Image-Guided Photothermal and Immune Therapy of Tumors via Melanin-Producing Genetically Engineered Bacteria
Abstract
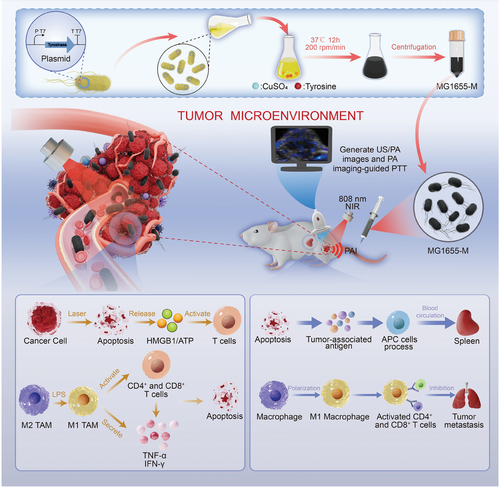
Photothermal therapy (PTT) is a new treatment modality for tumors. However, the efficient delivery of photothermal agents into tumors remains difficult, especially in hypoxic tumor regions. In this study, an approach to deliver melanin, a natural photothermal agent, into tumors using genetically engineered bacteria for image-guided photothermal and immune therapy is developed. An Escherichia coli MG1655 is transformed with a recombinant plasmid harboring a tyrosinase gene to produce melanin nanoparticles. Melanin-producing genetically engineered bacteria (MG1655-M) are systemically administered to 4T1 tumor-bearing mice. The tumor-targeting properties of MG1655-M in the hypoxic environment integrate the properties of hypoxia targeting, photoacoustic imaging, and photothermal therapeutic agents in an “all-in-one” manner. This eliminates the need for post-modification to achieve image-guided hypoxia-targeted cancer photothermal therapy. Tumor growth is significantly suppressed by irradiating the tumor with an 808 nm laser. Furthermore, strong antitumor immunity is triggered by PTT, thereby producing long-term immune memory effects that effectively inhibit tumor metastasis and recurrence. This work proposes a new photothermal and immune therapy guided by an “all-in-one” melanin-producing genetically engineered bacteria, which can offer broad potential applications in cancer treatment.
1 Introduction
Malignant tumors are the leading cause of death worldwide. Over the past few decades, conventional treatments for cancer such as surgery, drugs, and radiation have significantly improved. However, they still have some limitations, such as serious adverse effects, resistance to therapy, and low selectivity.[1, 2] The development of hypoxia may be a significant factor in tumor radiochemotherapy tolerance, a widespread phenomenon in solid tumors. Generally, hypoxic tumors require a normal radiation dose three times higher than that of normoxic tumors to achieve the desired cell death effect.[3] In addition, hypoxic tumors impede the clinical outcomes of chemotherapy owing to a lack of blood vessels and elevated interstitial fluid pressure, which limit the availability of various drug formulations.[4, 5] Although several sophisticated cargo-loaded nanoparticles have been developed to target drug delivery specifically to tumor sites, they still face challenges in reaching the hypoxic tumor region because of the lack of a self-propelling force.[6-8] Hypoxic tumors are highly aggressive and associated with poor patient prognosis.[9] Therefore, it is highly desirable to develop methods for delivering antitumor agents into the tumor-hypoxic regions to maximize their therapeutic efficacy.
Recently, owing to the low oxygen levels in solid tumors, bacteria-mediated tumor therapy has gained great attention as a way to deliver drugs.[10-12] Unlike other nonliving drug delivery systems, a few anaerobic and facultative anaerobic microorganisms, such as Escherichia coli, Salmonella, and Listeria, can actively target hypoxic, eutrophic, and immunosuppressive tumor microenvironments and overcome the barriers of high interstitial pressure.[13-15] They preferentially accumulate and proliferate inside the ischemic and hypoxic tumor regions. Additionally, bacterial growth can induce innate and adaptive immune reactions against tumor cells.[16-19] Importantly, bacteria can be engineered to produce or deliver antitumor agents according to the requirements of the clinical setting. For example, engineered bacteria expressing single-chain antibodies, such as CD47, PD–L1, or TNF–α, have been developed.[20-22] However, relying solely on engineered bacteria is ineffective for tumor removal. Tumor-targeting bacteria can be combined with other treatment modalities to improve their antitumor efficacy.[23] Recently, near-infrared photothermal therapy (PTT) has emerged as a new treatment modality for killing tumor cells via heating.[24, 25] Bacteria have been reported to deliver photothermal agents such as melanin, ICG, and polydopamine into tumors for photothermal therapy.[26-29] Enhanced antitumor efficacy can be achieved by regulating bacterial gene expression or carrying drug-loaded nanoparticles. Clinical Phase I studies suggest promising prospects for bacteria-based antitumor therapies.[30]
In this study, we genetically engineered E. coli MG1655, a tumor-targeting bacterial strain, to express a heterologous tyrosinase (tyr1) gene for imaging-guided PTT. Using genetically engineered bacteria, melanin nanoparticles can be biosynthesized under physiological conditions in the presence of tyrosine and Cu2+ ions. Without any chemical modification or additional payload loading, MG1655-producing melanin nanoparticles (MG1655-M) were systemically administered to tumor-bearing mice and actively delivered to the hypoxic regions. MG1655-M was tracked using photoacoustic (PA) imaging based on photothermally converted melanin nanoparticles. This information can be used to guide laser irradiation for PTT, effectively kill tumor cells, and prolong survival.[31] Furthermore, strong anticancer immune effects can be stimulated by heat-damaged tumor cells (which release tumor-related antigens) and exposure to bacterial lipopolysaccharides (LPS). Antigen-presenting cells (APCs) can uptake and process tumor-associated antigens, present them in conjunction with MHC molecules, home to the spleen, and activate antitumor immune protection to inhibit metastasis.[32, 33] This helped the polarization of macrophages from M2 to M1 phenotype, effectively activated CD4+ and CD8+ T-cells, and secreted TNF–α, IL–10, and IFN–γ.[34] In addition, PTT not only kills tumor cells directly but also induces immunogenic cell death (ICD) in tumor cells and releases damage-associated molecular patterns (DAMPs), such as HMGB1 and ATP. These DAMPs are recognized and presented by DCs, thereby activating T-cell immune responses.[35, 36] Eventually, immune memory effects are achieved, which significantly enhance the inhibitory effects against tumor metastasis and recurrence (Figure 1). This study proposes a novel photothermal immunotherapy guided by melanin-producing genetically engineered bacteria. The MG1655-M is believed to serve as a simple and smart hypoxia-targeted “photothermal agent” with wide potential for application in cancer treatment.
2 Results and Discussion
2.1 Melanin Biosynthesis and Characteristics of MG1655-M
The tyr1 gene is obtained from megaterium B and inserted downstream into the pJC-T7 vector of the strong T7 promoter (Figure 2A). The E. coli MG1655 cells were subsequently transformed using a recombinant plasmid. The tyr1 gene product was confirmed to have the expected molecular weight of 38.1 kDa (Figure S1, Supporting Information). Furthermore, tyr 1 expression did not affect the growth rate of genetically engineered bacteria (Figure S2A, Supporting Information). The growing medium is supplemented with 200 mg mL−1 CuSO4.5H2O and 8 mg L-tyrosine. The color of the culture gradually turned black within an incubation period of 4–48 h (Figure S2B, Supporting Information). In contrast, melanin was not synthesized by wild-type E. coli MG1655 in the absence of tyr 1 (Figure S2C, Supporting Information).
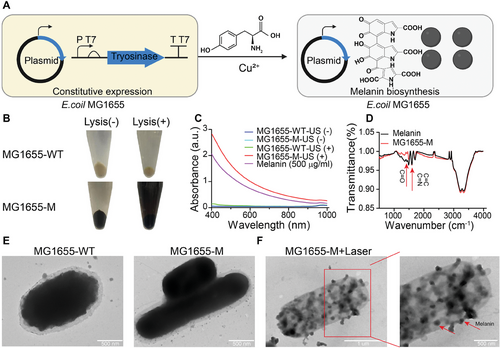
UV–vis absorption spectroscopy was performed to further demonstrate the presence of melanin in MG1655-M bacteria. Only the lysates of the ultrasound-treated MG1655-M cells with melanin exhibited optical spectrum characteristics similar to those of commercially available melanin nanoparticles (Figure 2B,C). The peaks at C═O (1350 cm−1), C═C, and C═N (1600 cm−1) of biosynthetic and commercial melanin were established by Fourier-transform infrared (FT-IR) spectroscopy, indicating the presence of chemical aromatic structures in melanin, which confirmed the production of melanin by MG1655-M (Figure 2D). The tyr 1 expression exhibits a negligible influence on the particle size of the bacteria and zeta potential (Figure S3A,B, Supporting Information). Transmission electron microscopy (TEM) images indicated that the morphology of MG1655-M was flat and elliptical, similar to that of MG1655-WT, whereas the higher contrast of the cytoplasm was significantly different from that of MG1655-WT (Figure 2E; Figure S3C, Supporting Information). Disintegrated melanin was observed after laser irradiation at 808 nm (Figure 2F). A concentration-dependent increase in the UV–vis absorbance intensity of the biosynthetic melanin nanoparticles was demonstrated using the following linear regression Equation: Y = 0.0625 X + 0.0407 (R2 = 0.9999, 808 nm) (Figure S4A,B, Supporting Information).
2.2 Photothermal Effect and Photoacoustic Imaging of MG1655-M In Vitro
The strong near-infrared (NIR) absorption of melanin nanoparticles in the 780–850 nm range motivated us to investigate the capability of MG1655-M as a photothermal agent.[37] The photothermal temperature curves at the same concentrations of commercial and biosynthetic melanin (Figure 3A) indicated that the photothermal properties of biosynthetic melanin were superior to those of commercial melanin. Furthermore, absorption spectra were obtained at different concentrations of MG1655-M using UV–visible absorption spectroscopy (Figure 3B). An 808 nm laser with a power density of 1.0 W cm−2 (300 s) was used to measure the photothermal conversion performance of MG1655-M at different concentrations. Compared to phosphate-buffered saline (PBS), an evident increase in temperature was observed with increasing concentrations of MG1655-M (Figure 3C). The temperature is maintained between 40 and 70 °C while keeping the bacterial concentration fixed at 108 CFU mL−1 by controlling the laser power in the range of 0.75–1.5 W cm−2 (Figure 3D). ≈42.3% is the calculated photothermal conversion efficiency (η) of MG1655-M (Figure S4C, Supporting Information).[38-40] In summary, these results indicate that the photothermal properties of MG1655-M are positively related to both the concentration of MG1655-M and the power density of the laser irradiation.
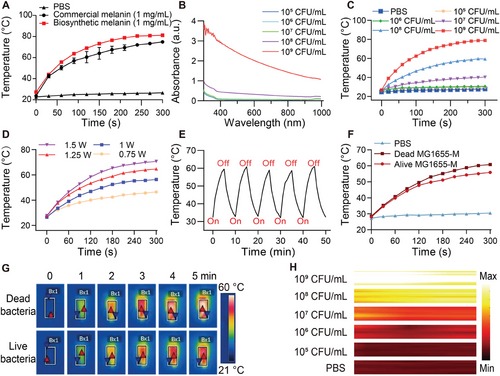
In addition, the photothermal stability of MG1655-M was evaluated during multiple exposures to an NIR laser (808 nm, 1 W cm−2) for five on/off cycles. The photothermal capability of MG1655-M remained unchanged after several NIR irradiation cycles (Figure 3E), demonstrating its high stability and excellent reproducibility. Additionally, the effects of heating on live and dead MG1655-M cells were investigated under the same NIR irradiation conditions to determine whether the photothermal effect could be attributed to bacterial activity. Similar heating effects were observed in both live and dead cells (Figure 3F,G). Furthermore, the MG1655-M was irradiated using the laser at different temperatures (30–65 °C) to determine if the photothermal effect had any detrimental impact on bacterial activity. The bacterial growth is observed normal at temperatures up to 55 °C (Figure S5, Supporting Information), indicating high thermal tolerance of these bacteria. In addition, strong PA signals were observed in MG1655-M cells with a clear dose-dependent signal enhancement (Figure 3H; Figure S6, Supporting Information).
2.3 In Vitro Tumor Cell Killing Experiment of MG1655-M
For the in vitro testing of the cytotoxicity of MG1655-M with and without laser irradiation, mouse breast cancer cells (4T1), human cervical cancer cells (HeLa), mouse prostate cancer cells (MB49), and human renal epithelial cells (293T) were treated with MG1655-M at different concentrations, followed by detection using a cell counting (CCK8) kit. After a 12-h incubation period with MG1655-M (1 × 109 CFU mL−1), the cell viability of the non-irradiated cells remained over 75%, indicating that the cell toxicity of MG1655-M was acceptable (Figure 4A–C; Figure S7A, Supporting Information). In contrast, the viabilities of 4T1, HeLa, MB49, and 293T cells were ≈10% after laser exposure to 1 W cm−2 at 808 nm for 5 min at this concentration. Many dead red blood cells in the MG1655-M + Laser group were stained with calcein-AM and propidium iodide (PI). In comparison, the green fluorescence of live cells was observed in the MG1655-M, Laser, and MG1655-WT groups (Figure 4D,E; Figure S7B, Supporting Information), indicating a good tumor cell-killing effect of MG1655-M in combination with laser irradiation. Flow cytometry confirmed 44.1% of apoptotic cells in the MG1655-M + Laser group, which was significantly higher than that in the groups treated with MG1655-M, Laser, or MG1655-WT (Figure 4F).
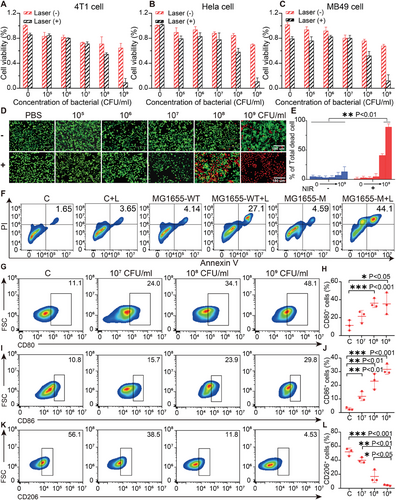
The effects of MG1655-M on macrophage activation were also investigated. The RAW 264.7 macrophages were subjected to incubation with diverse doses of bacteria (1 × 107, 1 × 108, or 1 × 109 CFU mL−1) for 24 h. The flow cytometry assay indicated that the expression of the M1 phenotype markers CD80 and CD86 was significantly increased in the 109 CFU mL−1 treatment group, with ≈29.8% CD80+ (Figure 4G,H) and 48.1% CD86+ macrophages (Figure 4I,J). In addition, there was a considerable decrease in the expression of the M2 phenotypic marker, CD206, in macrophages. (Figure 4K,L), confirming that M2 macrophages could be polarized to the M1 phenotype by the induction of MG1655-M. Macrophage polarization may contribute to the effects of LPS on bacterial surfaces.[41-43] Thus, MG1655-M effectively activated macrophages.
2.4 Tumor Targeting and Photoacoustic Imaging
To verify the tumor-targeting ability of MG1655-M, indocyanine green (ICG)-labeled live MG1655-M, heat-killed MG1655-M, and free ICG were injected into 4T1 tumor-bearing mice through the caudal vein, followed by imaging at different intervals using an IVIS spectral imaging system. Live MG1655-M exhibited effective tumor-targeting ability in 4T1 tumors, releasing robust fluorescence after injection 1 h (Figure 5A). The fluorescence intensity at the tumor site gradually increased over time, reaching twice that of the dead bacteria group after 48 h (Figure 5B). Fluorescence signals in different organs revealed that dead MG1655-M cells were primarily distributed in the liver and spleen. In comparison, live MG1655-M exhibited 3.77 times higher bacterial accumulation at the tumor site than dead MG1655-M (Figure S8, Supporting Information). A nutritious and hypoxic tumor microenvironment may be detected because various chemoreceptors are located on the surface of E. coli, thereby stimulating their active migration within the tumor region.[44, 45] Meanwhile, the bacteria settled in the hypoxic microenvironment of the tumor after injection, which could be easily observed with the naked eye. The tumors in mice injected with MG1655-M turned black within 2 days (Figure S9, Supporting Information), confirming that the bacteria targeted the tumor area.
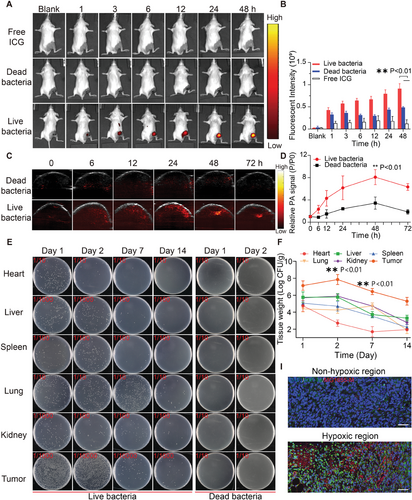
In addition to serving as PTT agents, melanin nanoparticles also serve as PA contrast agents.[46, 47] To determine the PA imaging capability of MG1655-M, 1 × 107 CFU of MG1655-M was injected intravenously into tumor-bearing mice, and the PA signal was recorded at different intervals in the tumor area. Quantitative analysis of the region of interest (ROI) PA signal intensity indicated that the maximum value of PA signal intensity at the tumor site was achieved 48 h after injection, with a 2.36-fold increase compared to the dead bacterial control group (Figure 5C,D).
Furthermore, 4T1-bearing mice were intravenously injected with 1 × 107 CFU of MG1655-M to investigate the distribution of MG1655-M after intravenous injection. Subsequently, several organs and tumors were collected at different intervals, homogenized, and plated on solid Luria–Bertani (LB) containing 100 µg mL−1 ampicillin. The colony count revealed the removal of MG1655-M from the heart, liver, spleen, lungs, and kidneys. However, the colony count in the tumor tissue increased exponentially over time, reached a maximum on day 2, and then gradually decreased (Figure 5E,F). Next, the efficiency of targeting ICG-labeled MG1655-M to tumor cells was evaluated using a fluorescence microscope. Interestingly, live MG1655-M labeled ICG (red fluorescence) was largely distributed in the center of the tumor, representing ischemic and anoxic regions because of a lack of nutrient supply. In contrast, dead MG1655-M cells primarily adhered to the peritumoral zone instead of the hypoxic region of the tumor, as detected by immunofluorescence staining (Figure 5G). Thus, MG1655-M can target, infiltrate, and settle within tumors owing to its hypoxic tumor microenvironment, which favors tumor treatment via PTT.[48, 49]
2.5 MG1655-M Mediated Tumor Photothermal Immunotherapy
A 4T1 subcutaneous tumor model was established and used to assess the antitumor efficacy of the MG1655-M + Laser in vivo. This procedure is shown in Figure 6A. Six groups were randomly selected: PBS (control), PBS + laser irradiation, MG1655-WT alone, MG1655-WT + laser irradiation, MG1655-M alone, and MG1655-M + laser treatment. 48 h after the intravenous injection of MG1655-M, the tumor was irradiated with an 808 nm laser (1 W cm−2) for 10 min. The tumor temperatures of the mice injected with MG1655-M were recorded using an infrared imager. This indicates a rapid increase to 57.1 °C, which is significantly higher than that observed with PBS and MG1655-WT (Figure S10, Supporting Information). The tumor volume of mice treated with the MG1655-M + Laser was less than 190 mm3 with the complete disappearance of the two tumors. In contrast, the tumor volumes in the other groups were greater than 1000 mm3 (Figure 6B; Figure S11A, Supporting Information). Furthermore, a slight antitumor effect was found in the MG1655-WT and MG1655-M groups, which may be due to the presence of LPS in the bacteria or nutrient competition due to bacterial growth.[50-52] More than 60% of the mice in the MG1655-M + Laser group survived for 60 days, whereas mice in the control group survived for only 30 days, indicating that the MG1655-M + Laser treatment significantly improved the survival rate (Figure 6C). Hematoxylin and eosin (H&E)-staining of tumor slices indicated that the MG1655-M + Laser group had fewer tumor cells, tissue necrosis, widespread nuclear shrinkage, and nuclear elimination (Figure 6D, upper row). To assess the maximum degree of apoptosis, terminal deoxynucleotidyl transferase TUNEL assays were performed (Figure 6D, bottom row). The administration of MG1655-WT and MG1655-M cells temporarily decreased the body weight of mice, which gradually recovered after a few days, indicating acceptable side effects in mice (Figure S11B, Supporting Information). However, the body temperature (rectal) of mice did not increase after MG1655-M injection and remained within the normal range (Figure S11C, Supporting Information).
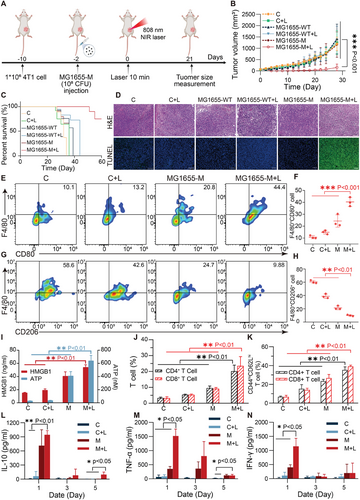
To further investigate the antitumor immune mechanisms, tumor-associated macrophages (TAM) were examined by flow cytometry. The analysis indicated an obvious increase in the CD80+ macrophages (40.33 ± 1.33%) and a considerable reduction in the CD206+ macrophages (9.93 ± 0.85%) in the tumors therapy with MG1655-M + Laser in contrast to those with the PBS group (M1:10.76 ± 1.09%, M2:60.4 ± 1.88%) (Figure 6E–H, Figure S12, Supporting Information). This suggests that TAM can effectively promote polarization from the M2 to M1 phenotype through MG1655-M + Laser treatment. Macrophage activation is ascribed to the bacteria-induced immunogenic cell death of tumor cells.[53, 54] Additionally, a significant increase in HMGB1 expression and ATP secretion (Figure 6I) was observed in the MG1655-M + Laser group, which activated the immune response of T cells. The MG1655-M + Laser group exhibited a remarkable increase in the number of CD4+ and CD8+ T cells in the tumors compared to the control and bacteria-only groups (Figure 6J; Figure S13, Supporting Information). Further analysis of T cell proliferation, exhaustion, and activation revealed a significant increase in CD3+CD4+CD44+CD62L+ and CD3+CD8+CD44+CD62L+ central memory T cells (TCM) in the MG1655-M + Laser group (Figure 6K; Figure S14, Supporting Information). In addition, the levels of TNF-α, IFN-γ, and IL-10 in tumors meaningfully increase in the MG1655-M + Laser group after 1 d and gradually recover to normal levels after 5 d (Figure 6L–N).
Notably, only a few lung metastases were observed in mice treated with the MG1655-M + Laser (Figure S15, Supporting Information), indicating that distant metastasis was effectively inhibited. To understand the systemic antitumor effect, on the 7th day after laser treatment, the mice were dissected to obtain spleens for the evaluation of immune cells. The consequence suggested the fraction of CD80+ macrophages in the MG1655-M + Laser group is 31.73 ± 1.37%, which is significantly higher than that in the PBS (11.33 ± 1.37%), PBS + Laser (13.83 ± 1.02%), and MG1655-M (21.03 ± 1.37%) groups. In contrast, the fraction of M2 macrophages (CD206+) in the MG1655-M + Laser group decreases to 8.73 ± 0.39%, which is significantly lower than that in the PBS group (34.93 ± 0.77%) (Figure S16, Supporting Information). Meanwhile, the fractions of CD4+ and CD8+ T-cells in the MG1655-M + L group are 26.93 ± 1.48% and 37.13 ± 2.04%, respectively, which are 5.1- and 4.2-time higher than that in the PBS group (Figure S17, Supporting Information). Literature suggests that cell debris can be produced due to apoptosis or necrosis of tumor cells, activate the immune system, and produce an immunological response.[55] Therefore, the MG1655-M + Laser produced a strong antitumor immune response. In contrast, tumor-related antigens and bacterial fragments play a role in the tumor microenvironment and activate immune responses. In addition, it promotes the maturation of dendritic cells (DCs) in tumors and the spleen, polarization of macrophages, and an increase in CD4+ and CD8+ T cells. It significantly increases the TCM content in tumors, thereby effectively enhancing its killing effects against distant tumors and metastases.
2.6 In Vivo Experiment of MG1655-M Mediated Orthotopic Tumor and Anti-Metastasis Treatment
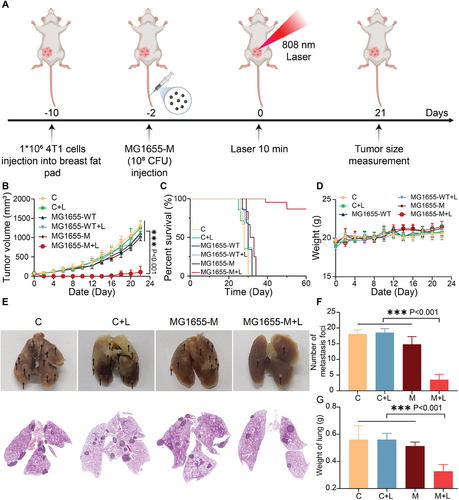
In subcutaneous tumor models, only a few lung metastases were observed in mice treated with the MG1655-M + Laser, indicating that distant metastasis could be effectively inhibited. To further evaluate the antimetastatic effects of the MG1655-M + laser, 4T1 cells were injected into the breast fat pads of mice to construct an orthotopic breast tumor model (Figure 7A). Owing to the high migration and invasion of 4T1 orthotopic breast tumors, MG1655-WT and MG1655-M cells exhibited negligible tumor growth inhibition. In contrast, the MG1655-M + Laser presented greater inhibition of tumor growth than the other groups (Figure 7B; Figure S18, Supporting Information). The two primary tumors were abolished in the MG1655-M + Laser group after treatment. Notably, combination therapy with MG1655-M+laser resulted in 60% animal survival at 60 days, which was significantly longer than that of the other groups (Figure 7C). No obvious differences were observed in the body weights of mice from all groups (Figure 7D). Furthermore, treatment with the MG1655-M + Laser reduced the number of lung metastases and the lung weight (Figure 7E–G). These results indicate that the MG1655-M+laser can effectively inhibit 4T1 tumor invasion and metastasis in vivo.
2.7 In Vivo Antitumor Immune Memory of MG1655-M
The MG1655-M + Laser effectively activated antitumor immunity. Therefore, experiments were designed to determine whether bacterial PTT of primary tumors could induce immune memory (Figure 8A). After 30 d of bacteria-mediated PTT, secondary 4T1 tumors were inoculated into mice in the control and challenge groups. The volume of secondary tumors and body weight of the mice were monitored. Tumors grew rapidly in mice that did not receive the therapy. Significantly slower tumor growth was observed in mice in which the first tumor was eliminated using bacteria-based PTT. Two mice did not exhibit any unexpected secondary tumor growth (Figure 8B; Figure S19, Supporting Information). All mice in the challenge group survived for more than 40 days after tumor recurrence (Figure 8C). In summary, bacteria-mediated PTT produced a strong immunological memory effect that prevented the invasion of recurrent tumors. The mechanism of immune memory induced by bacterial-mediated PTT, memory T cells, and related cytokines, including TNF-α, IFN-γ, and IL-10 were explained through flow cytometry and enzyme-linked immunosorbent assay (ELISA). On the 40th day after bacterial PTT treatment, TCM cells were collected from the spleens of mice with 4T1 tumors. The flow cytometry data revealed that the fraction of TCM cells (35.27 ± 2.75% for CD3+CD4+CD44+CD62L+ and 39.13 ± 1.05% for CD3+CD8+CD44+CD62L+) is significantly higher in mice that had the primary tumor removed by bacteria-mediated PTT than that in the control group (6.6 ± 0.76% for CD3+CD4+CD44+CD62L+, 6.67 ± 1.40% for CD3+CD8+CD44+CD62L+) (Figure 8D,E; Figure S20, Supporting Information). In addition, the serum TNF-α, IFN-γ, and IL-10 in the MG1655-M + Laser group were considerably higher than those in the control group (Figure 8F–H). Therefore, bacteria-based PTT significantly activates the immune system. When 4T1 cells attacked mice again, MG1655-M acted as an immune stimulant to enhance the general antitumor immune response activated by tumor-related antigens after PTT tumor therapy, providing long-term immune memory effects that prevented tumor recurrence (Figure 8I).

Considering the importance of the biosafety of MG1655-M for future clinical applications, we injected 108 CFU of MG1655-M and PBS through the caudal vein, followed by blood biochemistry analysis, blood cell counts, and H&E staining of the primary organs (heart, liver, spleen, lungs, and kidneys). Blood biochemistry and cell counts revealed that almost all indices were within the normal range. Only mean platelet volume and alkaline phosphatase levels were slightly higher than those in the PBS control (Figure S21, Supporting Information). Furthermore, standard hematological markers, such as liver function, kidney function, and blood count, showed little difference between the MG1655-M and PBS groups. (Figure S22, Supporting Information). The hemolysis rate at different concentrations of MG1655-M was <0.5% (Figure S23, Supporting Information). No histopathological changes were observed in major organs, including the heart, liver, spleen, lungs, and kidneys (Figure S24, Supporting Information). These results indicate that MG1655-M can be used as a PTT agent for tumor ablation because of its high biosafety and biocompatibility, which is in accordance with the literature. Earlier research based on the same bacterial species (E. coli MG1655) suggested that this species is harmless and has no impact on homeostasis.[56, 57]
3 Conclusion
In this study, we developed a genetically engineered bacterium (MG1655-M) capable of biosynthesizing melanin nanoparticles. Owing to its tumor-targeting properties, MG1655-M successfully colonized the hypoxic region of the tumor and effectively delivered melanin nanoparticles into the tumor. Interestingly, the delivery process can be tracked using photoacoustic imaging, thereby enabling the determination of the optimal time to guide follow-up photothermal therapy. More importantly, the MG1655-M + Laser can be used for photothermal tumor ablation and for producing strong antitumor immunity, thereby providing long-term protection from tumor recurrence. This study provides a universal platform to achieve efficient tumor-targeting delivery of photothermal agents, imaging-guided photothermal tumor ablation, and long-term immune memory effects, thereby opening new avenues for antitumor therapy.
4 Experimental Section
Preparation of Bacteria
Plasmid encoding tyr1 was directly transformed into competent cells of E. coli MG1655 and grown for 12 h at 37 °C in 2 mL LB medium (0283254, HuanKai Microbial) added to 200 mg mL−1 ampicillin (the first cultivation). Genetically modified bacteria, designated MG1655-M, were obtained. Overnight cultures were grown in LB medium (50 mL) with 200 mg mL−1 ampicillin for 24 h at 37 °C after being diluted 1000 times. The second crop was continuously infected with 200 mg mL−1 CuSO4.5H2O and 8 mg L-tyrosine for 12 h. The culture liquid was centrifuged at 8000 rpm for 10 min was used to centrifuge the culture liquid. The precipitated bacteria were subjected to three rounds of aseptic PBS washings at 8000 rpm for 5 min each.
Characterization of MG1655-M
The size distribution of MG1655-M was estimated by dynamic light scattering (DLS) using a Zetasizer (Malvern, USA). A TEM with an acceleration voltage of 100 kV (JEM-1200EX) was used to evaluate the morphological characteristics of MG1655-M. UV–vis spectra were obtained using a BioTek Synergy H1. The morphological and morphometric characteristics of MG1655-M were studied using scanning electron microscopy (SEM; Hitachi S4800). Fourier-transform infrared (FT-IR) spectroscopy (BRUKER) was used to obtain the Fourier-transform infrared (FT-IR) spectra of MG 1655-M.
Analysis of the Photothermal Performance of MG1655-M In Vitro
The photothermal effect of MG1655-M was measured under different conditions using an 808 nm laser and an FLIR E4 infrared camera. The MG1655-M samples were prepared at concentrations 105–109 CFU mL−1 and exposed to a constant laser power (1.0 W cm−2) for 5 min at room temperature (25 °C). Furthermore, the MG1655-M samples were prepared at a fixed concentration of 108 CFU mL−1 and varied the laser power in the range from 0.75 to 1.5 W cm−2 for 5 min at room temperature. The thermal camera recorded the temperature changes every 30 s during irradiation. The photothermal stability of MG1655-M was evaluated by subjecting a sample of 108 CFU mL−1 MG1655-M to cyclic laser power (1.0 W cm−2) for 5 min, followed by a natural cooling period until the temperature reached its initial value. This process was repeated a total of five times.
Efficiency Calculation of Photothermal Conversion of MG1655-M
Cytotoxicity of MG1655-M
HeLa, MB49, 4T1, and 293T cells were sowed onto 96 cell culture plates with 104 cells per well for the cell viability assay, and the plates were then incubated overnight in a CO2 incubator. The MG1655-M were distributed in DMEM without serum. MG1655-M at different doses (109, 108, 107, 106, and 105 CFU mL−1) was cultured for 12 h. Tumor cells were incubated for 12 h before washing three times with PBS. Cell proliferation, the cell counting kit 8 (CCK8; Shanghai Yishan Biotechnology), as directed by the manufacturer. Three replicates were performed for each treatment group.
In Vitro PTT Assays
Four groups were created using HeLa, MB49, 4T1, and 293T cells, which were seeded at a density of 104 cells/well in 96-well cell culture plates: PBS, PBS+Laser, MG1655-M, and MG1655-M + Laser. Cells in the cells were grown in a serum-free medium. The MG1655-M cells were treated with MG1655-M at concentrations of 109, 108, 107, 106, or 105 CFU mL−1. For the PBS + Laser and MG1655-M + Laser groups, cells were grown in serum-free media and exposed to the laser (1.0 W cm−2, 5 min). The tumor cells were then washed three times with PBS, and the CCK-8 test was used to examine cell viability. Calcein-AM and PI staining were used to determine the cell viability (YEASHEN, Beijing, China). Annexin V-fluorescein isothiocyanate/propidium iodide kit (YEASHEN, Beijing, China)
Immunological Activity of MG1655-M
The immunological activity of MG1655-M was determined by macrophage activation. Briefly, 1 mL of DMEM containing 10% FBS was used to culture RAW 264.7 macrophages for 12 h after they were seeded in a 24-well cell culture plate (5 × 104 cells/well). After being heated to 70 °C for 30 min, MG1655-M cells (109–107 CFU mL−1) were appended to the macrophages and permitted to incubate for 48 h. To identify the macrophage phenotype, the treated RAW 264.7 macrophages were subjected to flow cytometry analysis after staining with the appropriate antibodies, which included CD86-PE, CD80-BV421, and CD206-FITC (Biolegend, Cat. No. 105007, 104725, 1417071:100 dilution ratio) according to the manufacturer's instructions.
Breast Cancer Model
The Vital River Laboratory Animal Technology Co. (Beijing, China) was used to generate BALB/c mice. This study involved the use of animals was supported by the Institutional Animal Care and Use Committee (IACUC) of the Animal Experiment Center of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (SIAT-IACUC-220606-HCS-YF-A2157). When the tumor load reached 2000 cm3, the tumor-bearing mice were euthanized. When this limit was reached beyond the final measuring day, the mice were sacrificed right away. Tumor volume was calculated as (tumor width × tumor length2)/2. The mice were raised in an SPF habitat with adequate food, clean water, and a 12 h light/dark cycle. A xenograft tumor model was established using 4–6 weeks old BALB/c female mice. Subcutaneous injection of 100 µL basal medium including 1 × 106 breast cancer cells was performed on the mice's right thigh. After inoculation, tumor growth was tracked every two weeks, and therapy was started until the tumor grew to a size of 100 mm3. Using a nose cone, a mixture of 1%–2% isoflurane and oxygen was used to anesthetize the mice during the experiments. Following the experiments, all mice were sacrificed by asphyxiation with carbon dioxide.
Tumor Targeting of MG1655-M
MG1655-M concentration was adjusted to 1 × 108 CFU mL−1. After adding 40 µL of ICG dye (1 mg mL−1) to MG1655-M solution, the admixture was incubated in a CO2 incubator for 120 min. The bacterial suspension was washed with PBS until a clear supernatant was obtained. Three mice with tumors were injected with bacteria (5 × 107 CFU) through the caudal vein. The dead MG1655-M and free ICG served as the control, the dead MG1655-M ICG was treated for 30 min at 70 °C. Mice were scanned using a PerkinElmer IVIS system at predetermined intervals to assess the biological distribution of bacteria and tumor accumulation. Tumor-bearing mice were sacrificed 48 h after injecting ICG-labeled MG1655-M intravenously, and the tumors, heart, lungs, liver, kidneys, and spleen were excised for ex vivo fluorescence imaging. Meanwhile, sections of organs and tumors were cut into 5 mm thick sections and stained with DAPI and FITC-labeled anti-HIF-1α antibodies. Following the injection of MG1655-M, the number of bacteria in these prime organs was measured (CFU g−1 of tissue) at various intervals of 1, 2, 7, and 14 days. Organs from the mice, such as the kidneys, liver, spleen, heart, lungs, and tumors, were weighed, collected, and homogenized in sterile PBS. The tissue-grinding solution was diluted 10–10 000 times with PBS. LB plates containing ampicillin were covered with 100 µL of tissue grinding solution. The colonies developed after incubating at 37 °C for 12 h.
In Vitro PA Imaging of MG1655-M
A PA imaging system (VisualSonics) was used to observe tumor-bearing mice injected with bacteria through the caudal vein over time (0, 6, 12, 24, 48, and 72 h). To calculate the average tumor PA signals, the core zones of the tumors were chosen as ROI.
In Vivo Tumor Treatment of MG1655-M
When the tumor volume reached ≈100 mm3, 4T1 tumor-bearing mice were randomly divided into six groups (n = 5): control, control + laser, MG1655-WT, MG1655-WT + laser, MG1655-M, and MG1655-M + laser. Tumor-bearing mice of the MG1655-WT and MG1655-WT + Laser groups received an intravenous injection of MG1655-WT (108 CFU) in 100 µL PBS. Tumor-bearing mice of the MG1655-M and MG1655-M + Laser groups received intravenous injections of MG1655-M (108 CFU) in 100 µL PBS. A 100 µL injection of PBS was given to the control group. Following a 48-h injection of bacteria, mice in three groups (control, MG1655-WT + Laser, and MG1655-M + Lase) were administered isoflurane anesthesia and exposed to an 808 nm laser (1.0 W cm−2) for 10 min following systemic delivery. An FTIR thermal camera was used to monitor and document the tumor temperature and infrared radiation thermal images. The tumor volume and mouse weight were measured every second day. Tumor volume was computed as follows: tumor volume = (tumor length × tumor width2)/2. The survival time of the mice was recorded. Tumor samples were taken for TUNEL assays and H&E staining after the mice died.
Release of ATP and HMGB1
The tumors were removed and sliced into pieces on the third day of therapy. Intratumor levels of HMGB1 were detected using enzyme-linked immunosorbent assay (ELISA) kits (Wuhan Saiyu Biotechnology Co.), following the manufacturer's instructions. Intratumor levels of ATP were quantified using an Enhanced ATP Assay Kit (Shanghai Beyotime Biotechnology Co.), and ATP-derived chemiluminescence was detected using a Multi-Mode Plate Reader (LJL BioSystems).
Intratumoral Immune Cell Analysis
Considering that the MG1655-M and MG1655-WT groups had similar bacterial components and the MG1655-WT+Laser group received the same laser irradiation as the MG1655-M+Laser group, the MG1655-M and MG1655-M+L groups were examined, and the tumors were eliminated and sliced into pieces on the seventh day following therapy. The tissue was then digested for 4 h at 37 °C using collagenase V (2 mg mL−1, YEASEN) in Hank's balanced salt solution (HBSS). After obtaining a single-cell suspension, the cells underwent filtration using a 70 µm cell strainer and were then washed using a fluorescent-activated cell sorting (FACS) buffer. As per instructions of the manufacturer, the single-cell suspension was pre-blocked with the following surface antibodies from Biolegend: anti-CD45-AF700 (Cat No. 103128, dilution ratio 1:100), anti-CD3-APC (Cat No. 100236, dilution ratio 1:100), anti-CD4-FUTC (Cat No. 100406, dilution ratio 1:100), anti-CD8-PC.7 (Cat No. 100721, dilution ratio 1:100), anti-F4/80-FITC (Cat No. 123108, dilution ratio 1:100), and anti-CD80-APC (Cat No. 104714, dilution ratio 1:100). FlowJo software was used to process the data. On days 1, 3, and 5 following therapy, the intratumor levels of IFN-γ, TNF-α, and IL-10 were detected using ELISA kits (Dakewe) following the instructions.
Immune Memory Assessment
Following the 40-day elimination of primary 4T1 tumors using the MG1655-M + Laser, these mice were subcutaneously injected with another batch of 4T1 cells (1 × 106 CFU mL−1). The tumor size was then recorded. Spleens from the mice in various groups were stained with anti-CD3-APC, anti-CD4-FITC, and anti-CD8-PC.7 (Biolegend, Cat. No. 100236, 100406, 100721, dilution ratio 1:100), CD44-PE and CD62L-PC.5.5 (Biolegend, Cat. No. 103024, 104432, dilution ratio 1:50), according to the manufacturer's instructions. To analyze the fraction of memory T cells, the percentage of TCM cells was examined using flow cytometry.
Blood Biochemistry and Histological Analysis
Blood samples were collected for hematological analysis after 14 days to evaluate the in vivo toxicity of MG1655-M-based PTT. hematological indices, renal function, and liver function were measured. All histopathological analyses were performed in compliance with the accepted laboratory practices. The main organs (heart, liver, spleen, lungs, and kidneys) and tumors were removed and preserved in 4% paraformaldehyde for histological examination using H&E staining.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8.01 software. Data are displayed as mean ± SD. Statistical analyses were conducted using a one-way analysis of variance (ANOVA) with Tukey's post-hoc test and Student's t-test. *p < 0.05, **p < 0.01, ***p < 0.001 were considered statistically significant in analyses.
Acknowledgements
W.S. and Y.H. contributed equally to this work. The National Key Research and Development Program of China (2020YFA0908800), the National Natural Science Foundation of China (32171365, 82071929, 82202158), the Guangdong Innovation Platform of Translational Research for Cerebrovascular Diseases, and the Shenzhen Medical Research Fund (Grant No.2301012) are all gratefully acknowledged by the authors for their support of this research. The authors thank http://www.home-for-researchers.com for creating the image.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




