 30 MPa cannot be exceeded for starch weight fractions above 4–5 wt%, depending on the fractions of the other mixture compounds. The maximum compression strength for starch-containing briquettes was measured at 2 wt% bentonite, 2 wt% starch, 91 wt% Fe-carrier blend, and 5 wt% water. The results published in Lohmeier et al.[ 3 ] indicate that the compression strength for the starch-containing briquettes can potentially be increased if the water content in the mixture is decreased.
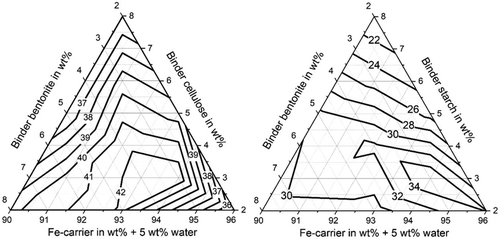
Figure 5 shows that abrasion resistances over 85% are exceeded for both organic binders in the residue mixture irrespective of the residue mixture composition, within the analyzed range of the mixture composition. The iso-abrasion-resistance curves are again interpolations between the measurement values obtained for the mixture compositions marked in Figure 1. For cellulose as organic binder the abrasion resistance values do not change remarkably with mixture composition; they are all between 90% and 93%. The briquettes containing starch as organic binder feature an overall higher abrasion resistance with values between 96% and 99%.
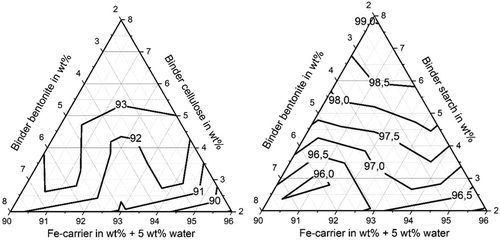
Figure 6 shows that the shatter strength is also over 85% for both organic binders in the residue mixture irrespective of the residue mixture composition. The iso-abrasion-resistance curves are again interpolations between the measurement values obtained for the mixture compositions marked in Figure 1. For cellulose as organic binder the shatter strength values are all between 97% and 99% and for the briquettes with starch as organic binder the values are all over 99%. The shatter strength values do not change remarkably with mixture composition. It can be seen by comparing Figure 4 with Figure 5 and 6 that the briquettes with greater compressive strength have qualitatively smaller abrasion and shatter strengths, which also confirms the previous studies.[ 3 ]
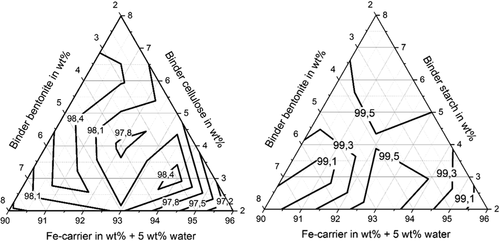
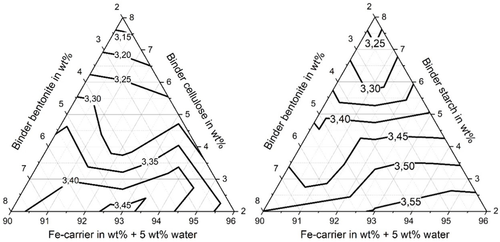
Figure 7 shows for the briquettes with starch and bentonite slightly higher briquette apparent densities (between 3.25 and 3.55 × g cm−3) as compared to the briquettes with cellulose and bentonite (between 3.15 and 3.45 × g cm−3). Furthermore, it can be seen that with increasing content of organic binder in the briquetting mixture, the apparent density of the briquettes decreases. This is because the organic binders have a lower true density than the residual mixture and bentonite. Table 3 lists in the lines “B/C” and “B/S” the residue/binder mixtures that resulted in the maximum strength values.
| Name reduction test | Binder [wt%] | Fe carrier [wt%] | Water [wt%] | Fetot [wt%] | Femet [wt%] |
ρ app [g·cm−3] |
R30(100) [%] |
S 20 [%] |
σ P [MPa] |
|---|---|---|---|---|---|---|---|---|---|
| B | 5 wt% bentonite | 90 | 5 | 71,1 | 28,1 | 3.681 | 87.1 | 92.9 | 41.5 |
| B/C | 5 wt% bentonite; 2 wt% cellulose | 88 | 5 | 69,5 | 27,5 | 3.470 | 90.9 | 98.1 | 42.2 |
| B/S | 2 wt% bentonite; 2 wt% starch | 91 | 5 | 71,9 | 28,4 | 3.585 | 95.9 | 99.0 | 34.1 |
| C | 3 wt% cellulose | 92 | 5 | 72,6 | 28,7 | 3.360 | 91.9 | 98.1 | 41.1 |
| S | 3 wt% starch | 93.4 | 3.6 | 72,7 | 28,8 | 3.517 | 95.5 | 98.8 | 42.1 |
The determination of the optimum mixture composition for the tests conducted here is based on the statistical mixture test plans with the program Statgraphics 18. The compressive strength, shatter strength, abrasion resistance, and apparent density are set as target values, each with equal importance, and the maximization of the target values is to be achieved in each case. For each target value, a mathematical model is calculated that describes the target value as a function of the mixture composition for the investigated test area. For the investigated range of mixture composition, the desirability can be calculated based on the mathematical models. The desirability is the product of all four target values, with normalization for each target value to the specified upper limit of the respective target value. The upper limit for shatter and abrasion resistance is set at 100%, for compressive strength at 50 MPa and for apparent density at 4 × g cm−3. If for a compound composition a shatter and abrasion resistance of 100%, compressive strength of 50 MPa, and an apparent density of 4 × g cm−3 are achieved, the desirability is 100%; if the achieved target quantities are lower, the desirability is correspondingly between 0% and 100%. Here, the Statgraphics 18 program applies a numerical search procedure to determine the local optimum over the entire range of mixture compositions studied. Maximizing all four target variables has advantages and disadvantages. One in favor is to determine the mix composition with which the best briquette strengths can be achieved. Another variant is to additionally aim at maximizing the proportion of Fe carriers or minimizing the necessary binder, which is advantageous with regard to high purity of the briquettes. However, as the briquettes should have sufficient cold strength as well as sufficient thermostability and reducibility, minimum binder content is disadvantageous. It is known that bentonite increases the thermal stability of the briquettes. Furthermore, the organic binders should improve the reducibility of the briquettes; low binder content or high Fe-carrier content is rather disadvantageous here. Additional briquettes were pressed from this mixture composition for the reduction tests presented in the following section.
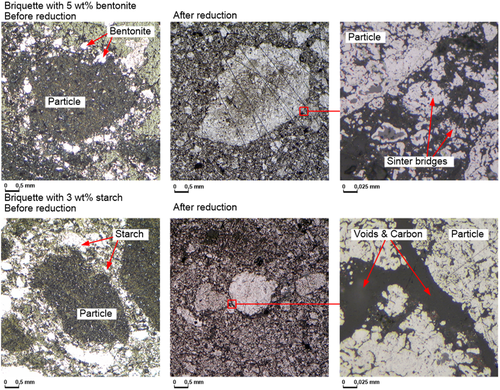
To explain the mechanism of binding during briquetting, microscopic images of the briquettes before and after reduction are shown in Figure 8 . Figure 8 shows exemplary sections of the structure of the briquette with 5 wt% bentonite as well as sections of the briquette structure of a briquette with 3 wt% starch before and after reduction. The binder (both bentonite and starch) accumulates around the individual particles, providing sufficient briquette strength (before reduction). For the briquettes with the combination of organic binder and bentonite, the microscopic investigations were not conducted. Starch and bentonite behave similarly during the briquetting process itself, in that they arrange themselves between the particles and hold them together due to cohesive forces within the binder. Using bentonite and an organic binder together, higher strengths can be achieved (Table 3) than when using the binders alone. In this case, the slightly higher strengths of the briquettes can be explained by the higher binder content.
3.2 Reducibility
Table 3 specifies in each line the composition of the briquettes that were reduced. It should be mentioned again that the briquettes used for the analysis of the mechanical properties and those used for the reduction featured different dimensions but were produced with the same pressing pressure and the same mixtures (see the materials section). In addition to the briquettes with the binder combination of bentonite/starch (B/S) and bentonite/cellulose (B/C), briquettes with solely bentonite (B) or solely cellulose (C) or solely starch (S) as binder were used for comparison. Furthermore, tests were conducted with reference iron ore pellets (P), which are the usual feed material to the Midrex shaft.
The briquettes B and the pellets P were completely intact after the reduction. During reduction experiments with briquettes B/S and S, the water inside the wash bottle turned yellowish already during the heating period with argon as purging gas. Later on, sugar crystallized from the aqueous phase in the wash bottle. After reduction the briquettes were not broken, but abraded at their corners and edges. During the experiments with briquettes B/C and C, a liquid phase condensed on the internal surface of the nonheated off-gas line. This condensate in connection with the very fine particles in the off-gas led to partial or complete blockade of the off-gas line, so the experiments with briquettes C had to be interrupted. After reduction/interruption, the briquettes were also not broken but abraded at the corners and edges. Summarizing, the briquettes with organic binder (with and without bentonite) did not break during reduction but showed strong abrasion at the edges, which will lead to problems when the charge is moved in the Midrex shaft. Starch saccharification is also critical for large-scale implementation, as off-gas cleaning is associated with challenges. In the comparative trials with pellets P, problems arose in one trial due to sintering of the pellets, which is not relevant in large-scale production because the bed is in motion.
The initial weight fraction of oxygen in the briquettes was calculated from Table 1. This fraction does not consider the oxygen contained in the binder but only the oxygen contained in the iron oxides of the residue. Assuming that all the weight loss measured with the weighing machine is due to the reduction of iron oxides to iron and the accompanying release of oxygen, the weight fraction of oxygen in the oxide form can be computed as a function of the reduction time. Figure 9 shows the temporal evolution of for all the samples, except for the sample C for which the tests had to be interrupted. The samples B, B/C, B/S, and S contain residue, which compared to the pellets P have already been reduced, at least in parts. Therefore, the initial value for the pellets P is the largest. The -values of the other samples vary just because of slightly different fractions of residue in the briquetted mixture. After 180 min of reduction, only the “S sample” features a smaller oxygen fraction compared to the other samples. The other samples have nearly all the same oxygen content, irrespective of their initial composition. By using the smaller briquette format and adding already reduced material, a similar weight fraction of oxygen can be achieved for the briquettes and the pellets after 3 h of reduction, so it is not a disadvantage to use the briquettes with bentonite in the reduction shaft at that residence time. This means that the use of organic binders and the associated problems can be avoided. The possible improvement in reducibility through the use of organic binders cannot be demonstrated on the basis of the reduction tests conducted. Only the briquettes with starch show a lower weight fraction of oxygen after reduction.
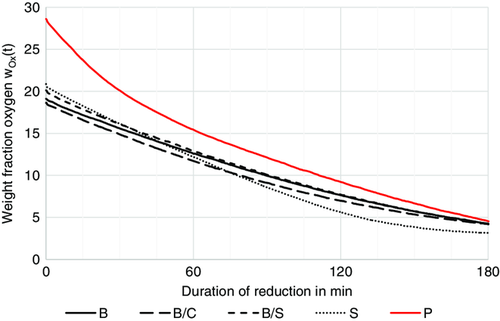
The microscopic images of the briquettes with starch and with bentonite (Figure 8) show the effect of the binders during reduction. In the case of the briquettes with starch, the thermal decomposition of the starch results in clearly visible voids and carbon remains between the particles, as expected. In the briquettes with bentonite, in contrast, no voids are visible and the binder remains in the briquette. However, the reduction tests showed that the formation of voids and their accessibility in the briquettes with starch are not sufficient to significantly improve oxygen release compared to the briquettes with bentonite.
3.3 Abrasion Resistance of Reduced Briquettes
Figure 10 shows that after 50 revolutions, the briquettes with bentonite are completely preserved and only slight edge abrasion is visible. The briquettes with pure organic binder have almost completely disintegrated. In the case of the binder combination bentonite/cellulose (B/C), some briquettes are still completely preserved and thus much more resistant than the briquettes with bentonite and starch. It should be noted that the briquettes with cellulose contain 5 wt% bentonite, whereas the briquettes with starch contain only 2 wt% bentonite. Higher bentonite content could certainly improve the stability of the starch briquettes under reduction conditions. A similar trend is also to be seen after 100 revolutions. Even though the addition of bentonite to the organic binders improves the strength of the briquettes somewhat, the abrasion resistance of the bentonite-bound briquettes is still very good after 100 revolutions, whereas all the other briquettes disintegrate. The organic binders cause the disintegration of the briquettes by suppressing the positive effect of the bentonite. For the briquettes with bentonite (B) and bentonite/cellulose (B/C), additional tests were conducted with 200 revolutions of the drum, confirming the previous findings. The reduced briquettes with pure bentonite as binder withstand the stresses in the abrasion drum even after 200 revolutions. Individual briquettes with the binder combination of bentonite and cellulose (B/C) also withstand this load, but most of these briquettes have completely disintegrated.
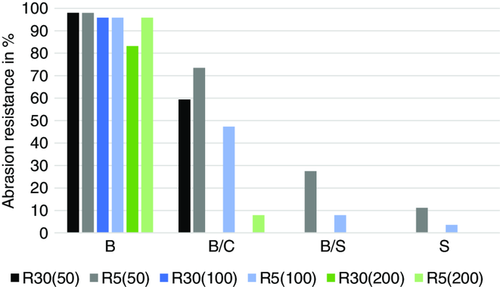
The microscopic images (Figure 8) can explain the different behavior of the briquettes with bentonite and organic binder. The binder accumulates around the individual particles, providing sufficient briquette strength before reduction. During reduction, the binders behave differently. The bentonite particles are still detectable after reduction and ensure the formation of sinter bridges between the now metallic iron particles. The organic binder, on the other hand, decomposes thermally, creating relatively large voids between the particles and leaving behind amorphous carbon. The voids between the metallic iron particles ensure that sintering is widely reduced. As a result, the briquettes break when subjected to mechanical stress. When an organic binder is used in combination with bentonite, voids as well as residues of carbon also remain in the briquette during reduction, which prevents sintering of the particles, as sufficient contact areas between the particles are required for this. The positive effect of the bentonite is suppressed, which confirms the findings on the thermal stability of briquettes with joint use of organic binder and bentonite.
3.4 Significance of the Binder for the Mechanical Stability of the Briquettes Under Elevated Temperatures
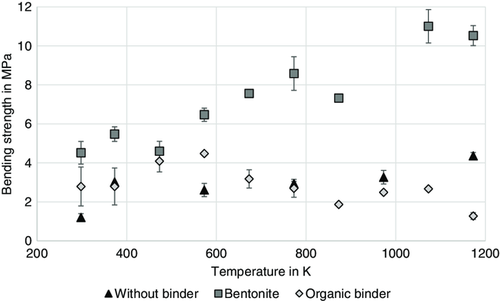
In the reduction tests, the strength of the briquettes can only be determined after cooling, which means that no statement can be made about the temperature-dependent behavior of the briquette strength. Therefore, tests were conducted in the Werner oven to be able to determine the mechanical strength of the briquettes also at elevated temperatures and to better observe the different behavior of briquettes with organic binder and bentonite. Figure 11 shows the temperature dependence of the bending strength of briquettes made from the residue mixture without binder, with the organic binder and with bentonite. For the briquettes without binder and with bentonite, an increase in bending strength with increasing temperature can be seen, with the strengths of the bentonite briquettes being significantly higher than those of the briquettes without binder. Bentonite has good sintering properties at low temperatures and thus favors the sintering processes in the briquette, which is why the bending strength also increases with increasing temperature for the briquettes without binder. For the briquettes with organic binder, an increase in bending strength can be seen up to about 573 K, after which there is a drop in bending strength. Accordingly, the results of the low-temperature disintegration test for bentonite and organic binder in Lohmeier et al.[ 3 ] are confirmed. The decrease in strength when using the organic binder and also the disintegration of the briquettes with the organic binder in the low-temperature disintegration tests can be justified by the thermal decomposition of the organic binders at about 573 K. The results of the test in the “Werner oven” therefore correspond very well with the data from the literature.[ 14, 30 ] The organic binder prevents sintering of the iron oxides and thus counteracts the occurring sintering mechanisms. It thus can be shown that the briquettes with the organic binder, unlike the briquettes with bentonite, are not mechanically stable at elevated temperatures and are therefore not suitable for use in the Midrex process. When the briquettes are used in the Midrex process, heating of the briquettes occurs and again the bentonite will contribute to the bonding within the briquette.[ 31, 32 ] The Na and Ca components act as fluxes and lower the melting point of some minerals in the agglomerate. This results in melting processes below the melting temperature, which lead to the emergence of additional bonds.[ 33 ] Consequently, the organic binders (used alone or also in combination with inorganic bentonite) are well suited in terms of cold strength of the briquettes, but the mechanical resistance of these briquettes at elevated temperatures is not given. Consequently, the use of organic binder in residual briquetting for the Midrex process cannot be recommended on the basis of these test results.
4 Conclusion
The objective of this research is the combined use of bentonite and an organic binder in the briquetting of residual materials from the Midrex process, with the goal of being able to produce briquettes that are mechanically stable at elevated temperatures and sufficiently reducible for reuse in the Midrex process. The following conclusions are drawn. 1) The use of an organic binder (alone and in combination with bentonite) is not useful. The mechanical stability of the briquettes under Midrex-relevant conditions is insufficient because the organic binders decompose thermally and thus suppress the sintering mechanisms in the residual mixture. A faster oxygen release during reduction compared to the briquettes with bentonite could not be demonstrated. 2) Briquetting of the residual materials with bentonite is possible. Because bentonite positively influences the sintering mechanisms occurring in the residue mixture, the briquettes are thermally stable under Midrex-relevant conditions. A blend composition consisting of 5 wt% bentonite, 5 wt% water, and 90 wt% Fe-carrier blend was found to be suitable for briquetting the residual materials in terms of mechanical strength under ambient conditions as well as under Midrex-relevant conditions. 3) To achieve sufficient metallization of the briquettes, it is necessary to use a residual mixture with sufficient amount of metallic iron (especially by adding HBI-screened fines). This enables a higher iron content of the briquettes before reduction, which means that less oxygen has to be released during reduction. The briquettes with 5 wt% bentonite as binder had a Fetot of 71.1 wt% and a Femet of 28.1 wt%, which were shown to be sufficient in the reduction tests. Furthermore, for sufficient metallization of the briquettes, the smallest possible briquette format must be selected. The tests were conducted with briquettes with a volume of 10 cm3, which proved to be suitable in terms of reducibility compared to previous tests with a larger briquette format. A continuation of the briquetting tests to further reduce the briquette format is reasonable as this will result in a further improvement of the oxygen release during the reduction. This could eliminate the use of already reduced components of the residual mixture. 4) For the large-scale implementation of residual briquetting, it is necessary to demonstrate the feasibility of briquetting on an industrial roller press. In particular, further optimization of briquetting should take place with regard to the briquette format. The smallest possible briquette size on industrial roller presses is 5 cm3. However, briquetting on roller presses at this small briquette size often presents technical challenges that need to be identified and eliminated.
Acknowledgements
The authors gratefully acknowledge the funding support of K1-MET GmbH, metallurgical competence center. The research program of the K1-MET competence center is supported by COMET (Competence Center for Excellent Technologies), the Austrian program for competence centers. COMET is funded by the Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation and Technology, the Federal Ministry for Digital and Economic Affairs, the Federal States of Upper Austria, Tyrol and Styria as well as the Styrian Business Promotion Agency (SFG). Apart from the public funding from COMET, this research project was partially financed by the scientific partner TU Bergakademie Freiberg (TUBAF), Institute of Thermal-, Environmental-, and Resources’ Process Engineering (ITUN) and the industrial partner voestalpine Stahl GmbH Linz. Furthermore, the authors would like to thank the Institute for Iron and Steel Technology of TU Bergakademie Freiberg for the opportunity to conduct the reduction tests and the Institute of Processing Machines and Recycling Systems Technology of TU Bergakademie Freiberg for the opportunity to conduct the microscopic investigations.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Research data are not shared.




