Dual-Drug Nanomedicine Assembly with Synergistic Anti-Aneurysmal Effects via Inflammation Suppression and Extracellular Matrix Stabilization
Abstract
Abdominal aortic aneurysm (AAA) represents a critical cardiovascular condition characterized by localized dilation of the abdominal aorta, carrying a significant risk of rupture and mortality. Current treatment options are limited, necessitating novel therapeutic approaches. This study investigates the potential of a pioneering nanodrug delivery system, RAP@PFB, in mitigating AAA progression. RAP@PFB integrates pentagalloyl glucose (PGG) and rapamycin (RAP) within a metal–organic-framework (MOF) structure through a facile assembly process, ensuring remarkable drug loading capacity and colloidal stability. The synergistic effects of PGG, a polyphenolic antioxidant, and RAP, an mTOR inhibitor, collectively regulate key players in AAA pathogenesis, such as macrophages and smooth muscle cells (SMCs). In macrophages, RAP@PFB efficiently scavenges various free radicals, suppresses inflammation, and promotes M1-to-M2 phenotype repolarization. In SMCs, it inhibits apoptosis and calcification, thereby stabilizing the extracellular matrix and reducing the risk of AAA rupture. Administered intravenously, RAP@PFB exhibits effective accumulation at the AAA site, demonstrating robust efficacy in reducing AAA progression through multiple mechanisms. Moreover, RAP@PFB demonstrates favorable biosafety profiles, supporting its potential translation into clinical applications for AAA therapy.
1 Introduction
Abdominal aortic aneurysm (AAA) presents a critical cardiovascular condition characterized by the persistent local dilation of the abdominal aorta, exceeding 50% of its normal diameter.[1, 2] Its primary complication, aneurysm rupture, carries a substantial risk of mortality. Clinically, an aneurysm's diameter serves as the foremost indicator for predicting rupture risk and guiding treatment decisions.[3, 4] Current surgical and interventional therapies primarily target large or symptomatic AAAs. However, there remains a glaring gap in treatment options for patients with small AAAs or those deemed unsuitable for surgical repair. Merely monitoring AAA via imaging not only imposes a financial burden on patients but, more significantly, exacerbates their psychological distress and diminishes their quality of life due to concerns regarding the lack of active treatment options. Despite exhaustive efforts by researchers exploring various drug therapies, none have demonstrated compelling benefits in clinical trials.[5] Thus, the development of effective drugs to prevent or decelerate AAA progression, rooted in its pathological characteristics, stands as a crucial supplement to the existing treatment paradigm for AAA and bears profound clinical significance.
The pathogenesis of AAA primarily stems from the chronic inflammation-induced degradation of the aortic wall.[6] Infiltration of inflammatory cells into the middle and outer layers of the aortic wall, particularly M1 pro-inflammatory macrophages, triggers the production of inflammatory mediators and reactive oxygen species (ROS), intensifying the inflammatory response.[7] This cascade of inflammation leads to the transformation of smooth muscle cells (SMCs) and their apoptosis, compromising matrix synthesis and repair capabilities.[8, 9] Furthermore, the excessive accumulation of ROS prompts the release of matrix metalloproteinases (MMPs) from inflammatory and vascular wall cells, resulting in a reduction in elastin content and cross-linking within the extracellular matrix (ECM), along with an increase in collagen cross-linking.[10] The decreased elastin content diminishes arterial wall elasticity, contributing to an increase in diameter, while heightened collagen content elevates local wall stress, heightening the risk of rupture.[11-13] These factors collectively culminate in the initiation and progression of AAA. Consequently, targeting the localized pathological processes of AAA by inhibiting the inflammatory cascade while preserving ECM stability represents a highly promising approach for drug therapy.
Drawing from these concepts, a multicenter clinical study employed a balloon catheter system to locally administer pentagalloyl glucose (PGG) to the aneurysm wall, demonstrating its potential in mitigating AAA.[14] PGG, a small molecule polyphenolic compound, exhibits free radical scavenging and anti-inflammatory properties.[15] Moreover, PGG shows promise in ECM regulation. First, PGG can associate with proline-rich proteins (e.g., elastin and collagen) through hydrophobic and hydrogen bonding interactions, thereby preserving the stability of the elastin layer critical for vascular function and biomechanical integrity.[16] Additionally, PGG may facilitate the repair of damaged arterial walls by enhancing elastin fiber synthesis.[17] Furthermore, PGG has been observed to elevate lysine oxidase (LOX) synthesis while reducing MMP-2 activity. Consequently, PGG possesses dual functionality in inflammation modulation and ECM regulation, rendering it a highly prospective therapeutic agent for AAA.
Despite promising attributes, the in vivo application of PGG for AAA treatment faces several challenges. Primarily, PGG's chemical properties are susceptible to instability and oxidation.[15] Upon systemic administration, PGG undergoes widespread distribution in the body, rapid metabolism, and clearance, necessitating frequent dosing.[18] Furthermore, PGG has shown limited efficacy in animal models and clinical trials.[14, 19, 20] A key contributing factor is its insufficient ability to regulate ECM, unable to counteract ECM degradation induced by continuous MMP secretion in an inflammatory milieu. Consequently, there remains a pressing need to develop a rational delivery system capable of enhancing PGG's stability and pharmacokinetic profile while addressing its pharmacological limitations.
In recent years, the advancement of nanomedicine has spurred exploration into its potential advantages in treating AAA.[21] Capitalizing on the phenomenon of endothelial inflammation and vascular wall leakage, nanomedicines offer the opportunity for passive targeting and accumulation at AAA sites.[22] Leveraging the abundant phenolic hydroxyl groups in the PGG structure, we have developed a metal-organic-framework (MOF) based nanomedicine delivery system. This system facilitates nano self-assembly through the coordination between PGG and Fe3+, enabling effective encapsulation of the hydrophobic drug rapamycin (RAP) with a remarkable drug loading capacity of up to 53.8%. RAP, an mTOR inhibitor with anti-inflammatory properties, has been successfully encapsulated within nanomedicine and released in response to the AAA lesion environment, demonstrating significant therapeutic efficacy.[23] Notably, we found that RAP could downregulate MMP2 expression,[24] thereby synergizing with PGG to enhance ECM stability (Scheme 1). This innovative dual-drug delivery system, termed RAP@PFB, exhibits rapid accumulation at AAA sites following intravenous injection, sustains prolonged retention, while smart drug release in response to the stimulus in AAA microenvironment. Through the combined actions of PGG and RAP, RAP@PFB demonstrates multifaceted pharmacological activities targeting AAA's pathological characteristics, including scavenging free radicals, exerting anti-inflammatory effects, inducing macrophage M1-TO-M2 repolarization, inhibiting SMC apoptosis and calcification, and modulating MMP2 secretion and activity. The robust therapeutic efficacy and high biosafety profile of RAP@PFB in animal studies underscore its potential for clinical translation, offering promising avenues for the efficient treatment of AAA.
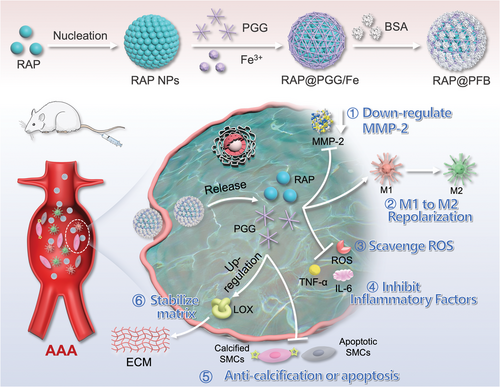
2 Results and Discussion
2.1 Preparation and Characterization of RAP@PFB Nanoparticles
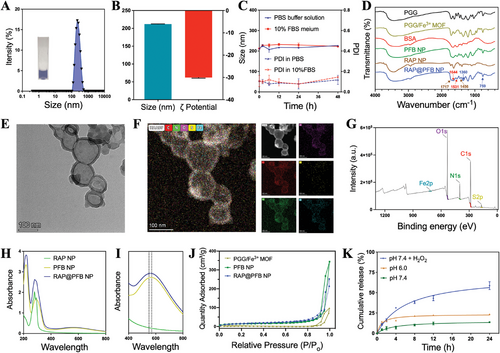
The RAP nanocore was synthesized employing a solvent exchange method to exploit the hydrophobic properties of RAP.[25, 26] Despite successful core formation, inherent poor colloidal stability led to rapid aggregation and precipitation of the nanoparticles (Figure S1, Supporting Information). To address this, a multifaceted approach involving the coordination of PGG and Fe3+ was adopted, leading to the formation of a MOF shell on the nanoparticle surface, followed by the adsorption of bovine serum albumin (BSA) protein (Figure 1A,B). This sequential modification significantly enhanced the colloidal stability of the nanoparticles, as evidenced by minimal changes in particle size and polydispersity index (PDI) under dynamic monitoring in both PBS buffer and complete culture medium containing 10% fetal bovine serum (FBS) (Figure 1C). Moreover, PGG showed significantly higher structural stability upon incorporation into the MOF, demonstrating the protective effect of the MOF against PGG oxidation (Figure S2, Supporting Information). This enhancement underscores the potential biomedical applicability of RAP@PFB nanoparticles. In parallel, BSA-modified PGG/Fe3+ coordination nanoparticles (PFB) were synthesized as a control group. From the FT–IR spectra (Figure 1D), RAP@PFB NPs, PFB NPs, PGG/Fe3+ MOF, and free PGG showed characteristic peaks at 759 and 1360 cm−1, which can be ascribed to C─H out-of-plane bending of the phenyl groups and phenolic ─OH bending from PGG, respectively. Two peaks at 1456 and 1717 cm−1 appear in the spectrum of RAP@PFB NP and RAP NP, attributable to C─H scissor bending vibrations and C═O carbonyl stretching vibrations in RAP, respectively. Moreover, the characteristic peaks of BSA at 1644 cm−1 (C═O stretch) and 1531 cm−1 (C─N stretching coupled with N─H bending mode) were seen in both free BSA and RAP@PFB NPs. All these results confirmed the structure of the nanoparticles. The X-ray diffraction (XRD) patterns of RAP@PFB NPs and RAP NPs are completely different from the characteristic diffraction patterns of rapamycin raw materials reported in the literature (Figure S3, Supporting Information),[27] and there are no characteristic diffraction peaks corresponding to RAP, indicating that RAP exists in an amorphous form in the nanoparticles.
To explore the assembly mechanisms between PGG and Fe3+, Replica Exchange with Solute Tempering molecular dynamics simulation technology were employed (Figure S4A, Supporting Information). Quantitative optimization analysis is carried out on the main conformations of clustering analysis, and the interactions between PGG molecules are mainly achieved through molecular nesting and stacking to achieve tight binding. After the addition of Fe3+, the overall structure of the composite remained tightly bound, and the position of Fe3+ formed stable interactions with two PGG molecules between the four phenolic hydroxyl groups (Figure S4B, Supporting Information). The main interactions between PGGs are through hydrogen bonds between phenolic hydroxyl groups and π–π stacking interactions between benzene rings, with a binding energy of −45.342 Kcal mol−1. When Fe3+ is added, the interaction between PGGs becomes stronger (visible pink area increases), and its binding energy increases to −50.014 Kcal mol−1 (Figure S4C, Supporting Information). This indicates that the addition of Fe3+ intensifies the stacking of PGG molecules, possibly due to the strong attraction of Fe3+ to phenolic hydroxyl groups. Therefore, Fe3+ can form very stable metal chelation bonds with the four phenolic hydroxyl groups in PGG molecules.
Transmission electron microscopy (TEM) analysis revealed distinct morphologies: regular spherical structure for RAP NPs, typical coordination polymerization cross-linked structures for PFB NPs, and a core-shell structure for RAP@PFB NPs (Figure 1E; Figure S5, Supporting Information). Elemental analysis confirmed the composition of RAP@PFB nanoparticles (Figure 1F), primarily comprising carbon, nitrogen, oxygen, iron, and sulfur. Specifically, iron predominantly localized at the peripheral region of the nanoparticles, corroborating the shell structure of the MOF, while sulfur originated from the BSA adsorption on the nanoparticle surface. Moreover, the BSA adsorption was further confirmed by Coomassie blue staining (Figure S6, Supporting Information). The existence of the elements C, N, O, S, and Fe in RAP@PFB was further confirmed using the results obtained from the analysis of X-ray photoelectron spectroscopy (XPS) (Figure 1G). The high-resolution XPS spectrum of Fe 2p (Figure S7, Supporting Information) has been observed with two peaks at 724.34 and 711.26 eV, which are associated to Fe (III) cation of Fe 2p1 and Fe 2p3, respectively. Also, the peaks at 732.96 and 716.9 eV are related to satellite peaks, which indicates that Fe (III) is the predominant form of iron state in RAP@PFB.
UV absorption spectroscopy characterized RAP@PFB nanoparticles, exhibiting characteristic absorption peaks of RAP at 278 nm (Figure 1H), along with absorptions at 550–570 nm attributed to the coordination between PGG and Fe3+ (Figure 1I), consistent with previous literature.[25] The N2 adsorption/desorption isotherms of PGG/Fe3+ MOF, PFB NP, and RAP@PFB NP are shown in Figure 1J, the Brunauer–Emmett–Teller assay revealed the specific surface area of the RAP@PFB nanoparticles was determined to be 54.61m2 g−1, which is significantly greater than the surface area of PGG/Fe3+ MOF (16.37 m2 g−1). Hence the larger surface area of the RAP@PFB nanoparticles is associated with the presence of RAP NP in the PGG/Fe3+ MOF. Furthermore, the Barrett-Joyner-Halenda method demonstrated that PGG/Fe3+ MOF has a mesoporous structure (Figure S8, Supporting Information). High-performance liquid chromatography (HPLC) quantification indicated a notable drug loading efficiency of 53.8%, showcasing the advantageous high drug loading capability of this self-assembly strategy.
Subsequent investigation of drug release kinetics demonstrated RAP's sustained release behavior at pH 7.4 (Figure 1K), with a cumulative release of 13.6% within 24 h. Notably, a decrease in pH to 6.0 expedited drug release, attributed to the dissociation of PGG/Fe3+ coordination bonds, highlighting the pH-responsive drug release characteristics of the nanoparticles, promising for intracellular drug delivery and therapeutic efficacy enhancement. The study reported abnormally high expression of ROS in AAA lesions, with ROS levels at AAA sites being 2.5 times higher than those at non-AAA sites.[9] To evaluate the response characteristics of nanoparticles to the AAA microenvironment, we measured the drug release behavior of RAP@PFB in the presence of H2O2. Under H2O2 conditions, drug release was further accelerated, with a cumulative release of approximately 58.6% after 24 h (Figure 1K). Therefore, RAP@PFB exhibits ROS-responsive drug release characteristics. To further elucidate the intelligent responsive drug release mechanism of the nanoparticles, we evaluated the stability of RAP@PFB under different conditions using TEM. At pH 7.4, RAP@PFB maintained its structural stability for 24 h; however, when the pH decreased or H2O2 was added, significant changes in the nanoparticle microstructure were observed (Figure S9, Supporting Information), indicating drug release caused by nanoparticle disintegration.
2.2 Capability of RAP@PFB in ROS Scavenging, Inflammation Inhibition, and Macrophage M1-to-M2 Repolarization
ROS play a pivotal role in the pathogenesis of AAA, eliciting immune cell infiltration and pro-inflammatory cytokine secretion, thereby exacerbating vascular inflammation and structural aberrations, ultimately contributing to AAA development and progression.[28, 29] Prompt ROS clearance presents a promising strategy to impede aneurysm formation and rupture, garnering significant interest among researchers.[30] Within our nanodrug delivery system, PGG assumes a pivotal role as a polyphenolic antioxidant endowed with ROS scavenging capabilities, thereby holding therapeutic potential in AAA management.
To ascertain the ROS scavenging efficacy of RAP@PFB, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was initially employed. DPPH radicals, characterized by characteristic UV–vis absorbance peaks at 517 nm, were utilized as a probe (Figure S10A, Supporting Information). Upon co-incubation of gradient concentrations of RAP@PFB for 40 min, a concentration-dependent decrease in the characteristic absorbance peak was observed Dynamic curve analysis revealed an impressive clearance rate (Figure S10B, Supporting Information), with RAP@PFB supplemented with 50 µM PGG achieving an 80% clearance rate against 0.5 mM DPPH within 5 min. We further evaluated the scavenging capacity of RAP@PFB against a variety of RONS, including H2O2, •OH, •O2−, and •NO. These radicals are intricately associated with vascular cell dysfunction, lipid and protein peroxidation, and DNA damage, all of which contribute to AAA pathogenesis.[31-33] The results showed concentration-dependent scavenging for all tested radicals (Figure S11A–D, Supporting Information), with RAP@PFB demonstrating superior efficacy in neutralizing these radicals than PFB (Figure S11E–H, Supporting Information). Electron spin resonance (ESR) spectroscopy analysis confirmed robust signals for both •OH and •O2− radicals. Following the addition of varying concentrations of RAP@PFB (Figure S12A,B, Supporting Information), a gradual decrease in signal intensity was observed, corroborating the broad-spectrum free radical scavenging activity of RAP@PFB. In summary, RAP@PFB exhibits notable ROS scavenging capabilities, underscoring its potential utility as an effective therapeutic agent in combating AAA by mitigating oxidative stress-induced vascular damage.
Following the confirmation of RAP@PFB's efficacy in scavenging ROS in vitro, we proceeded to evaluate its intracellular antioxidant activity. Initial assessment involved determining the cytotoxic effects of nanoparticles on RAW264.7 macrophages and SMCs through the MTT assay. Upon co-incubation with varying concentrations of RAP@PFB for 48 h, a concentration-dependent decrease in cell viability was observed (Figure S13, Supporting Information), predominantly attributed to the presence of RAP within the nanoparticle structure. Conversely, PFB nanoparticles exhibited negligible cytotoxicity across various concentrations.
Subsequent cell experiments necessitated the selection of low-toxic RAP@PFB concentrations, ensuring cellular viability. Accordingly, concentrations of 2.5 µM for RAP and 10 µM for PGG were chosen. To investigate the intracellular antioxidant activity, RAW264.7 cells were pre-treated with lipopolysaccharide (LPS), to induce high-level intracellular ROS generation. Utilizing the DCFH-DA probe, staining results revealed a significant elevation in intracellular ROS levels in RAW264.7 cells within the model group (Figure 2A). In contrast, treatment with PFB nanoparticles led to a reduction in cell fluorescence intensity, indicative of ROS clearance. Notably, RAP@PFB exhibited a more robust ROS clearance effect compared to PFB nanoparticles. Quantitative analysis via flow cytometry further corroborated the efficacy of both PFB and RAP@PFB in clearing intracellular ROS, with RAP@PFB demonstrating superior performance (Figure 2B; Figure S14A, Supporting Information). These findings underscore the significant intracellular ROS scavenging ability of RAP@PFB, suggesting its potential in protecting vascular wall cells from oxidative stress-induced damage.
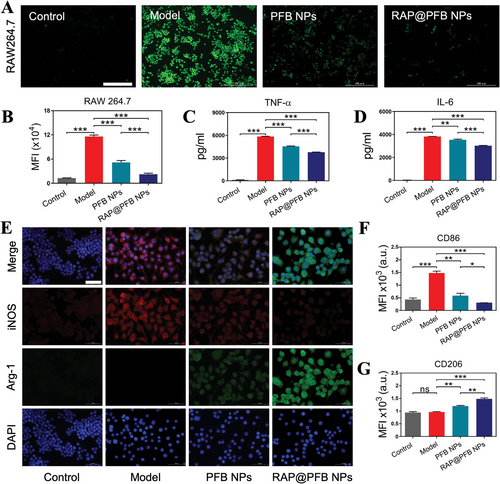
Having established the intracellular ROS scavenging activity of RAP@PFB, we proceeded to investigate its anti-inflammatory properties. Pre-treatment of RAW264.7 macrophages with LPS induced the secretion of inflammatory factors, notably tumor necrosis factor-alpha (TNF-α), which exhibited a significant upregulation post-LPS stimulation (Figure 2C). Treatment with PFB effectively attenuated TNF-α levels, attributed to its inherent ROS scavenging activity. Excessive recruitment of ROS precipitates macrophage infiltration and subsequent release of inflammatory cytokines, thereby driving vascular wall inflammation. In comparison, RAP@PFB demonstrated heightened anti-inflammatory efficacy, significantly inhibiting TNF-α secretion, attributable to the synergistic anti-inflammatory effects of PGG and RAP. Given the pivotal role of TNF-α in the pro-inflammatory cytokine cascade, its inhibition may influence the downstream expression of IL-6.[34] Subsequent evaluation revealed significant suppression of IL-6 expression by RAP@PFB (Figure 2D), further corroborating its broad anti-inflammatory effects.
Macrophages exhibit phenotypic plasticity, polarizing into distinct M1 (pro-inflammatory) and M2 (anti-inflammatory) phenotypes in response to environmental cues. Notably, AAA lesions are characterized by an abundance of M1 macrophages, which contribute to vascular wall weakening through enhanced expression of pro-inflammatory mediators and matrix-degrading proteases. Intervention targeting macrophage polarization presents a potential therapeutic strategy against AAA progression. Prior research has demonstrated that inhibiting pro-inflammatory cytokines and mitigating ROS levels can induce M1-to-M2 repolarization.[35-38] thereby impeding AAA formation. Leveraging the robust ROS scavenging and anti-inflammatory properties of RAP@PFB, we explored its regulatory effects on macrophage phenotype. Immunofluorescence staining of specific phenotype markers, inducible nitric oxide synthase (iNOS) for M1 and Arginase-1 (Arg-1) for M2, revealed LPS-induced enhancement of iNOS fluorescence intensity, indicative of M1 polarization, alongside diminished Arg-1 fluorescence, denoting weakened M2 phenotype (Figure 2E). Treatment with both PFB and RAP@PFB attenuated iNOS fluorescence while augmenting Arg-1 expression, suggestive of induced M1-to-M2 repolarization. To provide a quantitative characterization, the levels of CD86 (M1 marker) and CD206 (M2 marker) were measured by flow cytometry, and consistent results were observed (Figure 2F,G; Figure S14C,D, Supporting Information). Notably, RAP@PFB exerted a more pronounced regulatory effect on macrophage polarization. In conclusion, RAP@PFB nanoparticles demonstrate multifaceted anti-AAA effects, encompassing ROS clearance, inhibition of inflammatory mediator secretion, and induction of M1-to-M2 repolarization.
2.3 Effects of RAP@PFB on SMC Apoptosis, Calcification, and ECM Stability
SMCs play a pivotal role in preserving the structural integrity of the aorta.[39] Dysregulated accumulation of ROS in AAA instigates SMC apoptosis, precipitating the loss of SMCs within the arterial media and thereby exacerbating AAA progression. Leveraging the robust intracellular ROS scavenging activity of RAP@PFB (Figure 3A,B; Figure S14B, Supporting Information), we probed its anti-apoptotic effects on H2O2-treated SMCs. Flow cytometry analysis revealed significant anti-apoptotic efficacy for both PFB and RAP@PFB nanoparticles (Figure 3C,D), attributable to their potent free radical scavenging properties, with RAP@PFB exhibiting superior effectiveness.
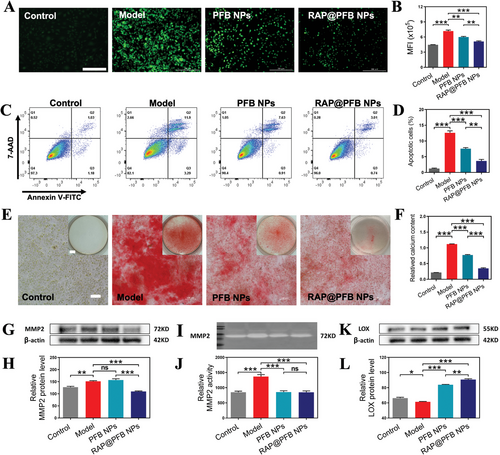
Moreover, SMCs are pivotal contributors to vascular calcification, wherein excessive apoptosis correlates with calcification occurrence.[40, 41] Furthermore, exposure to elevated levels of calcium/phosphate (Ca/P) prompts SMCs to secrete matrix vesicles, exacerbating vascular calcification.[42] Calcification escalation may elevate the risk of AAA rupture due to alterations in vascular mechanical properties.[43, 44] Pre-treatment of SMCs with nanoparticles followed by exposure to a calcifying milieu revealed substantial reductions in calcification deposition (Figure 3E,F), particularly pronounced in the RAP@PFB treatment group, potentially mediated by enhanced SMC autophagy facilitated by RAP. Studies have shown that RAP induces autophagy to inhibit the expression of the osteogenic marker Runx2 in SMCs, thereby increasing the expression of α-smooth muscle actin (α-SMA) protein and SM22α transcription levels in the functional phenotype of SMCs, thus inhibiting calcification.[45, 46] Additionally, RAP can inhibit cell aging and apoptosis, further contributing to its anti-calcification effects.[47] Our results were consistent with the previous studies.
In AAA lesions, the overexpression of MMPs by SMCs contributes to ECM degradation, thereby fostering AAA progression.[39, 48, 49] Hence, preserving ECM integrity emerges as a potential therapeutic strategy for mitigating AAA. In this study, we investigated the regulatory effects of nanoparticles on ECM in an inflammatory AAA microenvironment simulated using TNF-α to induce MMP2 overexpression in SMCs. Upon treatment with PFB nanoparticles, no significant alteration was observed in MMP2 expression levels (Figure 3G,H); however, MMP2 activity exhibited a noteworthy decrease (Figure 3I,J). This phenomenon can be attributed to the direct inhibitory effect of PGG present in the nanoparticles on MMP2 enzymatic activity. In contrast, RAP@PFB nanoparticles not only suppressed MMP2 activity but also downregulated MMP2 expression levels. This observation suggests a synergistic regulatory effect mediated by both RAP and PGG within the nanoparticle structure on MMP2.
Inflammatory mediators not only directly induce the upregulation of MMP2, but also modulate ECM integrity by downregulating LOX protein expression.[50, 51] Consequently, following induction by TNF-α, a significant downregulation of LOX protein expression was observed in SMCs (Figure 3K,L). LOX plays a pivotal role in catalyzing the cross-linking of collagen and elastin,[16, 52-54] thereby influencing the mechanical properties and resilience of collagen fibers. Notably, reduced LOX expression compromises ECM stability.[55, 56] However, upon treatment with nanoparticles, a marked upregulation of LOX protein expression was noted. Particularly, RAP@PFB nanoparticles demonstrated superior efficacy in this regard. Previous studies have indicated that PGG can stimulate the synthesis of mature elastin fibers and enhance elastin cross-linking by promoting LOX expression. Moreover, the anti-inflammatory properties of RAP@PFB contribute to the regulation of LOX protein expression by inhibiting inflammatory factor expression. These results demonstrate that RAP@PFB nanoparticles exert a dual regulatory effect on MMP2, inhibiting its enzymatic activity and downregulating its expression levels. Thus, the maintenance of ECM stability by RAP@PFB is achieved through multifaceted mechanisms, thereby mitigating AAA formation.
2.4 Enhanced Accumulation of RAP@PFB in AAA Lesions
Following the confirmation of the functionality of RAP@PFB at in vitro and cellular levels, its in vivo application was investigated. Preliminary assessment through an in vitro hemolysis test revealed that RAP@PFB exhibited favorable blood compatibility, with hemolysis rates consistently below 5% across varying concentrations (Figure S15, Supporting Information).
Liquid chromatography-mass spectrometry (LC-MS) method was used to measure pharmacokinetic profile of RAP. The blood circulation half-life (T1/2) of RAP in the free RAP group was 1.23 h, while in the RAP@PFB NP treatment group, it extended to 5.31 h, suggested that the encapsulation of RAP in MOF leads to prolonged in vivo circulation and delayed release (Figure S16, Supporting Information). A sustained release of RAP over 48 h was observed in RAP@PFB NPs, whose plasma concentration maintained a higher level than that of RAP suspension. Additionally, at the 48-h time point, the concentration of RAP in the RAP@PFB NP group was 12.97 ng ml−1, exceeding the plasma level required for primary immunosuppression in human transplant recipients.[57]
Subsequently, the in vivo targetability of RAP@PFB was evaluated utilizing a calcium chloride (CaCl2)-induced rat model of AAA (Figure 4A). Intravenous administration of RAP@PFB, labeled with Cy5.5 fluorescence, was performed via the tail vein. Subsequent imaging using the IVIS imaging system revealed negligible fluorescence signals in the abdominal aorta segment of normal mice after 6 h post-administration. In contrast, a pronounced fluorescence signal was observed in the lesion area of abdominal aneurysms, indicative of effective enrichment of nanoparticles at the AAA lesion site (Figure 4B). Endothelial dysfunction is one of the pathological features of AAA, allowing nanoparticles to penetrate the damaged vascular endothelium. Additionally, nanoparticles can be transported to and infiltrate the site of aortic injury through inflammatory cells such as neutrophils and monocytes/macrophages. Neovascularization in the middle and outer layers of the arteries also contributes to the accumulation of nanoparticles in aneurysm lesions.[58-60] These targeting mechanisms enable nanoparticles to effectively reach AAA sites despite the high shear force of blood flow in the aorta.
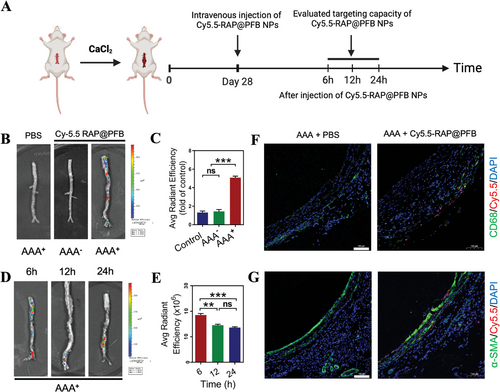
Further quantification of fluorescence intensity demonstrated a fourfold increase in fluorescence signal attributable to RAP@PFB at the AAA site compared to that of the normal mice control (Figure 4C). Dynamic monitoring of fluorescence kinetics revealed sustained retention of RAP@PFB in the affected area of the aneurysm for over 24 h (Figure 4D,E). Therefore, RAP@PFB nanoparticles exhibit enhanced accumulation at abdominal aortic aneurysm lesions, underscored by their ability to effectively target and persist within AAA lesions. To further elucidate the enrichment of nanoparticles at the AAA site, we performed co-localization studies using immunofluorescence for CD68+ macrophages and α-SMA+ SMCs (Figure 4F,G; Figure S17A,B, Supporting Information). The results demonstrated that the nanoparticles accumulated significantly in both the outer and middle layers of the arterial wall and effectively targeted lesion cells, particularly macrophages.
2.5 Therapeutic Efficacy of RAP@PFB in a Rat AAA Model
Upon verifying the effective targeting of aneurysm lesions by RAP@PFB, its in vivo anti-aneurysm efficacy was further evaluated (Figure S18, Supporting Information). A rat model of AAA was established, and rats were randomly allocated into three groups: PBS-treated, PFB-treated, and RAP@PFB-treated groups. Following treatment, the maximum inner diameter of the abdominal aorta was measured via in vivo ultrasound imaging, and the excised abdominal aortas underwent visual inspection to assess treatment outcomes (Figure 5A–C). Significant dilation of the abdominal aorta, surpassing the diameter of the control group by 1.5 times, was observed in the PBS-treated AAA rats, confirming the successful establishment of the disease model. Both RAP@PFB and PFB treatments exhibited alleviative effects on abdominal aortic aneurysm. Notably, RAP@PFB demonstrated superior therapeutic efficacy, corroborating the synergistic action of RAP and PGG in vivo (Figure 5D,E).
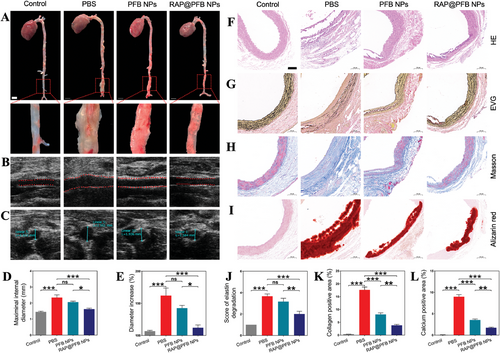
Further validation of the therapeutic effect was conducted through pathological analyses of AAA blood vessels. PBS-treated model group exhibited characteristic features of AAA progression (Figure 5F–I; Figure S19, Supporting Information), including arterial dilation, pronounced aneurysm wall thickening (as indicated by hematoxylin and eosin (HE) staining), rupture and degradation of elastin (demonstrated by Verhoeff's S-van Gieson (EVG) staining), severe calcification and deposition within the aneurysm's middle layer (revealed by Alizarin red staining), and excessive collagen deposition (illustrated by Masson staining). Following PFB treatment, although no significant reduction in elastin degradation was observed, a mitigated level of calcium deposition was noted, potentially attributed to PGG-mediated inhibition of elastin degradation and specific binding to potential calcification nucleation sites, thereby impeding calcium binding and calcification progression. The RAP@PFB group exhibited the most favorable therapeutic outcomes at the pathological level (Figure 5J–L), manifesting significant inhibition of elastin degradation, reduced collagen deposition, diminished vascular hardness, and decreased risk of rupture. Moreover, calcification was notably attenuated post-RAP@PFB treatment.
2.6 Therapeutic Mechanisms of RAP@PFB In Vivo
Following the elucidation of RAP@PFB's therapeutic efficacy, the underlying mechanisms of action were further investigated in vivo. To delineate the antioxidant and anti-inflammatory effects RAP@PFB, levels of pro-inflammatory factors (including TNF-α and IL-6) and ROS were assessed in arterial aneurysm tissue. Both RAP@PFB and PFB treatments led to significant reductions in TNF-α, IL-6, and ROS levels within the arterial aneurysm tissue (Figure 6A–C,G–I; Figures S20 and S21, Supporting Information). Moreover, the representative cytokines, including TNF-ɑ and IL-6, decreased significantly, demonstrating the anti-inflammatory effect of the nanoparticles (Figure S22, Supporting Information). Immunofluorescence staining of macrophage markers elucidated the polarization state of macrophages within the tissue. Specifically, the immune fluorescence signal of inducible iNOS exhibited a substantial increase following PBS treatment, indicative of M1-type macrophage characteristics (Figure 6D; Figure S23, Supporting Information). Conversely, treatment with nanoparticles resulted in a notable decrease in the iNOS fluorescence signal and a concomitant increase in the immune fluorescence signal of Arg-1(Figure 6J,K), suggesting a repolarization from M1 to M2 phenotype. Notably, RAP@PFB demonstrated enhanced pro-macrophage repolarization activity compared to PFB.
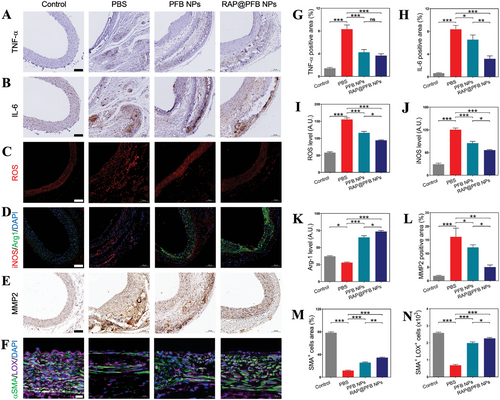
Furthermore, the anti-inflammatory and antioxidant properties of nanoparticles were associated with a downregulation in the expression of MMP-2 (Figure 6E,L; Figure S24, Supporting Information), a key contributor to ECM degradation in AAA. Immunostaining of α-SMA, a marker for SMCs, revealed a significant maintenance of functional smooth muscle cells following nanoparticle treatment. Additionally, upregulation of LOX expression within SMCs was observed, facilitating cross-linking and maturation of collagen and elastin within the ECM (Figure 6F,M,N; Figure S25, Supporting Information). Overall, the therapeutic effects of RAP@PFB against AAA are mediated through diverse mechanisms, including anti-inflammatory, antioxidant activities, promotion of macrophage M1-to-M2 repolarization, and regulation of MMP-2 and LOX expression, collectively contributing to the attenuation of AAA progression.
2.7 In Vivo Safety Evaluation of RAP@PFB
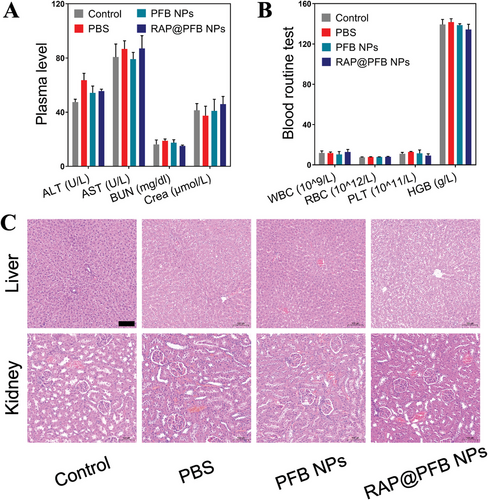
The biosafety profile of the nanomedicine was comprehensively assessed post-treatment. Evaluation encompassed key indicators of liver and kidney function (Figure 7A,B), including alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (Crea), as well as blood routine parameters such as white blood cells (WBC), red blood cells (RBC), platelets (PLT), and hemoglobin (HGB). Notably, all measured parameters remained within the normal physiological range, indicative of the absence of significant toxicological effects associated with nanoparticle administration. Histological examination of representative organs (Figure 7C), including the liver and kidneys, was conducted using HE staining to further assess potential pathological changes. Importantly, histological analysis revealed no discernible abnormalities or pathological alterations in the examined organs, affirming the high biocompatibility and translational potential of the nanoparticles. The findings from the in vivo safety evaluation underscore the favorable biosafety profile of RAP@PFB, thereby supporting its further clinical development and potential therapeutic application.
3 Conclusion
In conclusion, this study highlights the promising therapeutic potential of the novel nanodrug delivery system, RAP@PFB, in addressing AAA. By combining the antioxidant properties of PGG with the mTOR inhibitory effects of RAP within a MOF structure, RAP@PFB demonstrates multifaceted efficacy in mitigating AAA progression. Through comprehensive in vitro and in vivo evaluations, RAP@PFB exhibited remarkable capabilities in regulating inflammatory responses, promoting macrophage phenotype repolarization, inhibiting SMC apoptosis and calcification, and stabilizing the ECM. Notably, its favorable biosafety profile further supports its clinical translational potential as a promising therapeutic strategy for AAA. Moving forward, further investigations and clinical trials are warranted to validate and optimize the therapeutic efficacy of RAP@PFB, ultimately offering new hope for patients suffering from AAA.
4 Experimental Section
Materials
RAP and PGG were bought from Bide Pharmatech Co., Ltd. (Shanghai, China). Ferric chloride (FeCl3) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). BSA was obtained from Shanghai Macklin Biochemical Technology Co., Ltd. LPS was bought from Biosharp. (Hefei, China). DPPH and DCFH-DA were bought from MedChemExpress. Annexin V-FITC/7-AAD Apoptosis Detection Kit was purchased by Procell Life Science&Technology Co.,Ltd. (Wuhan, China). Dulbecco's modified Eagle's medium (DMEM), RPMI 1640 medium, and FBS were purchased from Gibco Life Technologies, Inc. (Grand Island, NY, USA). Mouse TNF-α, and IL-6 ELISA kit were purchased from Multisciences (Lianke) Biotech Co., Ltd. (Hangzhou, China).
Preparation of RAP@PFB NP
RAP NPs were prepared by quickly adding 50 µL of a RAP solution (20 mg mL−1, dissolved in ethanol) in 5 mL of ultrapure water under vigorous stirring for 5 min, followed by sonication for 2 min. Afterward, 100 µL of FeCl3 (10 mg mL−1) and 100 µL of PGG (40 mg mL−1, dissolved in dimethyl sulfoxide) were quickly added to the above RAP NPs. After stirring for 30 min, the RAP@PGG/Fe3+ was collected by centrifugation and washed with ultrapure water. Then, an equal volume of BSA solution (0.5 mg mL−1) was added to the above mixture and incubated at 4 °C overnight. Finally, the mixture was then centrifuged at 16 000 rpm for 15 min to collect RAP@PFB NP, and then resuspended in water. To prepare Cy5.5-loaded NPs (Cy5.5-RAP@PFB), 50 µL of Cy5.5 (10 mg mL−1, dissolved in dimethyl sulfoxide) and 50 µL of RAP (20 mg mL−1, dissolved in ethanol) were added to 5 mL of water to prepare a nanocore, and the shell layer was formed following the above-mentioned method.
Characterization of RAP@PFB NP
The particle size and the ζ potential of RAP@PFB NPs were determined by dynamic light scattering analysis using a Malvern Zetasizer Nano series (Nano ZS, Malvern instruments). The stability in different media (10 mM pH 7.4 PBS, cell culturing medium containing 10% FBS) was assessed by measuring particle size over 48 h incubation. The morphology and element distribution of RAP@PFB NPs were observed using transmission electron microscopy-energy dispersive spectrometry (TEM-EDS, Titan G2 60–300, FEI). The UV-vis spectra of RAP NPs, PFB NPs, and RAP@PFB NPs were measured by a UV–vis spectrophotometer (UV-2600, Shimadzu). The concentration of RAP was measured by HPLC (LC 2010A, Shimazu, Japan). The HPLC was equipped with an Agilent C18 column (250 mm × 4.6 mm, 5 µm). The mobile phase included 80% acetonitrile and 20% water, and RAP was detected by a UV detector at 278 nm. The release behavior profile of RAP was determined according to the previously reported methods.[21] RAP @PFB (1 mg) was dispersed in 5 mL phosphate buffered saline (PBS, 10 mM) media (pH 6.0 and 7.4 with or without H2O2, 1 mM) and shaken by a thermostatic shaker (100 rpm, 37 °C). Then, samples were collected at various time points (0, 1, 2, 4, 8, 12, 24 h; each time point has an individual solution), followed by centrifugation (3000 rpm, 5 min). The precipitation was dissolved in ethanol and filtered with a 0.22m poly(vinylidene difluoride) filter for HPLC analysis.
DPPH Radical Scavenging Activity
Different concentrations of PFB NPs or RAP@PFB NPs (20 µL, concentrations of RAP ranging from 0.25, 0.5, 1.25, 2.5, 5, to 12.5 µM and PGG from 1, 2, 5, 10, 20 to 50 µM) and acetate buffer (180 µL, 100 mM, pH 5.5) were added into DPPH ethanol solution (0.5 mM, 200 µL), followed by vigorous vortex in the dark. The UV absorbance at 517 nm was monitored every 5 min for 40 min, and UV spectra of 400–800 nm were measured at 40 min. A standard curve was prepared to quantify DPPH using UV–vis absorbance at 517 nm, based on which the DPPH scavenging percentage was calculated in the presence of various concentrations of NPs.
•OH and •O2− Radical Scavenging Activity
The •OH and •O2− scavenging activity of PFB NPs and RAP@PFB NPs (concentrations of PGG varying from 0.25, 0.5, 1, 2, 4 to 6 mg mL−1 and RAP from 0.0625, 0.125, 0.25, 0.5, 1 to 1.25 mg mL−1) were measured by using the hydroxyl free radical assay kit and inhibition and produce superoxide anion assay kit, respectively, according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, China). The hydroxyl radical and superoxide anion-scavenging capability of nanoparticles were calculated and compared. In addition, an ESR spectrometer (Bruker A300, Germany) was also employed to explore the •OH− and •O2− scavenging activity. Briefly, •OH− was produced in the Fenton reaction, while •O2− was generated by a mixture of xanthine and xanthine oxidase. The 5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide (BMPO) was used to trap the •OH− and •O2− via generation of BMPO/•OH and BMPO/•OOH, respectively. ESR spectra were recorded with or without addition of PFB NPs or RAP@PFB NPs at 19.62 mW microwave power and 1 G modulation amplitude.
•H2O2 Scavenging Activity
Briefly, PFB NPs and RAP@PFB NPs solutions (concentrations of PGG varying from 0.25, 0.5, 1, 2, 4 to 6 mg mL−1 and RAP from 0.0625, 0.125, 0.25, 0.5, 1 to 1.25 mg mL−1) were mixed with an excess amount of H2O2 (50 mM) at 37 °C for 24 h.[21] The residual H2O2 was measured by Hydrogen Peroxide Detection Kit (Nanjing Jiancheng Bioengineering Institute, China), with the absorbance being measured by a microplate reader at 405 nm. The H2O2-eliminating capability of nanoparticles were calculated and compared.
•NO Scavenging Activity
•NO was produced by sodium nitroprusside (SNP). Briefly, 100 µL of SNP (20 mM) and 100 µL of PFB NPs and RAP@PFB NPs solutions (concentrations of RAP ranging from 0.25, 0.5, 1.25, 2.5, 5 to 12.5 µM and PGG from 1, 2, 5, 10, 20 to 50 µM) were incubated with 200 µL of PBS solution (0.2 M, pH 7.4), followed by shaking at 37 °C for 1.5 h, the nitric oxide was measured by nitric oxide assay kit (Nanjing Jiancheng Bioengineering Institute, China). The absorbance was measured by a microplate reader (Infinite M200 Pro, Tecan) at 550 nm, and the nitric oxide-scavenging capability of PFB NPs and RAP@PFB NPs were calculated and compared.
Cell Culture
The murine RAW264.7 macrophage cells were cultured in RPMI-1640 complete medium and A10 rat aortic vascular SMCs were cultured in DMEM complete medium under a humidified atmosphere of 5% CO2 at 37 °C. The complete medium contains 10% FBS and 1% penicillin/streptomycin.
Cytotoxicity Assay
The RAW264.7 cells and SMCs were seeded in 96-well plates at a density of 5 × 103 cells per well. After overnight culturing, the cells were added with different concentrations of PFB NPs or RAP@PFB NPs (concentrations of RAP ranging from 0, 0.125, 0.25, 0.5, 1, 1.25 to 2.5 µM and PGG from 0, 0.5, 1, 2, 4, 5 to 10 µM) for 48 h incubation. Then, the medium was replaced by 100 µL of MTT solution (0.5 mg ml−1), followed by a 4 h incubation. Finally, 100 µL of DMSO was added to dissolve formazan crystals, and the absorbance at 570 nm was measured to calculate the cell viability.
Intracellular ROS Scavenging Activity
The intracellular ROS levels in RAW264.7 macrophages or SMCs induced by LPS (1 µg ml−1) or H2O2 (200 µM) were evaluated by using a fluorescent probe DCFH-DA. In brief, RAW264.7 cells or SMCs (2 × 105 cells per well) were seeded in 24-well plates and incubated for 12 h. The cell culture medium was replaced with a fresh medium containing different NPs (concentrations of 2.5 µM for RAP and 10 µM for PGG) and incubated for 12 h. After stimulation with LPS or H2O2 for 6 h, the fresh medium containing 10 µM DCFH-DA was added to each well and incubated at 37 °C for 30 min. Subsequently, cells were washed three times with PBS, and observed by fluorescence microscopy. To quantify the ROS level, the cells were seeded in 6 well plates at a density of 5 × 105 cells per well, and the same treatments were performed as described above. The cells were harvested, and the fluorescence intensity was measured by flow cytometry.
Anti-Inflammatory Effects and M1‑to‑M2 Polarization of the RAW264.7 Macrophages
The RAW264.7 cells (3 × 105 cells per well) were seeded in six-well plates and incubated overnight. Then, Cells were stimulated by LPS (1 µg mL−1) for 24 h and treated with PFB NPs or RAP@PFB NPs (concentrations of 2.5 µM for RAP and 10 µM for PGG) for 24 h. Subsequently, various inflammatory cytokines in culture supernatants were measured by ELISA. For immunofluorescence staining, the RAW264.7 cells were conducted to examine the level of iNOS (M1 marker) and Arg-1 (M2 marker). In brief, cells were fixed with 4% paraformaldehyde to allow incubation with primary antibodies at 4 °C overnight. Then, the secondary antibodies (with fluorescence-labeling) were added for 1 h incubation. The cell nuclei were co-stained with DAPI and then observed and imaged by fluorescence microscope. The proportion of RAW264.7 macrophage cells were analyzed by flow cytometry. The cells were harvested with the same treatments were performed as described above. The cells were stained with PE/Cy7-anti-CD86 (Elabscience, China), and APC-anti-CD206 (Elabscience, China) according to the protocol. Flow cytometry was performed with the flow cytometry. (BD Biosciences, U.S.A.).
In Vitro Apoptosis Assay of SMCs
Annexin V-FITC/7-AAD Apoptosis Detection Kit was conducted apoptosis analysis according to the manufacture's protocol. Specifically, SMCs were seeded in a 12-well plate at 2 × 105 cells per well, and incubated overnight. The medium was then replaced with a fresh growth medium containing various formulations (concentrations of 2.5 µM for RAP and 10 µM for PGG). After 12 h of incubation, cells were treated with 400 µM H2O2 for 6 h. Then, cells were washed with cold BioLegend cell staining buffer, digested with 0.25 wt% trypsin, and collected by centrifugation. After the cells were resuspended in 100 µL of Annexin V binding buffer with 2.5 µL of Annexin V and 5 µL of 7-AAD viability staining solution at 1 × 105 cells mL−1, they were vortexed gently and incubated in the dark for 15 min. Finally, 400 µL of Annexin V binding buffer was added for analysis by flow cytometry. (BD Biosciences, U.S.A.).
Detection of Calcification in SMCs
The degree of cell calcification was assessed by staining with Alizarin Red. Specifically, SMCs were seeded in a six-well plate at a density of 2 × 105 cells per well and incubated in 2 mL of growth medium for 12 h. Then different NPs (concentrations of 2.5 µM for RAP and 10 µM for PGG) were separately added and incubated with cells. After 24 h, cells were incubated in elevated Ca/P medium[21] for 48 h. Cells were washed with PBS, fixed with 4% paraformaldehyde, and stained with 0.2% (w/v) Alizarin red for 10 min at room temperature. Subsequently, cells were thoroughly washed with water and observed by optical microscopy. The Alizarin Red-stained area is proportional to the extent of calcium mineral deposition in the culture well. For quantitative analysis, deposited calcium was completely dissolved in 10% cetylpyridinium chloride (Macklin, Shanghai) and then the absorbance at 405 nm was quantified with a microplate reader (Infinite M200 Pro, Tecan).
MMP2 and LOX Regulation
The SMCs were incubated with TNF-α (10 ng ml−1) in serum-free medium for 24 h. Then, the cells were treated with different formulations (concentrations of 2.5 µM for RAP and 10 µM for PGG) for 24 h, the cells were harvested for analysis of MMP2 and LOX protein expression by Western blot. The cells were lysed in RIPA buffer (Servicebio, China). After quantification of the isolated proteins using a BCA protein quantification kit (Servicebio, China), equal amounts of proteins were loaded on 5% SDS-PAGE for separation, and then transferred to PVDF membranes. Afterward, the membranes were blocked for 1 h with 5% non-fat milk and then incubated with rabbit MMP2 monoclonal antibody (Abcam, U.S.A.) and rabbit LOX monoclonal antibody (Huabio, China) at 4 °C overnight, followed by incubating with HRP-conjugated secondary antibodies for 1 h at room temperature. Finally, the blots were detected by a western blotting detection system (BioSpectrum 300, UVP).
Zymography Assay
The conditioned medium was collected after the SMCs were treated in the same way as above, centrifuged, and loaded onto 10% acrylamide gel containing 1 mg ml−1 gelatin. After electrophoresis, the gels were washed with 2.5% Triton X-100 for 2 h and then incubated overnight at room temperature in a developing buffer (40 mM Tris-HCl, 10 mM CaCl2, and 0.01% NaN3; pH 8.0). Finally, the zymographic activities were revealed by staining with coomassie brilliant blue R-250 and quantified by densitometry of the corresponding bands.
Animal
Male Sprague-Dawley rats (250-300 g) were obtained from the Animal Center of the Central South University. All the animal care and experimental protocols were performed in accordance with the guidelines for the Care and Use of Laboratory Animals of Central South University (Changsha, China). All procedures and protocols were approved by the Animal Ethics Committee at Central South University. All animals were housed in standard cages under suitable light, temperature, and humidity environments, with ad libitum access to food and water. Animals were acclimatized to the laboratory for at least 3 days before further experiments.
Hemolysis Assay
Before in vivo experiment was conducted, the fresh red blood cells were isolated from the rat and centrifugated and washed with PBS for 3 times. Then, RBCs were diluted with PBS to 2% (v/v) suspension and incubated with an equal volume of water, PBS, or different formulations (concentrations of RAP ranging from 1.25, 2.5, 5, 12.5, 25, 50 µg mL−1 and PGG from 5, 10, 20, 50, 100 to 200 µg mL−1) at 25 °C for 3 h. After centrifugation, the hemolysis degrees of various groups were calculated by quantification of absorbance at 500 nm due to hemoglobin, and the samples were imaged by a cellphone camera.
Pharmacokinetics Study
The male SD rats were randomly assigned into two groups and injected with free RAP or RAP@PFB NPs intravenously at a RAP equivalent dose of 1 mg k−1g. The blood samples of 100 µL were collected from orbital at predetermined time points (5 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, 48 h) and stored at −80 °C. The concentration of RAP was measured by LC-MS method. Briefly, the aliquot of 50 µl of the whole blood sample was mixed with 150 µl methanol and vortexed for 1 min. After centrifugation at 15 000 rpm for 10 min at 4 °C, 10 µl of the supernatant extract was injected into the LC-MS system (Thermo Q Exactive) for analysis.
Establishment of an AAA model in Rats
In brief, after rats were anesthetized, the abdominal cavity was opened and the infrarenal abdominal aorta was exposed. The infrarenal abdominal aorta was treated by placing a piece of CaCl2-soaked (0.5 M) sterile cotton gauze (0.5 cm × 0.5 cm) on the aorta for 15 min. The treatment area was washed with warm saline and sutured post operation. Rats in the sham group were surgically treated with saline instead of CaCl2, following the similar procedures.
In Vivo Targeting Capability Profiles of RAP@PFB
At day 28 after CaCl2 induced AAA established, Cy5.5-RAP@PFB NPs (concentrations of 1 mg k−1g for RAP and 4 mg k−1g for PGG) in PBS was injected in AAA rats via the tail vein. Rats in the control group were subjected to i.v. injection of PBS. In addition, rats in the sham group (health rats) were treated by i.v. injection of Cy5.5-RAP@PFB NPs in PBS. At predefined time points (6 h, 12 h, and 24 h after injection), the rats were euthanized and the aortas including the thoracic aorta, abdominal aorta, and bilateral iliac arteries were harvested. After washing with PBS, the samples were observed on a PerkinElmer optical imaging system (IVIS Lumina, USA). The images were analyzed with Living Imaging Software. Furthermore, after 24 h of intravenous injection of Cy5.5-RAP@PFB NPs, the abdominal aorta lesions were excised and frozen in optimal cutting temperature (O.C.T.) compound. The section thickness was 6 µm. Cryosections of abdominal aortas were stained with anti-CD68 antibody (Servicebio, China.) or anti-α-SMA antibody (Abcam, U.S.A.) to further evaluate the tissue distribution of NPs. After the sections were incubated overnight at 4 °C, they were treated with corresponding Alexa Fluor488 secondary antibody (ThermoFisher Scientific, U.S.A.). Fluorescence images were acquired after nuclei were stained with DAPI.
Animals and Treatment
To study in vivo efficacies of different NPs in rats with AAA induced by CaCl2, male SD rats were randomly assigned into three groups. At day 1 after CaCl2-induced injury, PBS was i.v. injected in the model group, while different formulations (concentrations of 1 mg k−1g for RAP and 4 mg k−1g for PGG) were i.v. injected in randomly assigned rats via the tail vein twice a week for 4 weeks in other groups. In a control and model group rats were treated with PBS.
Ultrasound Imaging
After completion of different treatments, the maximum internal diameter of the abdominal aorta in each group was measured with the Vevo 3100 micro-ultrasound imaging system (Fujifilm VisualSonics, Canada) before euthanasia.
Histological Analysis
The abdominal aortas were exposed and harvested. The midsection of the abdominal aorta was made into paraffin sections (4 µm). The sections were separately stained with HE to observe general morphology, Masson Trichrome staining to assess collagen content, EVG staining to evaluate elastin degradation, and Alizarin Red staining to assess calcium content. Elastin degradation was scored as following criteria[8]: grade 1, no degradation and a well-organized lamina; grade 2, mild degradation with some interruptions or breaks in the lamina; grade 3, moderate degradation with multiple interruptions or breaks in the lamina; and grade 4, severe fragmentation or loss or aortic rupture.
Immunohistochemistry and Immunofluorescence Staining
For immunohistochemistry staining, paraffin-embedded sections (4 µm) were incubated with primary antibodies against TNF-α antibody (Proteintech, U.S.A.), IL-6 antibody (Boster, U.S.A.) and MMP2 antibody (Abcam, U.S.A.) overnight at 4 °C and incubated with a secondary antibody the next day. Then, the sections were stained for peroxidase activity with hydrogen peroxide and 3,3′-diaminobenzidine tetrahydrochloride as substrates, followed by light counterstaining with hematoxylin to identify the nuclei. A brown reaction product indicated the localization of TNF-α, IL-6, and MMP2.
For immunofluorescence Staining, the abdominal aorta sections were stained with iNOS (Servicebio, China), Arg-1 (Servicebio, China), α-SMA (Affinity, U.S.A.), and anti-LOX antibody (Huabio, China) antibodies overnight at 4 °C after continuous deparaffinization, rehydration, and antigen retrieval process. Afterward, the fluorescent-labeled secondary antibodies were added and incubated for 1 h. The sections were observed by a fluorescent microscope after staining the nuclei with DAPI.
In addition, the abdominal aorta was frozen directly in O.C.T. Compound and sectioned at a thickness of 4 µm. Cryosections of abdominal aortas were incubated with DHE (Sigma-Aldrich, U.S.A.) for 30 min. After washing with PBS three times, the cryosections were captured under the fluorescence microscope.
Biosafety Evaluation
After rats were euthanized, except for the aorta, blood samples were collected for blood routine tests and biochemical analysis to detect toxicity of the liver and the kidney. Moreover, the serum levels of TNF-α and IL-6 were quantified by ELISA. Major organs were harvested, including the liver and kidney. Histological sections were made and stained with HE and examined by optical microscopy.
Statistical Analysis
All the quantitative data were expressed as mean ± standard error of mean (SEM). Student's t-test and one-way analysis of variance (ANOVA) were used to assess the differences between two groups and among multiple groups, respectively. The significance was defined as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ns: no significance.
Acknowledgements
H.Z. and P.Z. contributed equally to this work. This work was supported by National Natural Science Foundation of China (no. 82170501) and Guangzhou Science and Technology Plan Project (2023A03J0355).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




