Evolutionary pattern of Metacaremys gen. nov. (Rodentia, Octodontidae) and its biochronological implications for the late Miocene and early Pliocene of southern South America
Abstract
We analyse the taxonomic status and diversity of the late Miocene Octodontoidea (Hystricognathi) Cercomys primitiva and related samples, a taxon previously known only from the holotype specimen. New findings associated with an extensive review of late Miocene and early Pliocene rodents have allowed us to recognize the occurrence of this and other related species in several localities of central and western Argentina, and in south-central Bolivia. We discuss the invalidity of Cercomys and propose the new genus Metacaremys, which includes the type species Metacaremys primitiva comb. nov. and two new species, Metacaremys calfucalel sp. nov. and Metacaremys dimi sp. nov. Osteological, brain and dental morphology show that the new genus is not related to Brazilian Echimyidae, as previously considered, but to the southern family Octodontidae. Although the molar morphology of this genus is quite conservative, comparison of the samples shows a variation in size. We discuss the plausible evolutionary pattern explaining this variation and the implied biochronological and biostratigraphical information. It is recognized as an anagenetic lineage in which an increase in size occurs from the oldest species, M. primitiva comb. nov. (early late Miocene, c. 9.23 Ma), to the youngest species, M. dimi sp. nov. (Miocene–Pliocene boundary, c. 5.28 Ma). Metacaremys calfucalel is intermediate in size and age between these two species. The polarity of this pattern of change is consistent with that shown by other partially synchronous independent lineages of octodontoids, thus providing new evidence for the biochronological and biostratigraphic scheme of the late Miocene and early Pliocene of southern South America.
The superfamily Octodontoidea is the most widely diversified clade among both New World and Old World hystricognath rodents (Woods & Kilpatrick 139; Wilson et al. 135). In South America, this clade is represented by 32 living genera of the families Abrocomidae, Ctenomyidae, Echimyidae and Octodontidae (Patton et al. 76; Emmons & Fabre 32), and more than 70 extinct genera of which nearly 20% remains to be formally described (Vucetich et al. 130; DHV unpub. data). In contrast to this extensive taxonomic diversification, octodontoids have remained morphologically conservative, the taxa of this group being unique among South American hystricognaths in retaining a rat-like appearance (Wilson et al. 135). This apparently narrow range of morphological innovation in combination with high diversity make the systematic interpretations, especially of fossils, unstable (e.g. Arnal & Vucetich 5; Verzi et al. 119).
A global Cenozoic trend of cooling and drying following the Middle Miocene Climatic Optimum (Denton 26; Zachos et al. 141, 142; Tripati et al. 0105), along with Andean orogeny, which promoted a rainshadow in most of southern South America, transformed the continent into multiple distinctive biomes (Pascual et al. 73; Palazzesi & Barreda 68; Le Roux 52; Upham & Patterson 0108). This promoted the acquisition by selection of derived, hypsodont molar morphologies in southern octodontoids as an adaptation to increasingly open environments (Pascual 69; Verzi 110; Verzi et al. 112, 117). The appearance of hypsodont lineages, and the extinction of those with more primitive low-crowned and rooted molars, marks the beginning of the stage of modernization of the families Abrocomidae, Ctenomyidae and Octodontidae since the late Miocene (Verzi et al. 117, 118, 119). This pattern is evident in the fossil record of southern South America, but it is especially apparent in the Cerro Azul Formation (sensu Folguera & Zárate 36) in central Argentina (Pascual et al. 71; Pascual 69; Reig 87). In this formation, a clear turnover in the composition of the octodontoid faunas is detected for the interval c. 9–5 Ma (late Miocene to early Pliocene). This turnover implies the increasing prevalence of hypsodont species, involving in situ gradual evolution of different lineages (Verzi et al. 114, 115, 116; Sostillo et al. 100).
Although the systematics at the supraspecies level of modern hypsodont octodontoids resulting from this turnover shows consensus, this is not the case for taxa with conservative molar morphologies from the same deposits (e.g. Chasichimys Pascual, 69, Chasicomys Pascual, 69, Neophanomys Rovereto, 92, Pampamys Verzi, Vucetich & Montalvo, 113; see Pascual 69; Reig 88; Arnal & Vucetich 5; Olivares et al. 66; Verzi et al. 117; Boivin et al. 11). In this context, the knowledge of the diversity of octodontoids from the late Miocene of central Argentina is still limited to partial reviews (e.g. Quintana 85; Verzi 110; Verzi et al. 114, 116; Olivares et al. 66, 67; Sostillo et al. 100), original descriptions (e.g. Rovereto 92; Pascual et al. 71; Pascual 69), or even to lists of taxa not formally described (Verzi 111). Such is the case for the late Miocene protohypsodont octodontoid Cercomys primitiva, which was previously known only by the holotype (Pascual 69). New findings, and a comprehensive review of late Miocene rodents, allowed us to recognize the presence of this and other related species in 16 localities of central and western Argentina, and in south-central Bolivia. Given that the name Cercomys is currently inapplicable (see below), we here propose a new genus for this sample. In addition, we revise the variation within this genus, describe two new species and discuss the evolutionary pattern of the lineage.
Because of their wide geographic distribution and high reproductive and rapid evolutionary rates (Vianey-Liaud et al. 123), rodents are an essential tool for the identification of biochronological and biostratigraphic units in the Neogene continental deposits, and therefore for the dating of sediments of this interval. Accordingly, aside from providing the taxonomic and evolutionary meaning of the variation of the new genus, we discuss its biochronological and biostratigraphical implications for the late Miocene and early Pliocene of southern South America.
Material and method
The 73 specimens studied here are housed in the following palaeontological collections (see below for explanation of abbreviations): PV-UNS, GHUNLPam, MMP; MLP; MD; MMH; IANIGLA; PVSJ. In addition, two specimens were compared using available illustrations (GEOBOL MH-41; CRILAR Pv 431).
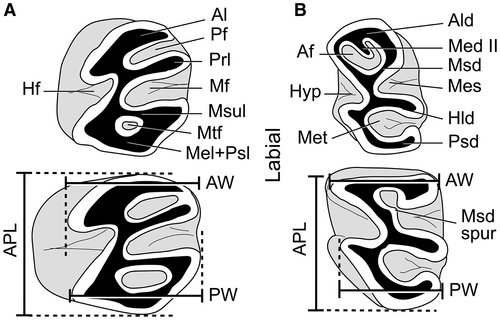
The nomenclature of craniomandibular traits follows Woods & Howland (138), Patterson & Wood (75), Wahlert (132), and Verzi et al. (119); the nomenclature of endocranial morphology follows Bertrand et al. (10). Dental nomenclature follows Verzi et al. (119) and modifications by Verzi et al. (121). We use quotation marks for ‘Acarechimys’ leucotheae Vucetich, Dozo, Arnal & Pérez, 131, according to Verzi et al. (120). The anteroposterior length (APL) of the available dental series (dp4–m3/DP4–M3) was measured, as well as the APL, anterior width (AW) and posterior width (PW) of each molariform (Fig. 1). To estimate the relative increase in size of m3 between species, we provide an m3/m1 length index using the ratio of APL m3 to APL m1 for the m1–3 series. All the measurements are expressed in millimetres and were taken on the occlusal plane of the teeth using Leica LAS Interactive Measurement, from photographs taken with a Leica DMC 2900 digital camera connected to a Leica MS5 stereomicroscope. Measurements of each specimen are listed in Piñero et al. (82, table S1). Additional photographs are included in Piñero et al. (82, figs S1–S2).
For each molar type, bivariate analyses were conducted to identify and visualize clusters of size using measurement data. Pairwise comparison of the mean values of groups (species) was carried out using analysis of variance (anova) and Fisher's least significant difference (LSD) test at a 5% significance level. The non-parametric Kruskal–Wallis test was used to test differences in the median values among the groups. Discriminant analyses were performed to test the reliability and statistical significance of these groups. A fitted linear regression model was used to determine if there was a relationship between dp4–m3 APL and group. The lower teeth were used for the measurements because they are the most abundant elements. These analyses were performed using Statgraphic Centurion XVI.I.
Given that dental wear influences the measurements, the latter were examined taking into account wear categories. The following scores were noted for each molariform: stage 0, no wear; stage 1, very slight wear (in which the enamel of the anterolophid and/or the anterior arm of the hypoconid is interrupted in the lower teeth, and the paraflexus and/or metaflexus are open in the upper teeth); stage 2, slight wear (in which the enamel of the lophids is continuous and the mesoflexid and metaflexid are open in the lower teeth, and the closure of parafossette is incipient in the upper teeth); stage 3, moderate wear (in which at least the metaflexid is almost closed in the lower teeth, and the parafossette is reduced in the upper teeth); stage 4, advanced wear (in which the mesofossettid and/or the metafossettid is completely formed in the lower teeth, and the parafossette and metafossette are absent or are present as a very small remnant in the upper teeth); and stage 5, very advanced wear (in which the mesofossettid and/or metafossettid are very reduced or absent in the lower teeth, and the closure of the mesofossette is complete in the upper teeth).
To comparatively analyse a natural endocast of the new genus, virtual endocasts of extant octodontoids were obtained. X-ray microcomputed tomography of the skull of selected octodontoids was acquired using a table top scanner (SkyScan/Bruker, model 1173). Pixel size was between 0.040 and 0.067 mm (see scan parameters in Piñero et al. 82, table S2). Three-dimensional virtual endocasts were generated from these data following a threshold-based 2D segmentation procedure with 3D Slicer software (Fedorov et al. 34). The 3D surface models for each virtual endocast were generated and saved in PLY file format.
Institutional abbreviations
CRILAR, Centro Regional de Investigaciones Científicas y Transferencia Tecnológica de La Rioja, Argentina; GEOBOL, Servicio Geológico de Bolivia, La Paz, Bolivia; GHUNLPam, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, Santa Rosa, Argentina; IANIGLA, Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales, Mendoza, Argentina; IMCN, Instituto y Museo de Ciencias Naturales, San Juan, Argentina; MACN, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires, Argentina; MD, Museo Municipal de Ciencias Naturales ‘Carlos Darwin’, Punta Alta, Argentina; MN-UFRJ, Museu Nacional, Universidade Federal do Rio de Janeiro, Brazil; MMH, Museo Municipal de Ciencias Naturales ‘Vicente Di Martino’, Monte Hermoso, Argentina; MMP, Museo Municipal de Ciencias Naturales ‘Lorenzo Scaglia’, Mar del Plata, Argentina; MLP, Museo de La Plata, La Plata, Argentina; PVSJ, Instituto y Museo de Ciencias Naturales, Universidad Nacional de San Juan, San Juan, Argentina; PV-UNS, Departamento de Geología, Universidad Nacional del Sur, Bahía Blanca, Argentina.
Systematic palaeontology
Order RODENTIA Bowdich, 13Suborder HYSTRICOGNATHI Tullberg, 0107Parvorder CAVIOMORPHA Wood, 137Superfamily OCTODONTOIDEA Waterhouse, 134Family OCTODONTIDAE Waterhouse, 134Genus METACAREMYS nov.Figures 2–5; Piñero et al. (82, figs S1–S2)
LSID
urn:lsid:zoobank.org:act:56D865C2-81BC-4F74-9778-0C507877D7EB
Derivation of name
Greek, meta, beyond, and Acaremys Ameghino, 2, referring to its similar, although more derived, morphology with respect to this genus.
Type species
Cercomys primitiva Pascual, 69; from Arroyo Chasicó, Argentina.
Included species
The type species, M. calfucalel sp. nov. and Metacaremys dimi sp. nov.
Emended diagnosis
Octodontoid larger than Neophanomys, Acaremys minutissimus Ameghino, 2 and ‘Acarechimys’ leucotheae. Cranium with the tip of the lateral process of the supraoccipital dorsal to the mastoid process. Mandible proportionally lower than that of Neophanomys, with the chin process slightly more posterior than in A. minutissimus, at the level of the anterior part of the dp4. Masseteric crest descending from the posterior part of the notch for the tendon of m. masseter medialis, pars infraorbitalis. Notch subhorizontal. Lateral crest straight, descending toward the masseteric notch following the same direction as the anterior margin of coronoid apophysis. Lower incisor very slender, with enamel more labially extended than in A. minutissimus and Neophanomys. Fourth deciduous molars (DP4/dp4) functional throughout life. Upper molars tetralophodont, with the mesoflexus/fossette more persistent and wider than the paraflexus/fossette and metaflexus/fossette. Mesolophule originated from the mure, and with its labial end joined to the medial wall of the metacone area in M1–2. DP4 with the mesolophule not reduced. Juvenile dp4 pentalophodont, with a principal oblique spur of the metalophulid II, and the mesolophid anterolingually oriented; adult dp4 bilobed or suboval. m1–3 trilophodont in non-senile specimens; atypically, remnant tetralophodonty in m1; m1–2 with protoconid and hypoconid areas labially extended and acuminate, and mesoflexid more persistent than metaflexid.
Description
Small octodontoid, similar in size to Acaremys murinus Ameghino, 2 and larger than A. minutissimus, ‘Acarechimys’ leucotheae and Neophanomys. The anterior zygomatic root originates at the level of the anterior portion of the DP4. The specimen PVSJ-LT103 preserves a fragment of palate, and an associated natural brain endocast with a partial auditory region. On this specimen, the mesopterygoid fossa extends to the level of the middle part of the M2. Although the auditory region does not preserve the lateral process of the supraoccipital, the existing fissure in its place indicates that this process was short and located dorsal to the mastoid process. The endocast preserves most of the cerebral hemispheres, part of the right transverse sinus and the confluence of sinuses, and part of the cerebellum. Nevertheless, this preservation is partial and limits the full understanding the neuroanatomy of this genus. In dorsal view, the morphology of the endocast is similar to the virtual brain endocast of Aconaemys Ameghino, 3 and Octomys Thomas, 0103 (Fig. 2). As in the latter, the hemispheres are somewhat wider posterior to the trace of the frontoparietal suture than anterior to this trace. Hemispheres are markedly widened posterior to the suture trace in Abrocoma Waterhouse, 133 whereas in echimyids their greatest width is roughly at the level of this trace (Fig. 2). A slight concavity is present at the level of the orbital region, as it occurs in Abrocoma, Aconaemys and Octomys, and unlike in echimyids, in which it is not visible in dorsal view. In lateral view, this concavity is similar to that of Aconaemys and Octomys. The presence or absence of exposure of the midbrain could not be determined on the endocast of Metacaremys because of a lack of preservation. In the cerebellum, only the vermis is visible whereas the lateral lobes are partially covered by bone. The virtual endocasts are available in MorphoSource (Piñero et al. 83).
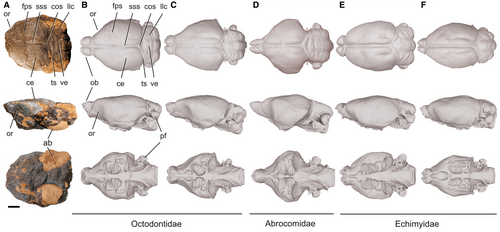
The corpus of the mandible (Fig. 3) is lower than that of Neophanomys; the chin process is at the level of the anterior part of the dp4, slightly more posterior than in A. minutissimus, in which it is anterior to the dp4. The notch for the tendon of the m. masseter medialis is developed as a semicircular to elliptical, subhorizontal step; the masseteric crest descends posteroventrally from the posterior part of the notch, and as such its orientation is decoupled from that of the notch. This morphology is like that of Acaremys and modern octodontids, and different to that of Acarechimys Patterson in Pascual, 69, Ameghinomys Verzi, Olivares & Morgan, 120, Caviocricetus Vucetich & Verzi, 128, and other stem abrocomids in which the notch lies on the origin of the masseteric crest and descends posteroventrally together with the crest. In Metacaremys, the lateral crest descends straight towards the posterior part of the notch, as it occurs in stem and modern octodontids, ctenomyids and echimyids. Different to this configuration, the lateral crest is positioned more ventrally along the mandibular body in stem abrocomids, and descends more abruptly than the anterior margin of the coronoid apophysis, describing a curve that rises at its anterior extreme, which corresponds to the anterior end of the masseteric notch. This variation in the masseteric morphology in octodontoids, associated with the insertion of the m. masseter medialis, provides potentially valuable systematic and phylogenetic information. The lateral crest is the most dorsal and medial site of insertion for the fibres of the m. masseter medialis, pars zygomatico-mandibularis; in those species in which this crest is lower, a more ventral arrangement of this fibre package would be expected.
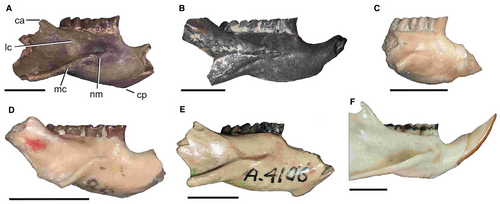
The lower incisor is very slender and has a curved anterior face, similar to that of Neophanomys. The occlusal surface is long; its anterolingual enamelled edge is sharp, while the anterolabial one is smoothly curved. The enamel extends on the labial side more than in A. minutissimus and Neophanomys, and occupies at least half the thickness of the tooth. The base is deeply inserted, behind the m3.
The molars are higher crowned than those of A. minutissimus, A. murinus, ‘Acarechimys’ leucotheae and Galileomys Vucetich & Kramarz, 126. The DP4 and dp4 are functional throughout life.
The discovery of the associated palate and mandible fragments PVSJ-LT30 (Piñero et al. 82, fig. S1G, I) at the Loma de Las Tapias site (Loma de Las Tapias Formation, western Argentina), provides unequivocal information on the morphology of the upper molars of Metacaremys. This allows the maxillary remains and upper molars from this and other localities to be assigned to this genus (see below). The DP4–M3 are tetralophodont. The mesoflexus/fossette of these molars is more persistent than the paraflexus/fossette or the metaflexus/fossette, and its bottom is wider; accordingly, the mure is long. The mesoflexus (or the bottom of the mesofossette) of specimens from the Huayquerian (late late Miocene to early Pliocene) is wider than that of specimens from the Chasicoan (early late Miocene). This morphology is shared with stem octodontids Acaremys, ‘A’. leucotheae, Neophanomys, and Platypittamys Wood, 136, and differs from that of Acarechimys and Ameghinomys in which the bottom of the mesoflexus is narrower and, consequently, the mure is short. In Caviocricetus and Dudumus Arnal, Kramarz, Vucetich & Vieytes, 6, the bottom of both the mesoflexus and the para- and metaflexus is wide. The adult DP4 has an inflection on the anterior lingual surface that generates a projection on the anterior face; a similar morphology is present in Neophanomys. The lingual side of the protocone area of the M1 is straight, so that together with the antero- and protoloph they form a triangular-like anterior lobe. In the M2, the lingual side of the protocone is slightly more curved. All these molars have flattened occlusal surfaces. Nevertheless, in the juvenile M1 GHUNLPam 6924 (stage 0; Piñero et al. 82, table S1) from Cerro La Bota (Cerro Azul Formation, central Argentina), the protocone and metacone areas are cusp like (Piñero et al. 82, fig. S1B). The morphology of this molar is very similar to that of the M1 of the likewise juvenile MLP 15-410, lectotype of A. murinus. Both have the anteroloph and mesolophule shorter than the protoloph and posteroloph, with their ends oriented towards the paracone and metacone areas, respectively.
The juvenile dp4 is pentalophodont. The metalophulid II is represented by two spurs originating on the anterolophid, the labial one being more developed. The mesolophid is a strong, anterolingually oriented crest as in ‘Acarechimys’ leucotheae, Acaremys minutissimus and Dudumus. In more worn dp4, these lophids, anterior to the hypolophid, form a rounded to subrhombic lobe with a central anterofossettid; the oldest specimens have a simple, suboval dp4 without flexids. The m1–3 are trilophodont, with the flexids wider and with their closure more synchronous than in Chasichimys. The protoconid and hypoconid areas of m1–2 are more labially extended and pointed than in A. leucotheae, A. minutissimus and Chasichimys, as also observed in Acaremys.
Occurrence
Lower upper Miocene and lower Pliocene of central Argentina; upper Miocene of western and north-western Argentina, and south-central Bolivia.
Metacaremys primitiva (Pascual, 69) comb. nov.Figures 4, 5A–H; Piñero et al. (82)
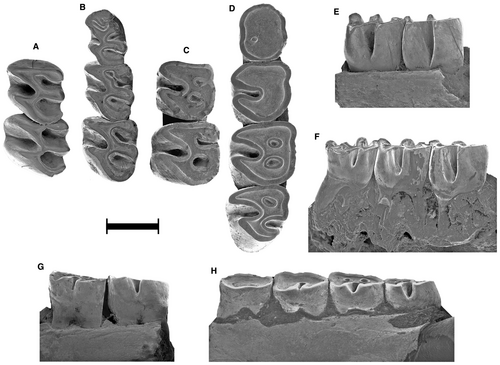

Holotype
MMP 317-M, left mandibular fragment with m1–2 and dp4 alveolus (Fig. 4C, G).
Type locality
Arroyo Chasicó, Buenos Aires Province, central Argentina. Cerro Azul Formation, Chasicoan, lower upper Miocene.
Referred material
See Piñero et al. (82).
Emended diagnosis
The smallest species of Metacaremys. m1–2 with occasional presence of mesolophid spur. Mesoflexid more persistent than metaflexid, especially in the m1. Bottom of the metaflexid/fossettid of the m2 curved.
Measurements
See Table 1 and Piñero et al. (82).
| Species | Molar | APL | AW | PW | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Min. | Mean | Max. | SD | n | Min. | Mean | Max. | σ | n | Min. | Mean | Max. | SD | ||
| M. primitiva | 6 | 8.69 | 8.93 | 9.14 | 0.174 | |||||||||||
| M. calfucalel | Dp4–m3 | 4 | 10.01 | 10.35 | 10.71 | 0.347 | ||||||||||
| M. dimi | 1 | 11.87 | ||||||||||||||
| M. primitiva | Dp4 | 18 | 1.82 | 2.22 | 2.43 | 0.156 | 17 | 1.16 | 1.39 | 1.68 | 0.147 | 18 | 1.34 | 1.58 | 1.81 | 0.136 |
| M. calfucalel | 8 | 2.67 | 2.79 | 2.89 | 0.075 | 7 | 1.41 | 1.54 | 1.74 | 0.104 | 8 | 1.66 | 1.81 | 1.97 | 0.103 | |
| M. dimi | 2 | 2.46 | 2.83 | 3.20 | 0.518 | 2 | 1.54 | 1.74 | 1.93 | 0.277 | 2 | 1.86 | 1.98 | 2.10 | 0.165 | |
| M. primitiva | m1 | 29 | 1.94 | 2.25 | 2.49 | 0.121 | 28 | 1.71 | 1.97 | 2.22 | 0.141 | 28 | 1.69 | 1.96 | 2.29 | 0.179 |
| M. calfucalel | 16 | 2.39 | 2.62 | 2.88 | 0.128 | 15 | 1.53 | 2.06 | 2.34 | 0.196 | 16 | 1.78 | 2.17 | 2.46 | 0.179 | |
| M. dimi | 3 | 2.74 | 2.96 | 3.33 | 0.326 | 3 | 2.53 | 2.59 | 2.64 | 0.055 | 3 | 2.55 | 2.62 | 2.70 | 0.077 | |
| M. primitiva | 26 | 2.10 | 2.43 | 2.62 | 0.151 | 25 | 1.70 | 2.21 | 2.63 | 0.210 | 25 | 1.69 | 2.18 | 2.59 | 0.230 | |
| M. calfucalel | m2 | 12 | 2.49 | 2.77 | 3.14 | 0.199 | 10 | 1.85 | 2.33 | 2.91 | 0.332 | 11 | 1.83 | 2.34 | 2.90 | 0.321 |
| M. dimi | 3 | 3.29 | 3.35 | 3.45 | 0.092 | 3 | 3.12 | 3.20 | 3.31 | 0.098 | 3 | 3.11 | 3.24 | 3.32 | 0.110 | |
| M. primitiva | m3 | 14 | 1.68 | 2.17 | 2.39 | 0.216 | 13 | 1.34 | 2.07 | 2.32 | 0.269 | 13 | 1.33 | 1.85 | 2.08 | 0.209 |
| M. calfucalel | 9 | 2.35 | 2.51 | 2.79 | 0.155 | 9 | 1.56 | 2.12 | 2.59 | 0.298 | 9 | 1.36 | 1.93 | 2.23 | 0.260 | |
| M. dimi | 2 | 3.23 | 3.40 | 3.58 | 0.241 | 2 | 2.95 | 2.97 | 2.99 | 0.028 | 2 | 2.83 | 2.84 | 2.85 | 0.018 | |
| M. primitiva | DP4–M3 | 3 | 7.719 | 8.147 | 8.362 | 0.371 | ||||||||||
| M. calfucalel | 2 | 8.64 | 9.26 | 9.87 | 0.867 | |||||||||||
| M. primitiva | 7 | 1.93 | 2.06 | 2.20 | 0.093 | 6 | 1.21 | 1.62 | 2.05 | 0.27 | 7 | 1.51 | 1.82 | 2.05 | 0.210 | |
| M. calfucalel | DP4 | 3 | 2.20 | 2.14 | 2.26 | 0.056 | 3 | 2.23 | 2.33 | 2.51 | 0.16 | 3 | 1.19 | 1.92 | 2.39 | 0.641 |
| M. dimi | 1 | 2.51 | 1 | 2.451 | 1 | 2.52 | ||||||||||
| M. primitiva | 7 | 1.95 | 2.12 | 2.26 | 0.120 | 7 | 1.96 | 2.16 | 2.36 | 0.180 | 7 | 1.97 | 2.10 | 2.36 | 0.141 | |
| M. calfucalel | M1 | 4 | 2.29 | 2.45 | 2.64 | 0.157 | 4 | 2.25 | 2.52 | 2.84 | 0.254 | 4 | 2.20 | 2.35 | 2.52 | 0.151 |
| M. dimi | 1 | 2.64 | 1 | 2.61 | 1 | 2.72 | ||||||||||
| M. primitiva | 6 | 2.02 | 2.16 | 2.32 | 0.101 | 5 | 1.92 | 2.06 | 2.41 | 0.20 | 4 | 1.81 | 2.00 | 2.18 | 0.180 | |
| M. calfucalel | M2 | 2 | 2.26 | 2.54 | 2.83 | 0.406 | 2 | 2.49 | 2.68 | 2.86 | 0.26 | 2 | 2.24 | 2.50 | 2.77 | 0.368 |
| M. dimi | 1 | 2.81 | 1 | 2.66 | 1 | 2.52 | ||||||||||
| M. primitiva | M3 | 4 | 1.52 | 1.70 | 1.91 | 0.213 | 4 | 1.62 | 1.84 | 2.17 | 0.249 | 4 | 1.23 | 1.42 | 1.77 | 0.251 |
| M. calfucalel | 2 | 2.16 | 2.34 | 2.53 | 0.262 | 2 | 2.34 | 2.49 | 2.64 | 0.212 | 2 | 1.78 | 1.95 | 2.12 | 0.240 | |
| Mandible | Dm | |||||||||||||||
| M. primitiva | 11 | 5.25 | 6.02 | 6.75 | 0.240 | |||||||||||
| M. calfucalel | 6 | 5.50 | 6.78 | 7.31 | 0.080 | |||||||||||
| M. dimi | 1 | 7.66 | ||||||||||||||
- Abbreviations: APL, anteroposterior length; AW, anterior width; Dm, Depth of mandible below m1; PW, posterior width; SD, standard deviation.
Description
Metacaremys primitiva is the smallest species of the genus. The relative size of the upper permanent molars is M2 > M1 > M3, except for MMP 1501-M (holotype of Chasicomys cangapoli; Fig. 5B) in which M1 and M2 are subequal (see Quintana 85).
The scarcely worn dp4 is pentalophodont. The metalophulid II of the dp4 of MMH-CH 84-4-104 (Fig. 5E) and MMP 1507-M (Fig. 4B) is represented by two spurs, the labial one being more developed. In GHUNLPam 21986 (Piñero et al. 82, fig. S1R) and CRILAR Pv 431 (Piñero et al. 82, fig. S1H), this lophid is represented by a single labial spur, which is more transverse in the latter. In the dp4 of more worn specimens, the lophids anterior to the hypolophid form a rounded to subrhombic lobe, with one (Fig. 5F and Piñero et al. 82, fig. S1P) or two (Fig. 5C and Piñero et al. 82, fig. S1R) central anterofossettid/s. The oldest specimens have a simple, suboval dp4 without flexids/fossettids (Fig. 5H and Piñero et al. 82, fig. S1K, Y). The m1–3 are trilophodont in juvenile stages. The m2 is the largest molar, whereas m1 and m3 are similar in size. The closure of lingual flexids is slightly asynchronous, especially in the m1. As it occurs in protohypsodont octodontids and ctenomyids, the mesoflexid is more persistent than the metaflexid in m1–2; this is more evident on the m1. The bottom of the metaflexid on the m1–2 is curved. Lingual flexids/fossettids are lost in senile specimens; the hypoflexid is significantly more persistent than the lingual flexids/fossettids. In labial view, the hypoflexid extends along most of the crown height, and it is separated from the roots by a low basal zone lacking flexids (Fig. 4). A spur of the mesolophid is present on the m1 of MMP 1507-M (Fig. 4B) and of MLP 58-IX-3-24 (Piñero et al. 82, fig. S1Q), and is also present on the isolated m1 or m2 of GHUNLPam 6922 and 6929. On GHUNLPam 6922 (Fig. 5D), this spur is in contact with the expanded lingual end of the anterolophid (which includes the lingual portion of the mesolophid), both delimiting a reduced anterofossettid as in Neophanomys recens Verzi et al., 116 GHUNLPam 19604. This variation shows how the trilophodonty of Metacaremys is derived by reduction from a tetralophodont morphology. A similar trend to the reduction of the mesolophid can be seen in the lectotype of Acaremys tricarinatus Ameghino, 4 MACN A-4113. An anteroposterior cristid joins the anterolophid with the hypolophid on the m2–3 of GHUNLPam 303 (Piñero et al. 82, fig. S1U), but it represents a very particular case. On the m3 of M. primitiva comb. nov., the metaflexid is more persistent than the mesoflexid.
Remarks
Quintana (85) erected Chasicomys cangapoli based on a single maxilla with DP4–M3 from the Vivero Member of Cerro Azul Formation in Arroyo Chasicó (MMP 1501-M; Fig. 5B). Newly recovered material from Loma de Las Tapias (Loma de las Tapias Formation, western Argentina), including an associated palate and mandible (Piñero et al. 82, fig. S1G, I), allowed us to determine that Chasicomys cangapoli is a junior synonym of Cercomys primitiva (i.e. M. primitiva comb. nov.). In addition, in his review of the late Miocene octodontoids of Arroyo Chasicó, Pascual (69) included the specimen MLP 55-IV-28-3 (Piñero et al. 82, fig. S1V) into the variation of Chasichimys bonaerense. Verzi (110) noted that this specimen belonged to a different octodontoid genus. The reanalysis of this material in the context of variation of the currently available samples suggests that it should be assigned to M. primitiva. Finally, we here assign to M. primitiva comb. nov. the specimens CRILAR Pv 431 and GEOBOL MH-441, originally reported as Octodontidae indet. (Brandoni et al. 14) and cf. Sciamys sp. (Villarroel & Marshall 124), respectively.
Occurrence
Late Miocene deposits of western, north-western and central Argentina, and south-central Bolivia. Cerro Azul Formation (Chasicoan and early Huayquerian, lower upper Miocene, central Argentina); Loma de las Tapias Formation, Arenisca Albardón Member (Chasicoan, lower upper Miocene, western Argentina); Salicas Formation (upper Miocene, north-western Argentina); Estratos Muyu Huasi, middle levels (upper Miocene, south-central Bolivia). Localities and fossil-bearing deposits are detailed in Piñero et al. (82) (see Fig. 6).
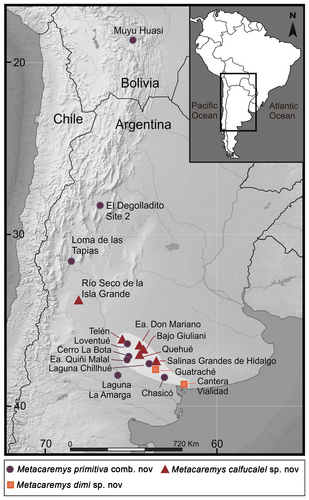
The holotype comes from the lower levels (Vivero Member) of the ‘Arroyo Chasicó Formation’ (currently, Cerro Azul Formation), Chasicoan, lower upper Miocene, central Argentina.
Metacaremys calfucalel sp. nov.Figure 5I–P; Piñero et al. (82)
LSID
urn:lsid:zoobank.org:act:EDE8E45C-E778-4646-854A-08C5E09A52BB
Derivation of name
From the Mapuche, Kallfü, azul (blue) and calel, cerro (hill), in reference to Cerro Azul Formation, the main fossil-bearing deposit of the sample.
Holotype
GHUNLPam 5039, right mandibular fragment with dp4–m3 (Fig. 5M).
Type locality
Estancia Don Mariano, La Pampa Province, central Argentina. Cerro Azul Formation, Huayquerian, upper upper Miocene.
Referred material
See Piñero et al. (82).
Diagnosis
Medium-sized species of Metacaremys with slightly asynchronous closure of lingual flexids, the mesoflexid being more persistent. Bottom of metaflexid of m2 straight and wider than in M. primitiva comb. nov. Average APL of dp4–m3 around 15% longer than in M. primitiva comb. nov. Average APL of m2 and m3 around 20% and 30% shorter, respectively, than in Metacaremys dimi sp. nov. Bottom of mesoflexus/fossette of the M1 slightly wider (mure anteroposteriorly longer) than in M. primitiva. Spur of the mesolophid absent in m1–2.
Measurements
Description
Medium-sized species of Metacaremys, nearly 15% larger than M. primitiva. The morphology of incisors and molars of M. calfucalel sp. nov. is quite similar to that of M. primitiva comb. nov. The mesoflexus/fossette of the M1 is slightly wider, and the mure longer, than in the last species. The two complete DP4–M3 series (GHUNLPam 18889 and 18417; Fig. 5I and J, respectively) have the M2 larger than the subequal M1 and M3. The m1–2 lack the mesolophid spur. Closure of the lingual flexids in these molars is slightly asynchronous, with the metaflexid being somewhat less persistent than the mesoflexid. The bottom of the metaflexid of m2 is straight and wider than in M. primitiva comb. nov. The m1 and m3 are similar in size and smaller than the m2.
Remarks
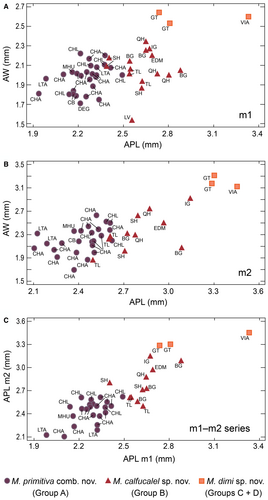
The molar size of the specimens from Río Seco de la Isla Grande, Estancia Don Mariano, and Bajo Giuliani fall within the maximum values of variation of this species. In contrast, specimens from Loventué, Telén and Salinas Grandes de Hidalgo occupy the minimum values of the size range (Fig. 7; Piñero et al. 82, table S1).
Occurrence
Late Miocene deposits of central and western Argentina. Cerro Azul Formation (Huayquerian, upper upper Miocene, central Argentina); Tunuyán Formation (upper upper Miocene, western Argentina). Localities and fossil-bearing deposits are detailed in Piñero et al. (82) (Fig. 6).
Metacaremys dimi sp. nov.Figure 5Q–T
LSID
urn:lsid:zoobank.org:act:FFB8C17D-617C-40ED-8B65-7C07A26AFE4B
Derivation of name
Named in memory of ‘Dim’, Vicente Di Martino (1940-2011), an enthusiastic naturalist and director of the Museo Municipal de Ciencias Naturales de Monte Hermoso, who actively preserved the palaeontological patrimony of southern Buenos Aires Province.
Holotype
MMH-GUA 20-01, left mandibular fragment with intra-alveolar portion of incisor and dp4–m3 (Fig. 5S).
Type locality
Guatraché, La Pampa Province, central Argentina. Cerro Azul Formation, Huayquerian, upper upper Miocene.
Referred material
See Piñero et al. (82).
Diagnosis
Large species of Metacaremys with almost synchronous closure of lingual flexids. Bottom of the metaflexid straight and wider than in M. primitiva comb. nov. Average APL of m1 and m2 c. 15% longer than in M. calfucalel sp. nov. Average APL of m3 nearly 30% longer than in the latter species. Average AW of the lower molars between 20% and 30% wider than in M. calfucalel sp. nov. Value of the m3/m1 APL index >1.1.
Measurements
Description
Metacaremys dimi sp. nov. is a large species of Metacaremys, nearly 20% larger than M. calfucalel sp. nov. The morphology of incisors and molars of M. dimi sp. nov. is quite similar to that of M. calfucalel sp. nov. The upper molars have an anteroposteriorly wider mesoflexus, and longer mure, than in M. primitiva comb. nov. The closure of the lingual flexids in lower molars is almost synchronous. The m3 is proportionally larger than in M. primitiva comb. nov. and M. calfucalel sp. nov. The AW of the molars of M. dimi sp. nov. is also larger than that of the last two species. The m3 is longer and wider than the m1, whereas the m2 is the widest molar.
Remarks
The specimen PV-UNS-6022 from Cantera Vialidad is larger than GHUNLPam 8323 and MMH-GUA 20-01 from Guatraché (Fig. 5R–T).
Occurrence
Late late Miocene and early Pliocene deposits of central Argentina. Cerro Azul Formation. Localities and fossil-bearing deposits are detailed in Piñero et al. (82) (see Fig. 6).
Quantitative analysis
The comparison of specimens from different localities showed a variation in size. Bivariate analyses on each type of lower tooth, comparing APL and AW, support the recognition of four groups (Fig. 7A, B): M. primitiva (group A), M. calfucalel (group B), and M. dimi (groups C + D). Variance and discriminant analyses support the reliability of these groups and demonstrate significant differences between them (Tables 2, 3).
| anova | Fisher's LSD test | Kruskal–Wallis test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-value | p-value | Group | n | Mean | Std error | 95% CI | Between-group comparison (G-G) | Mean difference | Group | n | Mean rank | p-value | ||
| Lower bound | Upper bound | |||||||||||||
| dp4 APL | 96.55 | <0.0001 | A | 18 | 2.218 | 0.032 | 2.170 | 2.265 | A-B | 0.573* | A | 18 | 9.5 | 0.0002 |
| B | 8 | 2.791 | 0.048 | 2.720 | 2.862 | B | 8 | 23.5 | ||||||
| C | 1 | 19.0 | ||||||||||||
| D | 1 | 28.0 | ||||||||||||
| m1 APL | 94.70 | <0.0001 | A | 30 | 2.249 | 0.022 | 2.218 | 2.281 | A-B | 0.368* | A | 30 | 15.67 | <0.0001 |
| B | 16 | 2.618 | 0.031 | 2.574 | 2.662 | B | 16 | 38.37 | ||||||
| C | 2 | 46.0 | ||||||||||||
| D | 1 | 49.0 | ||||||||||||
| m1 AW | 14.68 | <0.0001 | A | 29 | 1.971 | 0.029 | 1.929 | 2.013 | A-B | 0.086 | A | 29 | 19.78 | 0.0104 |
| B | 15 | 2.057 | 0.041 | 1.999 | 2.116 | A-C | 0.618* | B | 15 | 27.77 | ||||
| C | 2 | 2.589 | 0.112 | 2.429 | 2.749 | B-C | 0.532* | C | 2 | 45.5 | ||||
| m2 APL | 37.91 | <0.0001 | A | 26 | 2.430 | 0.033 | 2.383 | 2.477 | A-B | 0.351* | A | 26 | 14.36 | <0.0001 |
| B | 13 | 2.781 | 0.046 | 2.714 | 2.847 | B | 13 | 31.27 | ||||||
| C | 2 | 40.5 | ||||||||||||
| D | 1 | 42.0 | ||||||||||||
| m2 AW | 15.90 | <0.0001 | A | 25 | 2.210 | 0.050 | 2.138 | 2.282 | A-B | 0.130 | A | 25 | 17.36 | 0.0408 |
| B | 11 | 2.341 | 0.075 | 2.232 | 2.449 | A-C | 1.033* | B | 11 | 21.09 | ||||
| C | 2 | 3.243 | 0.177 | 2.989 | 3.498 | B-C | 0.903* | C | 2 | 37.5 | ||||
| m3 APL | 16.51 | 0.0006 | A | 14 | 2.172 | 0.052 | 2.096 | 2.249 | A-B | 0.338* | A | 14 | 7.75 | 0.0002 |
| B | 9 | 2.510 | 0.065 | 2.415 | 2.606 | B | 9 | 18.61 | ||||||
| C | 2 | 24.5 | ||||||||||||
| m3 AW | 9.54 | 0.0011 | A | 13 | 2.066 | 0.076 | 1.954 | 2.178 | A-B | 0.059 | A | 13 | 11.62 | 0.071 |
| B | 9 | 2.125 | 0.091 | 1.990 | 2.259 | A-C | 0.903* | B | 9 | 11.33 | ||||
| C | 2 | 2.969 | 0.194 | 2.684 | 3.254 | B-C | 0.844* | C | 2 | 23.5 | ||||
| dp4–m3 APL | 77.50 | <0.0001 | A | 6 | 8.932 | 0.103 | 8.763 | 9.100 | A-B | 1.423* | A | 6 | 3.5 | 0.0187 |
| B | 4 | 10.35 | 0.127 | 10.157 | 10.561 | A-C | 2.936* | B | 4 | 8.5 | ||||
| C | 1 | 11.87 | 0.253 | 11.450 | 12.280 | B-C | 1.513* | C | 1 | 11.0 | ||||
| m3 APL/m1 APL | 7.88 | 0.0042 | A | 10 | 0.948 | 0.029 | 0.903 | 0.992 | A-B | 0.016 | ||||
| B | 7 | 0.964 | 0.035 | 0.911 | 1.017 | A-C | 0.282* | |||||||
| C | 2 | 1.229 | 0.066 | 1.131 | 1.328 | B-C | 0.265* | |||||||
- Abbreviations: APL, anteroposterior length; AW, anterior width; CI, confidence interval; G, group; n, number of specimens. Group A, M. primitiva; group B, M. calfucalel; groups C + D, M. dimi.
- * Significant at p < 0.05. Bold, p < 0.05.
| Group | n | Predicted group membership, n (%) | Specimens correctly classified (%) | p-value | Standardized discriminant function | ||
|---|---|---|---|---|---|---|---|
| B | A | ||||||
| dp4 | A | 17 | 0 (0) | 17 (100) | 100 | <0.0001 | −0.978772 × APL (mm) − 0.0135686 × AW (mm) − 0.0433297 × PW (mm) |
| B | 7 | 7 (100) | 0 (0) | ||||
| m1 | A | 27 | 1 (3.70) | 26 (96.30) | 92.86 | <0.0001 | 0.969034 × APL (mm) − 0.183361 × AW (mm) + 0.183477 × PW (mm) |
| B | 15 | 13 (86.67) | 2 (13.33) | ||||
| m2 | A | 25 | 1 (4.0) | 24 (96.0) | 94.44 | <0.0001 | 1.12487 × APL (mm) − 0.316179 × AW (mm) − 0.0220582 × PW (mm) |
| B | 11 | 10 (90.91) | 1 (9.09) | ||||
| m3 | A | 13 | 1 (7.69) | 12 (92.31) | 86.36 | 0.0032 | 1.17022 × APL (mm) − 1.06131 × AW (mm) + 0.569797 × PW (mm) |
| B | 9 | 7 (77.78) | 2 (22.22) | ||||
| m1–m2 | A | 23 | 1 (4.35) | 22 (95.65) | 94.12 | <0.0001 | 0.92173 × AP (mm) m1 + 0.130077 × AP (mm) m2 |
| B | 11 | 10 (90.91) | 1 (9.09) | ||||
- Abbreviations: APL, anteroposterior length; AW, anterior width; n, number of cases; PW, posterior width. Group A, M. primitiva; group B, M. calfucalel.
- Bold, p < 0.05.
Scatter and box-and-whisker plots showed that APL was the best variable for delimiting groups (Figs 7, 8). These diagrams also showed a gradual increase in size from M. primitiva to M. calfucalel. Outliers in box-and-whisker plots usually correspond to molars at early or very advanced stages of wear (Fig. 8A–D). Similarly, the small size of the single dp4 of M. dimi (group C) is due to its very advanced wear stage (Fig. 8A). AW values are quite similar in M. primitiva and M. calfucalel, whereas the increase in relative width is not correlated with APL. Fisher's LSD test indicated no significant differences (p > 0.05) in the AW of m1, m2 and m3 between M. primitiva and M. calfucalel, but did indicate significant differences (p < 0.05) in the AW of m1, m2 and m3 between M. calfucalel and M. dimi (Table 2). Therefore, an increase in AW with respect to other groups is evident only in M. dimi. The maximum APL values of M. primitiva overlap with the minimum values of M. calfucalel. These minimum values usually correspond to individuals at early stage of wear of M. calfucalel (m2 and m3). Statistical analyses indicated significant differences in the APL values of all lower teeth between M. primitiva and M. calfucalel (Fisher’s LSD test; p < 0.05), a well as between the four groups (Kruskal-Wallis test; p < 0.05) (Table 2). Discriminant analyses on M. primitiva and M. calfucalel (including the variables of APL, AW, and PW) showed that 100% of the 24 dp4 could be correctly classified, as well as 92.86% of the 42 m1, 94.44% of the 36 m2, and 86.36% of the 22 m3 (Table 3). The percentage of correct classification increases in some cases when individuals are selected according to their stage of wear. For m1 at a stage of slight/moderate wear (≤ stage 3) and for m2 at a stage of moderate/advanced wear (≥ stage 3), 95% and 100% of cases were correctly classified, respectively.
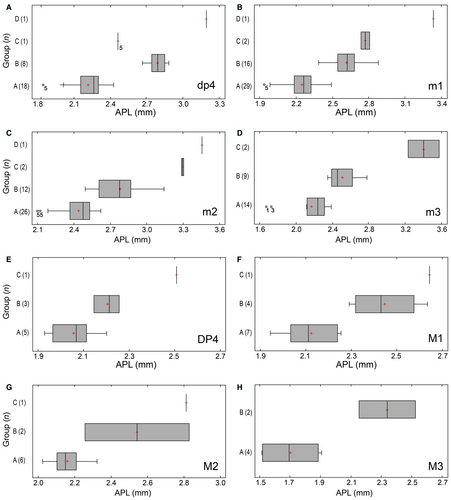
Scatter plots of m1 APL versus m2 APL (for m1–2 series) also showed four well-demarcated clusters (see above; Fig. 7C). Four of 11 m2 of M. calfucalel overlap the maximum APL values of M. primitiva, but all of them correspond to specimens at early stages of wear (≤stage 2). The separation between groups is much clearer when observing m1 APL, in that only one specimen from M. calfucalel overlaps with those from M. primitiva (GHUNLPam 8261, Salinas Grandes de Hidalgo). Discriminant analysis (excluding groups C and D) allowed the correct classification of 94% of the 34 specimens (Table 3). Only two specimens were incorrectly classified (GHUNLPam 307 from Laguna Chillhué; GHUNLPam 8261 from Salinas Grandes de Hidalgo).
For the dp4–m3 series, the range of the APL values did not overlap and there were significant differences between M. primitiva, M. calfucalel and M. dimi (Fisher's LSD test; p < 0.05; Kruskal–Wallis test; p = 0.018). Discriminant analysis correctly classified 100% of the 11 specimens. Linear regression analysis showed a strong (R = 0.9749) and significant (p < 0.001) relationship between dp4–m3 APL and group. The fitted model was able to explain 95% of length variability (Piñero et al. 82, fig. S3). It demonstrates that there is a progressive increase in size from M. primitiva to M. dimi.
There was no significant difference (p > 0.05) in the mean m3/m1 length index between M. primitiva and M. calfucalel. However, a significant increase in size of the m3 with respect to the m1 was detected in M. dimi, for which the mean value differed significantly (p < 0.05) from the means of the former two species (Table 2).
The upper teeth increase in size proportionally to the lower ones, and their scatter and box-and-whisker plots produced the same groups (Fig. 8E–H). However, no statistical analysis can be applied because the number of specimens is small.
Although specimen PV-UNS-6022 representing group D is undoubtedly larger than those assigned to group C, we include all these specimens into a single species, M. dimi, until additional material is available.
Discussion
Affinities and evolutionary pattern of Metacaremys gen. nov.
The taxon Cercomys Cuvier, 24 has a complex history (Petter 79; Pessôa et al. 77). Cuvier (24) published the description of this genus and its type species, Cercomys cunicularius, preceded by a plate illustrating its external appearance. Later, Cuvier (25) provided clear illustrations of the cranium in dorsal view, the articulated cranium and mandible in lateral view, and the left DP4–M2, which were consistent with the original description. Günther (44), Goldman (42) and Thomas (0102) agreed that the skull and teeth described and illustrated by Cuvier (25) are indistinguishable from species currently included in Proechimys Allen, 1 (‘Echimys’ in Günther 44). This is very clear in the illustrations of the molars, and especially in the illustration of the skull, which has a typical strong extension of the premaxilla anterior to the incisors (Cuvier 25: p. 451, pl. 18, fig. 1; pl. 19, figs 1–2). However, Thomas (0102), based primarily on the fact that the spineless fur figured by Cuvier (24) is not consistent with molar morphology, stated: ‘In searching for an animal with spineless fur to which the original figure of Cercomys cunicularius could be assigned, I have naturally thought of Thrichomys apereoides…’. This proposal by Thomas (0102) led to the replacement of Thrichomys Trouessart, 0106 by Cercomys Cuvier, 24 for more than 60 years (e.g. Ellerman 31; Simpson 99; Wood 137; see Pessôa et al. 77). This is the sense in which the name was used by Pascual (69) when considering Cercomys primitiva as an extinct species of the extant genus Thrichomys. Petter (79) revised the type material of C. cunicularius and noted that this is a compound consisting of a skin of the Cricetidae Nectomys squamipes (Brants, 15), a cranium of a species of the Echimyidae Proechimys, and a mandible assignable to a species of the Echimyidae Echimys Cuvier, 23. Accordingly, Petter (79) pointed out that the Thomas (0102) proposal cannot be accepted.
Indeed, the problem of the validity of Cercomys Cuvier, 24 (or Cuvier, 25) depends not on specimens but on the status of its type species Cercomys cunicularius. This requires, primarily, determination of the correspondence of the specimens that make up the type with species from Proechimys, Echimys and Nectomys. In this context, whatever the decision regarding the validity or possible application of Cercomys, ‘Cercomys’ primitiva cannot be included in this genus.
Beyond the name, Pascual (69) interpreted that the lineage of the living echimyid Thrichomys had been represented in the late Miocene of central Argentina. Reig (87, 88) accepted this proposal. In general, there has been some consensus in assigning many of the extinct octodontoids to Echimyidae (e.g. Patterson & Pascual 74; Patterson & Wood 75; Vucetich & Verzi 127; McKenna & Bell 62; Carvalho & Salles 17; see Verzi et al. 119, table 2), partly because the living species of this family have a conservative molar morphology that is at least superficially similar to that of ancient octodontoids. Nevertheless, Metacaremys gen. nov. shares diagnostic characters with stem (the early–middle Miocene Acaremys and the late Miocene Neophanomys) and crown Octodontidae, in particular the masseteric crest descending from the posterior part of the notch for the tendon of m. masseter medialis, the mesoflexus/fossette more persistent and wider than the paraflexus/fossette and metaflexus/fossette in upper molars, and the mesoflexid more persistent than the metaflexid in lower molars (Verzi et al. 119). Molars of Metacaremys gen. nov., lower crowned and consequently with less occlusal simplification than in the remaining late Miocene octodontids, show a lophate morphology similar to that of Acaremys minutus and Acaremys minutissimus (Verzi et al. 120, 121). Hypsodont molars are a key adaptation to open environments (see below). In the living fauna, octodontoids from open environments of southern South America are represented by species with euhypsodont (rootless) molars, with the single exception of the semiaquatic echimyid Myocastor (Vucetich & Verzi 129). In this sense, the record of Metacaremys gen. nov., along with other octodontoids with rooted molars and conservative occlusal morphologies (Verzi et al. 119, fig. 10), suggests that environmental regions suitable for these morphologies could have been more widespread in southern South America during the late Miocene than today.
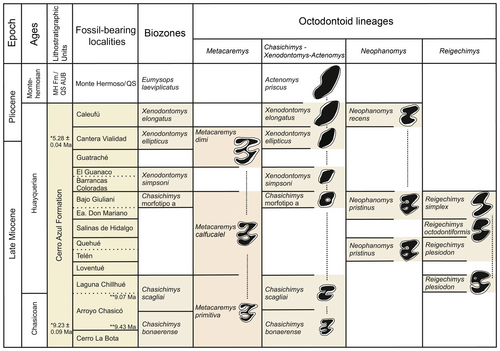
Quali-quantitative analyses of molars of Metacaremys show a variation in measurements through the available sample. When interpreted in the context of the available numerical ages and biochronological evidence provided by other octodontoid lineages of the late Miocene to early Pliocene (Fig. 9), a constant increase in size is seen. This trend is essentially continuous, and the recognition of discrete, named species is here favoured to facilitate systematic and biostratigraphic analyses rather than to represent a biological pattern or process (see Barnosky & Bell 7). Indeed, species of Metacaremys seem to represent a single, undivided lineage with directional change (sensu Hunt 46) through a c. 4 myr period from the late Miocene to the Miocene–Pliocene boundary (Fig. 9). Different to other late Miocene octodontoid lineages from the same deposits also following directional evolution, Metacaremys shows no changes in hypsodonty (in contrast to those in the ctenomyid Chasichimys and the octodontid Neophanomys) nor in occlusal simplification (in contrast to that in Chasichimys, Nephanomys and the echimyid Reigechimys Verzi et al., 112) (Fig. 5; Verzi 110; Verzi et al. 114, 116; Sostillo et al. 100). Nevertheless, in Metacaremys dimi sp. nov., the closing of the lingual flexids in lower molars seems to be somewhat more synchronous than in Metacaremys primitiva comb. nov. and Metacaremys calfucalel sp. nov. This suggests that a trend towards occlusal simplification could have occurred incipiently in Metacaremys as well. The recognition of this new lineage supports the assumption that directional change is common in the evolutionary history of rodents (Chaline & Mein 19; Chaline 18; Schmidt-Kittler & Vianey-Liaud 95; Chaline et al. 20; Martin 59, 60; Vianey-Liaud & Michaux 122; Barnosky & Bell 7; Lister 54 and literature therein).
Changes affecting molar morphology are usually interpreted as ecological adaptations that reveal significant selective processes (Gomes Rodrigues et al. 43). Tooth shape may be associated with diet and used to evaluate environment changes along the lineages (Fortelius et al. 39; Renaud et al. 91). In this sense, increased tooth height or hypsodonty may be considered as a selective advantage, providing adaptation in response to increasing demands for wear tolerance and functional durability in progressively more open and arid environments (Jernvall & Fortelius 49; Fortelius et al. 39). The turnover of octodontoid faunas in the Cerro Azul Formation, where Metacaremys is recorded, shows a trend towards the increase of hypsodont taxa. From the oldest exposure of this formation to the youngest one, octodontoid rodents with rootless (i.e. euhypsodont) molars increase in dominance while protohypsodont rodents decrease (Verzi et al. 116, fig. 8). This trend among octodontoids is coincident with the increasing aridity and consequent development of open environments detected on both a regional (Pascual & Ortiz Jaureguizar 70; Palazzesi & Barreda 68; Le Roux 52; Hynek et al. 47; Carrapa et al. 16; Domingo et al. 30) and global scale (Janis 48; Denton 26; Zachos et al. 141; Tripati et al. 0105). Different to the gross direction of this turnover, Metacaremys shows no changes in hypsodonty but instead has progressively larger and broader molars. This direction of evolution is similar to that of the European Stephanomys Schaub, 94 (Renaud et al. 91), and the European and North African Paraethomys Petter, 78 (Renaud et al. 90; Piñero & Agustí 80; Piñero & Verzi 81). These murid lineages display parallel evolution toward larger and wider molars, as part of a common adaptive (and channelized by common constraints) response to grass eating in increasingly open environments (Renaud & Auffray 89). Acquisition of wider molars could be functionally related to a relative increase in chewing surface and increased grinding efficiency (Renaud et al. 91; Van Dam 0109; Renaud & Auffray 89). Nevertheless, molar size is highly correlated with body mass in rodents (e.g. Legendre 53; Moncunill-Solé et al. 63), and an increase in the overall size of Metacaremys cannot be ruled out. The available mandibular measurements support this assumption (Table 1 and Piñero et al. 82, table S1). In such a case, the palaeoecological interpretation should be integrated into the more comprehensive issue of the relationship between body mass and type of diet in herbivorous mammals (McArthur 61).
Biochronology, biostratigraphy, and the Miocene–Pliocene boundary in southern South America
Knowledge of the biochronology, biostratigraphy and chronostratigraphy of the late Miocene and early Pliocene of southern South America is essentially (although not exclusively) based on faunas and fossil-bearing deposits of central and northern Argentina (Rovereto 92; Kraglievich 51; Pascual et al. 72; Bondesio et al. 12; Marshall & Patterson 55; Marshall et al. 57, 58; Yrigoyen 140; Goin et al. 41; Tauber 0101; Zárate 143; Cione & Tonni 21; Verzi et al. 115; Reguero & Candela 86; Contreras & Baraldo 22; Brandoni et al. 14; Esteban et al. 33; Deschamps & Tomassini 28; Beilinson et al. 8; Garrido et al. 40; Nasif et al. 65). The composition of these faunas resulted in the recognition of two South American Land Mammal Ages (SALMAs): Chasicoan, based on faunas from the lower upper Miocene, and Huayquerian, based on those from the upper upper Miocene (Pascual et al. 72; Marshall et al. 57). Subsequently, this scheme was enhanced by the recognition of different biostratigraphic units (Cione & Tonni 21; Deschamps 27; Verzi et al. 115; Reguero & Candela 86; Esteban et al. 33; Deschamps & Tomassini 28).
In Figure 9, the stratigraphic distribution of Metacaremys is shown in the context of the biozones and available numerical ages for the Cerro Azul Formation. Exposures of this formation and their faunal content belong to a sedimentary and faunistic cycle that followed the withdrawal of the widespread Paranaense marine transgression (Hernández et al. 45; Pascual et al. 73; Ruskin et al. 93). This time interval encompasses the Chasicoan and Huayquerian SALMAs, although the definition of the Huayquerian is a subject of debate and remains to be defined (Forasiepi et al. 38). Here, we retain the term ‘Huayquerian’, as commonly used in South American stratigraphy and chronology, to refer to the late Miocene and early Pliocene interval between the Chasicoan and Montehermosan SALMAs (Marshall et al. 57; Deschamps et al. 29). Thus, we interpret the Chasichimys bonaerense and Chasichimys scagliai biozones as representing early and late Chasicoan, respectively, and the remaining biozones as different steps of the Huayquerian (Verzi et al. 115).
Increasing stratigraphic and chronological evidence has allowed us to understand and to more precisely constrain the Cerro Azul Formation (Schultz et al. 96, 97; Zárate et al. 144; Folguera & Zárate 36, 37; Deschamps & Tomassini 28; Beilinson et al. 8; Montalvo et al. 64). Numerical ages and magnestostratigraphy constrain the outcrops of this formation in Arroyo Chasicó to the interval c. 9.43 to <9.02 Ma (Schultz et al. 96, 97; Zárate et al. 144). The recent discovery of a specimen of Chasichimys scagliai Pascual, 69 (C. Oliva & DHV unpub. data) in the lithofacies association 3 in Arroyo Chasicó locality (sensu Zárate et al. 144; previously Las Barrancas Member, Fidalgo et al. 35) confirms the stratigraphic succession of the two biozones recognized in this locality. Moreover, a recent palaeontological study of the Cerro La Bota site supported the Chasicoan age of this fauna; in this locality, Metacaremys primitiva is recorded together with Chasichimys bonaerense (Montalvo et al. 64). With respect to the Huayquerian levels of the Cerro Azul Formation, impact glasses from the level of Cantera Vialidad bearing Xenodontomys ellipticus Kraglievich, 50 were dated at 5.28 ± 0.04 Ma (Schultz et al. 96, 97); X. ellipticus was also recorded in overlying levels associated with M. dimi sp. nov. (Deschamps et al. 29; Deschamps & Tomassini 28). Thus, the association of X. ellipticus and M. dimi sp. nov. becomes a key biostratigraphic indicator to identify the Miocene–Pliocene boundary in the continental deposits of central Argentina. A recent detailed study of the geology, magnetostratigraphy and palaeontology of the Quequén Salado river basin (central-eastern Argentina) allowed the levels bearing X. ellipticus to be constrained to the interval 5.23–5.89 Ma (Beilinson et al. 8). In this locality, the stratigraphic superposition of the X. ellipticus Biozone (Huayquerian, late Miocene to early Pliocene) and the Eumysops laeviplicatus Biozone (Montehermosan, early Pliocene), which includes the record of most derived species of the Xenodontomys–Actenomys lineage (see Verzi et al. 117), was documented for the first time by Beilinson et al. (8).
Accordingly, the Cerro Azul Formation spans the period 9.4 Ma to <5.3 Ma (Fig. 9). During this interval, the Metacaremys lineage experienced a progressive increase in size, from the smallest species, M. primitiva comb. nov., recorded in the lower upper Miocene (Chasicoan), to the largest species, M. dimi sp. nov., recorded at the Miocene–Pliocene boundary (Huayquerian). In the interval in which the limited variation of M. calfucalel sp. nov. is recorded, the echimyid lineage of Reigechimys shows marked changes, the occurrence of which emphasizes the species-specific nature of evolutionary pathways and rates (Lister 54).
It is worth noting that Metacaremys is not recorded in the lower Pliocene Montehermosan from Farola Monte Hermoso and Quequén Salado river basin (central Argentina), even though the rich octodontoid rodent faunas of these localities have been intensively studied (e.g. Deschamps et al. 29; Tomassini et al. 0104; Beilinson et al., 8). This suggests that the lineage could have become extinct in the Pampean Region during or after the Huayquerian SALMA (late Miocene or early Pliocene).
The record of Metacaremys in deposits other than the Cerro Azul Formation is at least partially consistent with or even supports our biochronological and biostratigraphical scheme. In the Loma de Las Tapias Formation (western Argentina), M. primitiva comb. nov. is recorded in the lower levels of the Arenisca Albardón Member, the estimated age of which is c. 8.2 Ma (based on radiometric dating and a local history of magnetic polarity; Bercowski et al. 9; Contreras & Baraldo 22). The vertebrate fauna of these levels is recognized as Assemblage A and assigned to the Chasicoan (Contreras & Baraldo 22). In lower levels of the Tunuyán Formation, near the boundary with the underlying Huayquerías Formation (western Argentina), M. calfucalel sp. nov. is recorded as overlying a tuff dated at 5.8 ± 0.41 Ma (Garrido et al. 40). Ongoing studies on well-constrained vertebrate faunas recently recovered from Huayquerías and Tunuyán formations (Prevosti et al. 84) will allow assessment of this presumed correlation based on the presence of Metacaremys.
The biochronological meaning of the record of M. primitiva comb. nov. in El Degolladito Site 2 of the Salicas Formation (north-western Argentina) and ‘Estrato Muyu-Huasi’ (south-central Bolivia) should be viewed with caution, given that faunas from both deposits have been primarily assigned to the Huayquerian (Tauber 0101; Brandoni et al. 14; Villarroel & Marshall 124; Marshall & Sempere 56, respectively).
Conclusion
We erect the genus Metacaremys gen. nov. and the species Metacaremys calfucalel sp. nov. and Metacaremys dimi sp. nov., and transfer ‘Cercomys’ primitiva to this genus (Metacaremys primitiva comb. nov.). The Metacaremys species are here interpreted as members of a single, undivided lineage with directional evolution marked by size increase from the early late Miocene to the Miocene–Pliocene boundary (c. 4 myr of recorded evolution). This directional change in Metacaremys is congruent with that observed for other octodontoid lineages from the same deposits, that is, Chasichimys–Xenodontomys (Ctenomyidae), Neophanomys (Octodontidae) and Reigechimys (Echimyidae), which could testify to a selective morphological advantage for open areas during the late Miocene climatic deterioration. This pattern allows the refining of biochronological and biostratigraphic interpretations for the late Neogene of southern South America. In particular, Metacaremys dimi sp. nov. and Xenodontomys ellipticus are key species for identifying the Miocene–Pliocene boundary in the continental record of central and western Argentina. The new taxa described here expand our understanding of the late Miocene octodontoid faunas from South America. In addition, they can contribute significantly to the definition of a late Neogene biozonation based on caviomorph successions for a wider regional geographic frame, including Argentina and other parts of South America.
Acknowledgements
This research was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT PICT 2016-2881), the Facultad de Ciencias Exactas y Naturales UNLPam [FCEyN 06G (UNLPam)], and the Secretaría General de Ciencia y Tecnología, Universidad Nacional del Sur (PGI 24/H154). PP and AFV are beneficiaries of a postdoctoral and doctoral fellowship, respectively, from the Argentinian Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). We thank the curators for granting access to specimens under their care: A. Martinelli, M. Ezcurra, L. Chornogubsky (MACN), E. Tonni, M. Reguero (MLP), A. Forasiepi, S. Devincenzi (IANIGLA), F. Scaglia, M. Taglioretti (MMP), R. Caputo (MD), N. Sánchez, C. Oliva (MMH). We also thank A. Forasiepi and F. Prevosti for actively facilitating access to specimens from Huayquerías (to DHV); and D. Brandoni for kindly providing photographs of the specimen CRILAR Pv 431. T. dos Santos, R. Lopes, I. Perez and S. Dos Reis generously and actively provided us with computed tomography images of echimyids. B. Epele from YTEC (YPF-CONICET, Argentina) assisted with the computed tomography of abrocomids and octodontids. M. Arnal assisted DHV in early stages of the work. We are especially grateful to L. Marivaux and another anonymous reviewer, and the editors L. Hautier, S. Thomas and E. Rubano for their valuable revisions and comments that greatly improved the manuscript.
Open Research
Data archiving statement
Data for this study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.mkkwh70z3.
Digital reconstructions and image stacks of specimens used in this work are available in MorphoSource (see Dryad for details): https://doi.org/10.17602/m2/m348696; https://doi.org/10.17602/m2/m168179; https://doi.org/10.17602/m2/m348683; https://doi.org/10.17602/m2/m168174; https://doi.org/10.17602/m2/m348640; https://doi.org/10.17602/m2/m168169; https://doi.org/10.17602/m2/m168180; https://doi.org/10.17602/m2/m168181
This published work and the nomenclatural acts it contains, have been registered in ZooBank: http://zoobank.org/references/D8BE3B6F-C42B-47D2-ACE2-76133BC94963




