Highly Fluorinated Peptide Probes with Enhanced In Vivo Stability for 19F-MRI
Abstract
A labeling strategy for in vivo 19F-MRI (magnetic resonance imaging) based on highly fluorinated, short hydrophilic peptide probes, is developed. As dual-purpose probes, they are functionalized further by a fluorophore and an alkyne moiety for bioconjugation. High fluorination is achieved by three perfluoro-tert-butyl groups, introduced into asparagine analogues by chemically stable amide bond linkages. d-amino acids and β-alanine in the sequences endow the peptide probes with low cytotoxicity and high serum stability. This design also yielded unstructured peptides, rendering all 27 19F substitutions chemically equivalent, giving rise to a single 19F-NMR resonance with <10 Hz linewidth. The resulting performance in 19F-MRI is demonstrated for six different peptide probes. Using fluorescence microscopy, these probes are found to exhibit high stability and long circulation times in living zebrafish embryos. Furthermore, the probes can be conjugated to bovine serum albumin with only amoderate increase in 19F-NMR linewidth to ≈30 Hz. Overall, these peptide probes are hence suitable for in vivo 19F-MRI applications.
1 Introduction
In vivo imaging of fluorine-containing compounds is of high medical relevance as nearly 30% of the newly approved drugs contain at least one fluorine,[1] half of which carry a CF3 group.[2, 3] Fluorine is often selectively incorporated into the structure of a candidate small-molecule therapeutic to modulate the physicochemical properties and improve metabolic stability.[4, 5] Due to its high gyromagnetic ratio and absence of natural background signal, 19F has been used as a highly sensitive label for NMR spectroscopy,[6, 7] for example, to study self-assembly of peptides on lipid membranes,[8] dynamics of receptors,[9] and tracking of drug molecules in cells.[10] Furthermore, the favorable properties of 19F have been exploited in magnetic resonance imaging (MRI) already in the 1970s.[11] Unlike the abundant protons (1H), 19F allows selective labeling and, therefore, high contrast in imaging. However, single, double, or even triple 19F probes as used in 19F-NMR spectroscopy are insufficient in MRI, and a higher local 19F concentration is required to overcome the low sensitivity. To resolve this restriction, the most straightforward strategy is to increase the amount of fluorine in a single molecule. Consequently, perfluorocarbons were applied in the first 19F-MRI experiments,[11] and to date, are used in many applications,[12] such as angiogenesis imaging,[13] visualization of inflammation,[14] immune cell cancer therapy,[15] hepatic uptake,[16] activatable targeting,[17, 18] and theranostics.[19, 20] However, because of their extremely high hydrophobicity, the use of perfluorocarbons relies on the construction of nanoparticles with hydrophilic shells in order to achieve biocompatibility, which may cause retention and stability problems in the body.[21] Another approach has employed liposomal formulations of hydrophilic fluorine-rich organic compounds to overcome the hydrophobicity.[22] As an alternative with tunable hydrophilicity, peptide-based 19F-MRI probes have been suggested. Kirberger et al., for example, have reported peptide probes composed of alternating trifluoro-acetylated l-lysines (TFA-lysine) and d-lysines to endow the peptide with stability against proteolysis.[23] Nonetheless, stability beyond ≈24 h has still been an issue due to slow hydrolysis of the trifluoroacetamide bond at physiological pH. In their study the authors chose the amino acid sequence to obtain an unfolded peptide in aqueous environment, which lead to an identical chemical shift of all 19F-labels. Avoiding secondary structure and hence chemical shift dispersion assured a high intensity in 19F-NMR and therefore increased the detectability in 19F-MRI.
Our study intended to develop hydrolytically and proteolytically stable peptide probes and increase the number of 19F even further, as signal intensity is the major challenge in 19F-MRI. However, a mere extension of the peptide length bears the risk that the peptide will eventually adopt a secondary structure, leading to a spread in local environments and hence 19F chemical shift. Equally, a larger size of the peptide probe can influence the biological activity of any labeled biomolecule. In order to realize a short peptide probe with nonetheless maximized 19F signal while keeping its size compact, we used perfluoro-tert-butyl groups as functional units to label several amino acids in a peptide. Each moiety possesses nine fluorine atoms, thus yielding a threefold higher signal as compared to commonly used CF3 labels. As in the case of CF3, all 19F atoms in a perfluoro-tert-butyl group are chemically equivalent due to their rapid axial rotation on the NMR-time scale,[24] contributing additionally to an intense signal in 19F-MRI. Furthermore, the additional hydrophobicity introduced by the high fluorination was compensated by the incorporation of polar amino acids in the peptides, as well as by endowing the fluorinated amino acid with multiple polar groups. A similar approach has been followed by Taylor et al., who used polymers containing nonafluorooxy-ethylacrylate and poly(ethylene glycol) acrylate units to tune the hydrophilicity.[25] Compared to partially fluorinated polymers, using peptide-based 19F-MRI probes offers better control on the synthesis, leading to a platform that allows more precise tuning of spectroscopic properties and generating well-defined products with higher biocompatibility.
In this paper, we describe six fluorinated peptide probes employing perfluoro-tert-butyl groups as 19F labels, which boost the magnetic resonance signal of 19F by massive fluorination. The hydrophobicity associated with fluorination is overcome by introducing multiple cationic or anionic residues, and proteolytic degradation of the peptides in vivo is decreased by including d-amino acids and β-alanine in the sequence. Currently, only a few perfluoro-tert-butyl amino acids have been published, such as serine, homoserine, tyrosine, and hydroxyproline analogues synthesized through the functionalization of an OH group.[24, 26-28] For the first time, here we introduce a perfluoro-tert-butyl moiety into aspartic acid to form an asparagine analogue (AsnF9) with nine magnetically equivalent fluorine atoms. In this amino acid, the fluorinated moiety is connected via a sufficiently long linker, not only to reduce susceptibility to racemization (which the electronegative fluorine nucleus may cause), but also to distance the bulky C4F9 group from the carboxylate to maintain a high reactivity during peptide synthesis. In addition, we aimed for favorable relaxation properties due to the high mobility of the C4F9 group at the end of the elongated side chain.
We connected a nonafluoro-tert-butyl precursor to the aspartic acid side chain via an amide bond. For this, we chose ethanolamine as a linker between the amino acid fragment and the fluorine-rich moiety. The resulting sterically hindered amide bond is unlikely to be hydrolyzed or enzymatically cleaved under physiological conditions. The final perfluoro-tert-butyl containing α-amino acid derivative 6 (AsnF9) was obtained in only four steps from commercially available starting materials using standard chemistry protocols (Figure 1, Scheme S1 and Figures S1–S9, Supporting Information). Starting from N-Boc (tert-butoxycarbonyl) ethanolamine 1, precursor 3 was obtained via previously described Mitsunobu reaction[29] with nonafluoro-tert-butanol and subsequent Boc-deprotection of the intermediate 2. 1-tert-butyl-ester N-Fmoc (fluorenylmethoxycarbonyl) protected aspartic acid 4 was coupled to precursor 3 using amide coupling reagent PyAOP ((7-azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate) in the presence of base to obtain intermediate 5. Final trifluoroacetic acid (TFA)-mediated cleavage of the tert-butyl protection gave product 6 (N-Fmoc-AsnF9) with an overall yield of 28%, suitable for solid-phase peptide synthesis.
Ideally, our goal is to obtain a single narrow 19F-NMR resonance, which can be achieved if all CF3 groups are in an essentially identical chemical environment. To this aim, our design of the peptides avoids any secondary structure and places all 19F labels in identical repeat units. The resulting four peptide probes (P1–P4) consist of three identical repeat units—each of which carries a fluorinated AsnF9 and a charged amino acid—plus two further amino acids at the N-terminus for fluorescent labeling and bioconjugation (Table 1 and structures in Figure S10, Supporting Information). In addition, to investigate the influence of the peptide length on the 19F-NMR signal, P5 and P6 have been synthesized as short probes with only a single repeat unit.
| Peptide | Sequencea) |
|---|---|
| P1 | Sar-Pra-(Bal-glu-AsnF9)3-Gly-OH |
| P2 | Sar-Pra-(glu-AsnF9)3-Gly-OH |
| P3 | Sar-Pra-(Bal-lys-AsnF9)3-Gly-OH |
| P4 | Sar-Pra-(lys-AsnF9)3-Gly-OH |
| P5 | Sar-Pra-glu-AsnF9-Gly-OH |
| P6 | Sar-Pra-lys-AsnF9-Gly-OH |
- a) Sar = sarcosine, Pra = l-propargylglycine, Bal = ß-alanine, glu = d-glutamic acid, lys = d-lysine, AsnF9 = perfluoro-tert-butyl-modified l-asparagine.
The amino acids in the repeat unit were selected considering the following peptide properties allowing an optimized use for in vivo 19F-MRI: 1) high solubility, 2) prevention of secondary structure, 3) proteolytic stability, 4) tag for bioconjugation, and 5) fluorescence label.
1) High water-solubility was achieved by including d-lysine or d-glutamic acid as cationic or anionic amino acids. 2) A mixture of d- and l-amino acid stereoisomers was chosen to prevent the formation of α-helical secondary structures. This way, we envisaged to avoid peptide folding, and therefore prevent different 19F-NMR chemical shifts and hence multiple NMR signals. In addition, β-alanine was included in the repeat unit of P1 and P3 to evaluate a possible improvement of the backbone flexibility of the probes.[30] 3) Importantly, d-amino acids also offer a stronger resistance against proteolytic degradation, as many enzymes only recognize the naturally abundant l-isomers. 4) Furthermore, we introduced a propargylglycine with an alkyne function, in order to allow coupling of other molecules to the peptide via “click” reaction on the N-terminal side of the peptides. 5) To monitor the peptide distribution in zebrafish embryos using fluorescence microscopy, we attached rhodamine B to the N-terminus of aliquots of all peptides in a second series of fluorescent products Rho-P1 to Rho-P6 (Figures S13–S15, Supporting Information). In order to ensure the functionality of the dye in its active open form, a sarcosine was introduced at the N-terminus to then be adjacent to the fluorophore.[31] To simplify the synthesis, an additional glycine was added at the C-terminus.
2 Results and Discussion
The peptides were synthesized manually on solid support following the standard Fmoc protocol. The identity and purity of the peptide probes were confirmed by mass spectrometry (matrix-assisted laser-induced desorption/ionization, MALDI-MS) and reverse-phase high-performance liquid chromatography (RP-HPLC) (see Table S1 and Figures S11–S12, Supporting Information). We observed a polarity decreasing in the order of P6 > P5 > P3 > P4 > P1 > P2, as can be judged from increasing retention times when eluted by a linear gradient of acetonitrile/water in RP-HPLC (Figure S12, Supporting Information). These results illustrate a decrease in hydrophilicity with increasing number of fluorine atoms per peptide length. Notably, β-alanine increased the hydrophilicity of the peptide probes, whereas d-glutamic acid had a smaller contribution to the improvement of the polarity than d-lysine. As the fluorinated peptide probes have different lipophilicity and charge, they are expected to show different biodistribution and pharmacokinetics in vivo.
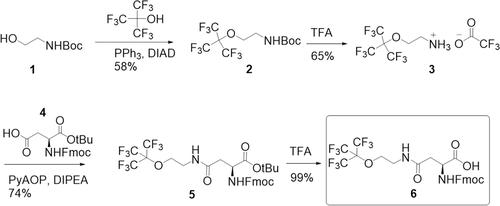
19F-NMR spectroscopy was applied to characterize the probes in terms of how well they will be suited for 19F-MRI. Encouragingly, when dissolved in PBS (phosphate buffer saline, pH 7.4) at a 19F concentration of 5.4 mm, a single resonance or splitting into a set of very close signals (P3) at around −69.3 ppm with a very narrow linewidth of ≈8 Hz was observed for all peptide probes, demonstrating that all fluorine nuclei are in a similar chemical environment (Figure 2A, Supporting Information). Furthermore, all peptides, except for P1, were soluble in methanol, where P5 and P6 exhibited single sharp peaks at around −70.7 ppm with the same narrow linewidth as in PBS. The peak splitting of P2–P4 with a slight increase in the chemical shift span in methanol indicates that the 19F labeled side chains are in slightly different environments, possibly due to formation of some local secondary structure or aggregation in the organic solvent. By comparison, the rhodamine-labeled peptides (Rho-peptides) showed a broadening of the 19F-NMR peak in PBS, demonstrating a higher tendency to fold or aggregate (Figure S16, Supporting Information). Their larger linewidth or chemical shift dispersion is presumably related to the more hydrophobic character of the rhodamine-labeled constructs, which is also visible in the longer retention times in RP-HPLC and significantly decreased T2 (Figure S15 and Table S2, Supporting Information).
At a 19F concentration of 5.4 mm, the 19F linewidth of the peptides is found to increase as P1 ≈ P3 < P2 ≈ P4 < P5 ≈ P6, implying that β-alanine leads to a reduction of the effective 19F-NMR linewidth and an increase in signal intensity (Figure 2A). On the other hand, the 19F signal obtained for the shorter peptides P5 and P6, measured at a concentration of 0.6 mm, which corresponds to the same fluorine concentration as for P1–P4, interestingly exhibited slightly broader linewidth and lower intensity than the longer peptides, potentially due to the higher net hydrophobicity of the shorter peptides. In in vivo applications the linewidths may be broadened further due to intermolecular contacts with molecular constituents of the tissue. However, our label design avoids such additional line broadening as we included charged residues in the peptide sequence. As has been reported previously,[23] labels equipped with a high degree of polarity lead to linewidths compatible with 19F MRI also in biological tissue. Hence, using charged residues, as in our case, should avoid intermolecular interactions and line broadening even more. Moreover, our modular design with positively or negatively charged residues provides a range of options allowing to optimize the peptide label with respect of the linewidth.
In addition, we measured T1 and T2 19F-NMR relaxation times to assess the performance of the peptides in 19F-MRI, where a short T1 and long T2 value would be desirable. T1 was found to be in the range of 1.0–1.3 s, which compares well with T1 of other compounds labeled with trifluoromethyl groups.[22] T2 values in the range of 200–300 ms were determined, which are longer than typical values for fluorine-labeled molecules of the size of the peptides and hence favorable for 19F-MRI (Figure 2B). We note at this point, that T2 relaxation is not the only factor contributing to the linewidths (Figure 2A), as apparent from the linewidths exceeding the relaxation-related linewidth estimate of 1/(π T2).
We compared the performance of the six peptides as 19F-MRI labels using a microcoil imaging setup operating at 11.7 T.[32] Peptides were dissolved in 6 mm aqueous CuSO4 to enhance T1 relaxation and decrease the recycle delay mainly to facilitate the setup procedure and in the development process. (We note here, that the usage of shorter flip angles in the FLASH imaging sequence reduces the necessity of signal recovery by T1 relaxation.[33]) The peptide concentrations, 25 mm in the case of P1–P4 and 75 mm in the case of P5–P6, were adjusted to achieve a fluorine concentration of 675 mm, and NaOH was added in the case of the anionic peptides P1 and P2 to improve their solubility. (Figure 2C). For the images, a volume of 7 mm × 7 mm × 5 mm divided into 32 × 32 × 20 voxels was scanned with 10 240 repetitions in 30 min. All peptides exhibited a similar imaging performance. Only for P3 a slight reduction in intensity was observed, which may be related to the signal dispersion already seen in the 19F-NMR spectra. Hence, we have demonstrated here the use of our peptide-based probes in in vitro 19F-MRI on a submillimeter-scale, which offers the prospect of using 19F-MRI to image tissue and organs or small-sized animal models.
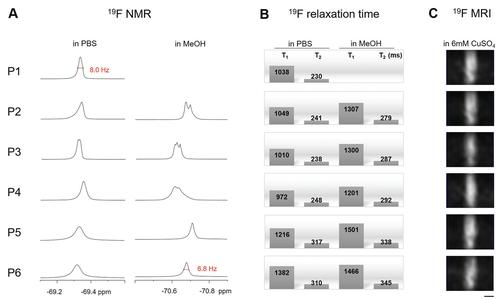
As sufficient sensitivity is expected to be one of the key factors for the envisaged 19F-MRI in vivo applications, we estimated the limit of detection from the signal/noise ratio obtained at different peptide concentrations (Figure 3). We used solution NMR to avoid that a potentially lower sensitivity of the microcoil setup used for our 19F MRI would not lead to representative signal/noise values, and considered the small voxel volume observed in MRI and different signal recovery requirements inherent in the used FLASH imaging sequence in the analysis. For an order of magnitude estimate, it should be sufficient to assume that the signal scales proportionally with the sample volume, neglecting potential hardware related differences in performance. In this case we can then relate the signal obtained from a 5 mm tube using NMR spectroscopy to a generalized MRI setting which in general has a different size. As a typical 19F-MRI in vivo application, we assumed the acquisition of a 2D image of a slice, as, for example, acquired in 19F-MRI in vivo experiments using nanoparticle label on mice or zebrafish.[16, 34] To relate the signal to noise values obtained by solution NMR of a 5 mm tube sample, we considered a 64 × 64 pixel image matrix of a slice covering 1/10 of the sample, and a FLASH sequence with a 20° flip angle (yielding 34% signal) and a typical repetition time of 10 ms.[33] (We note at this point that due to the small flip angle the repetition time of the FLASH sequence does not depend much on the T1 relaxation time.) Taking an MRI experiment of a mouse with a field of view of 3 cm × 3 cm × 6 cm as an example, these settings would correspond to a slice thickness along the long axis of 6 mm, and a resolution of ≈470 µm × 470 µm, yielding a voxel volume of 1.3 µL. Assuming a signal/noise ratio of 5 as minimum for a detectable signal, and that the MRI signal is lower by a factor of ≈1/2000 (=1/10 × 1/64 × 0.34), a signal/noise ratio of ≈10 000 would be required in a 1D NMR spectrum of ≈1000 repetitions to correspond to the detection of an MRI 2D image within 10 min (the duration of ≈1000 repetitions of 64 spatial increments). For the longer peptides P1–P4, this limiting signal/noise ratio is reached with peptide concentrations of ≈2 mm (Figure 3). In biological tissue the signal may be reduced due to further line broadening. However, as our estimate was derived at a rather low magnetic field of 7 T (300 MHz) and many current MRI applications are performed at higher fields, the estimate of ≈1 mm peptide concentration should be a realistic figure.
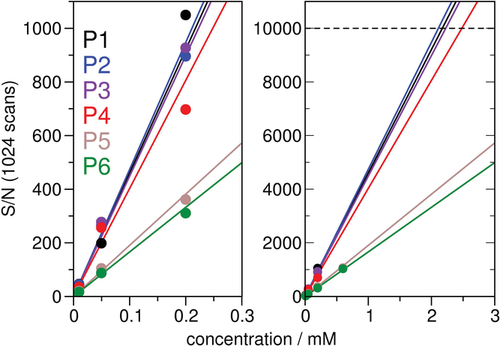
Chemical and biological stability, as well as absence of toxicity are essential for in vivo MRI imaging applications, which was evaluated for all peptide-based labels. All peptides were found to be stable in PBS for more than one week at room temperature (Figure S17, Supporting Information). We further investigated the behavior of peptides in bovine blood serum and plasma, and observed over 60% intact peptides after 7 days incubation (Figure 4A and Figures S18–S20, Supporting Information). Hence, the long-term stability renders the peptides promising candidates for in vivo applications or even long-term tracking of drugs. For example, biomolecules such as antibodies tend to require an extended time to accumulate in the target tissue or organ.[35] We note at this point that the rhodamine-labeled peptides were found to degrade or co-precipitate with serum protein more rapidly (Figures S21–S23, Supporting Information), which might be related to their higher hydrophobicity. Rhodamine labeling (used here for dual-purpose fluorescent monitoring, see below) may thus alter the propensity for degradation or precipitation processes, which would however not impede any application of the peptides in 19F-MRI where rhodamine labeling is obviously not needed.
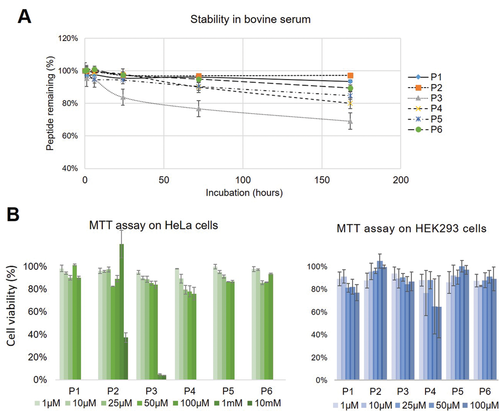
To evaluate a potential toxicity in light of in vivo applications, we conducted MTT assays on HeLa and HEK293 cells for all six peptides. A viability of more than 75% in the case of HeLa cells after 24 h incubation with up to 0.1 mm of the peptides (Figure 4B) was observed. For P2, an extension of the test series to 10 mm showed a viability of 100% at 1 mm peptide concentration, which was reduced to ≈40% at 10 mm. In the case of HEK293 cells, except for P4, all peptides showed an MTT-assay signal corresponding to a cell viability of more than 80%. The MTT assay may only represent one aspect of toxicity, and give only a rough indication of limiting toxic concentrations, as it probes the influence on particular cell cultures, but not on an organism level. However, the concentrations where the peptide was still found to be non-toxic and minimum concentrations needed for 19F MRI detection are within the same ballpark of roughly in the ≈1 mm range. Furthermore, the modular design allows to build peptides with different properties, such as positive or negative charge, and this way provides means to optimize for low toxicity in the desired application. Thus, the seemingly low cytotoxicity and versatile building concept of these peptides provides a good basis for in vivo 19F-MRI at the cellular level.
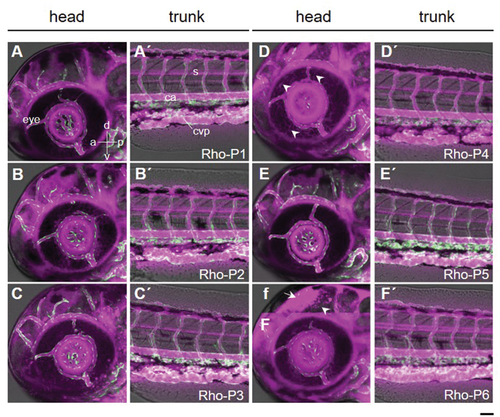
In order to assess the potential of the peptide probes for in vivo 19F-MRI, we applied the rhodamine-labeled peptides (Rho-P1 to Rho-P6) to zebrafish embryos and followed their distribution and clearance using fluorescence microscopy. A 1 mg ml−1 Rho-peptide solution was intravenously injected into 3 day-post-fertilization (dpf) zebrafish embryos. The rhodamine-labeled peptides were observed to circulate stably in the blood stream (Figure 5). The absence of accumulation in the caudal vein plexus, an embryonic tissue functionally equivalent to the fetal liver in mammals, indicates that the peptides were not recognized by macrophages and scavenger endothelial cells.[36] The Rho-peptides were also observed in the anterior chamber of the eye and optic tectum in the brain, suggesting their unique ability to cross the blood–aqueous and blood–brain barrier, respectively. Notable findings are the presence of fiber-like structures in the corneal epithelium (arrowheads in Figure 5D) with Rho-P4, and large amounts of Rho-P6 along the radial glia fiber (arrowhead in Figure 5f). Furthermore, the half-life of the rhodamine-labeled peptides in the zebrafish embryo was analyzed by fitting an exponential decay (with offset) model to the fluorescence intensity change over a period of 19 h. Among the peptides, Rho-P5 and Rho-P6 had the shortest half-life of 1.75 h and the longest of 30.55 h, respectively, while Rho-P1 to Rho-P4 possess similar half-life times between 7–15 h (Figure S24, Supporting Information). In terms of biodegradation, Rho-P6 and Rho-P4, having longer lifetimes in blood circulation, are the most promising candidates as dual probes to be used in correlative 19F-MRI and fluorescence imaging.
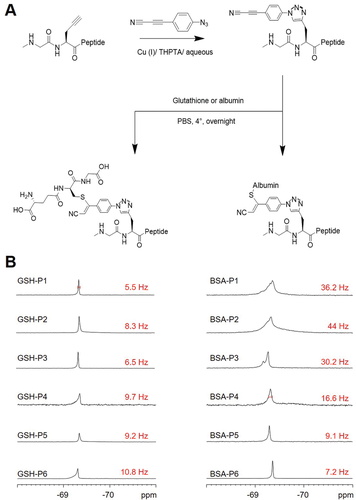
With a view to possible in vivo 19F-MRI applications involving molecular targeting, we explored the coupling of the peptides to small molecules and proteins. The peptide or protein part of such bioconjugates can be chosen to possess an affinity for specific targets in the organism or specific tissue regions, then allowing, for example, molecular tracking or visualization of uptake of different body parts.[16] For a proof-of-principle validation, two thiol-containing targets, glutathione (GSH) and bovine serum albumin (BSA), were labeled using a combination of thiol-alkyne addition and copper-mediated azide-alkyne “click” chemistry. (In an in vivo application, the coupling would be performed prior administration to the organism.) We built the bridge between the alkyne function of the fluorinated peptide and the conjugation partner with commercially available 3-(4-azidophenyl)propiolonitrile (APN-azide) as cross-linker, as it has been reported that APN-based modifications avoid side products and produce conjugates that are stable in the blood stream.[37] APN-azide was attached to the peptide probe using previously described conditions,[38] and subsequently purified via RP-HPLC (Figure 6A, Table S3, and Figure S25, Supporting Information). Afterward, BSA and GSH were conjugated to the peptides under mild reaction conditions (in PBS at 4 °C overnight) by coupling the thiols of the cysteine residues with the second functional end of the APN linker of the peptides. GSH-conjugates were readily purified using RP-HPLC, whereas the unreacted peptide was removed by dialysis in the case of the BSA-conjugates. After validation of the bioconjugates by MALDI-MS, 19F-NMR spectra and relaxation times were acquired (Figure 6B, Figures S26–S28, and Table S4, Supporting Information). Both conjugates gave rise to a single 19F-NMR signal in aqueous solution. While the chemical shift did not change significantly, the linewidth of the BSA-conjugate at half-height appears to broaden to various extents (Figure 6B, right). In the case of the GSH-conjugates, the linewidth was comparable to that of the free peptide (Figure 5B, left). Also, the relaxation times changed in the case of the BSA-conjugates, T1 was reduced by half, and T2 was found to be 5–20 fold shorter than that of the free peptides (Table S5, Supporting Information). Given the high molecular weight of the BSA-conjugates, these changes in relaxation behavior are in line with our predictions. Nevertheless, the 19F-NMR linewidth of 16.6 Hz still lies within the range of other probes that have been used successfully in 19F-MRI,[25] thus rendering also the new peptide conjugates as suitable labels for 19F-MRI. Our dual-purpose peptide-based fluorine probes thus offer a general modular approach to label proteins, in order to track them via fluorescence and 19F-MRI, either in parallel or subsequently. The APN-based cross-linker can be easily changed to functionalities other than thiols, if further types of functional groups are to be targeted.
3 Conclusion
In conclusion, we have designed, synthesized, and characterized highly fluorinated peptide probes for 19F-MRI. Introducing an asparagine analogue carrying a perfluoro-tert-butyl group as fluorine label into peptides, as well as d-stereoisomers of charged amino acids, we were able to design MRI probes endowed with high water solubility and stability against degradation. Due to our design of the peptide sequence—which avoids secondary structure formation and which is based on equal repeat units—a dispersion of 19F chemical shifts could be prevented and high intensity and small linewidth were achieved. Low cytotoxicity, high biostability, and tissue penetration have been demonstrated in cell assays and live zebrafish embryos, and stable bioconjugates could be achieved with BSA and GSH. Hence, optimized 19F-NMR signal and biocompatibility render the AsnF9 based peptide probes highly promising candidates for in vivo applications of 19F-MRI.
4 Experimental Section
Synthetic Procedures
Details of the synthesis and chemical analysis of the fluorine-labeled amino acid and the peptides are given in the Supporting Information.
19F-NMR and Relaxation Time Measurements
Liquid-state 19F-NMR spectra were obtained using a 300 MHz Bruker Avance spectrometer (Bruker Biospin, Ettlingen) with a multiple channel (1H/2H/19F/13C/15N) probe. Peptide samples were dissolved in methanol and phosphate buffered saline (PBS) buffer with pH 7.4. The 19F-NMR spectra were acquired using a single excitation pulse sequence of 15 µs and an acquisition time of 0.66 s. Around 1000 scans, separated by a recycle delay of 3 s, were averaged. The 19F-NMR spectra were referenced indirectly using the 1H-NMR signal of the solvent (methanol, set to 4.78 and 3.31 ppm, or H2O, set to 4.8 ppm) and processed using a line broadening of 1 Hz. All measurements were performed at room temperature (20 °C). The 19F-NMR spin-lattice relaxation time T1 was acquired using the inversion recovery method with delay times of 0.000001, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, and 10 s. Eleven spectra were obtained using a 90°-pulse of 15 µs and a recycle delay of 10 s. 19F-NMR spin–spin relaxation times T2 were acquired using a Hahn echo sequence with delay times of 0.00001, 0.001, 0.002, 0.005, 0.010, 0.020, 0.050, 0.1, 0.2, 0.5, 1, and 2 s. Twelve spectra were obtained using a 90°-pulse of 15 µs and a recycle delay of 5 s. Approximately 36–64 scans were accumulated in the T1 and T2 experiments. T1 and T2 relaxation times were analyzed using the relaxation fitting functions within the software Topspin 4.1.1 (Bruker).
MRI
19F-MRI images were obtained using a 500 MHz Bruker Avance III widebore spectrometer (Bruker Biospin, Ettlingen). Peptides were dissolved in 6 mm CuSO4 using a peptide concentration corresponding to a 19F concentration of 675 mm. 65 mm NaOH was added to the solutions of P1 and P2 to improve solubility. The peptide solutions were filled in one side sealed capillaries with inner diameter of 1.6 mm and outer diameter of 2.0 mm. The capillaries were then inserted into a 2.2 mm inner diameter glass tube surrounded by a custom-made dual-tuned 1H/19F micro saddle coil.[32] Experiments were carried out at 20 °C with an external water flow for cooling the gradient coils. 19F-MRI of the peptides was carried out using a FLASH sequence with adjusted parameters (echo time: 2.72 ms, repetition time: 5.76 ms, flip angle = 45°, image size: 32 × 32 pixels, field of view: 7 × 7 mm, resolution 0.219 × 0.219 mm, slice sickness: 1 mm). Images were obtained of coronal planes and averaged over 10 000 scans. Images were acquired using the ParaVision 6.0.1 software and post-processed using pixel binning (averaging shrink factor of 32) by Fiji/ImageJ.
Digestion Assay
The stability of the peptides was assessed using an assay based on digestion in bovine serum and bovine plasma. As a first step, commercial fetal bovine serum was temperature-equilibrated at 37 °C for 15 min. 0.6 mg of peptide in 100 µL of PBS buffer (pH 7.4) was added to the serum to a final concentration of 50 mm. At 0, 1, 6, 24, 72, and 168 h, 100 µL aliquots were taken and mixed with 500 µL MeOH for 20 min on ice. Serum proteins were precipitated and removed by centrifugation at 13 000 rpm for 15 min. The supernatant was immediately analyzed by analytical RP-HPLC using a linear gradient of solvent A/B ratios of 5:95—95:5 in 25 min (A: 97% H2O, 3% ACN, 0.1% TFA; B: 90% ACN, 10% H2O, 0.1% TFA), or stored in −80 °C freezer for further analysis. The measurements were carried out three times. The digestion assay in bovine plasma was performed using the same procedure.
Cytotoxicity Assay
A MTT assay on HeLa cells and HEK 293 cells was carried out to evaluate the toxicity of the peptides. Cells were plated in 96-well plates at a density of 15 000 cells per well and grown overnight. Before adding the peptide solutions, the previous cell media were discarded and incubated with DMEM pure for 30 min. The adherent cells were washed with PBS. The peptide solutions were then successively added into 96-well plates, and filled up with pure DMEM to the working volume of 200 µL. After the incubation of the plate at 37 °C for 24 h, 100 µL MTT solution was added to the washed cell and incubated for 2–4 h until the visibility of purple formazan. Afterward, MTT solutions were removed and cells were washed with PBS. 100 µL DMSO was added into each well to dissolve the purple crystals. The optical densities were measured at 570 nm and the value of a blank sample was subtracted. Assays were carried out three times.
Zebrafish Experiments
All zebrafish husbandry and experiments were performed in accordance with the German animal protection regulations. Danio rerio transgenic line Tg(kdrl:EGFP)s843Tg was used to visualize blood vessels. Embryos were maintained at 28 °C as previously described.[39]
Intravenous Peptide Injection
Zebrafish embryos at 3 dpf were anesthetized in 643 µM MS-222 (tricaine methanesulfonate) and embedded in 0.8% low melting-agarose. Rhodamine B labeled peptides were dissolved in PBS buffer (pH 7.4) at a concentration of 1 mg mL−1 (0.5 mm for P1–P4 and 1.3 mm for P5–P6, respectively). A few microliters of peptide solution were transferred into a pulled glass capillary and 10–20 nL was injected into the cardinal vein by a micro-injector (Eppendorf FemtoJet) at room temperature.
Fluorescence Imaging
Injected embryos were observed under a Leica TCS SP5 upright confocal microscope (Leica Microsystems) with excitation lasers at 488 and 561 nm for the detection of EGFP (498–528 nm) and rhodamine B (574–634 nm), respectively. Bright field transmission images were also acquired. A “HCX APO L U-V-I 20.0 × 0.50 WATER UV” objective lens was used. Time-lapse imaging in the head and trunk regions of the zebrafish embryos was performed using the resonant scanning mode of the microscope (bidirectional scanning at 8000 Hz) for 18 embryos injected with six different peptides, repeating the measurement three times for each peptide. The images were analyzed by Fiji/ImageJ.
Acknowledgements
The authors acknowledge the financial support by the strategy fund of KIT (division 1), by the DFG instrument grant 121384/58-1, by the GRK 2039 programme, by EU H2020-MSCA-RISE through the PELICO (grant 690973) and ALISE (grant 101007256) projects, and by BMBF through the VIP+ funding. The authors thank Markus Schmitt for technical support and Ronja Kammerichs for help in cell experiments. The MRI measurements were supported by the Karlsruhe Nano and Micro Facility (KNMFi), a Helmholtz Research Infrastructure at KIT. N.M., O.N., and J.G.K. acknowledge partial financial support of the Helmholtz Association through the programme “Materials Systems Engineering” and through the ERC Synergy Grant “HiSCORE” 951459, while S.L.G., S.A., M.T., and A.S.U. are part of the programme “Natural, Artificial, and Cognitive Information Processing.”
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




