From Precursor Chemistry to Gas Sensors: Plasma-Enhanced Atomic Layer Deposition Process Engineering for Zinc Oxide Layers from a Nonpyrophoric Zinc Precursor for Gas Barrier and Sensor Applications
Abstract
The identification of bis-3-(N,N-dimethylamino)propyl zinc ([Zn(DMP)2], BDMPZ) as a safe and potential alternative to the highly pyrophoric diethyl zinc (DEZ) as atomic layer deposition (ALD) precursor for ZnO thin films is reported. Owing to the intramolecular stabilization, BDMPZ is a thermally stable, volatile, nonpyrophoric solid compound, however, it possesses a high reactivity due to the presence of Zn-C and Zn-N bonds in this complex. Employing this precursor, a new oxygen plasma enhanced (PE)ALD process in the deposition temperature range of 60 and 160 °C is developed. The resulting ZnO thin films are uniform, smooth, stoichiometric, and highly transparent. The deposition on polyethylene terephthalate (PET) at 60 °C results in dense and compact ZnO layers for a thickness as low as 7.5 nm with encouraging oxygen transmission rates (OTR) compared to the bare PET substrates. As a representative application of the ZnO layers, the gas sensing properties are investigated. A high response toward NO2 is observed without cross-sensitivities against NH3 and CO. Thus, the new PEALD process employing BDMPZ has the potential to be a safe substitute to the commonly used DEZ processes.
1 Introduction
Zinc(II) oxide (ZnO) is a widely used thin film material in a range of thin film applications as it offers an outstanding set of functional properties.[1] With a direct allowed band gap of 3.37 eV it is an ultraviolet (UV)-emitting photoluminescent material that can be used in UV-light emitting diodes (LEDs).[2] Furthermore, it is electrically conductive and thus, can be used as channel material in thin film transistors (TFTs)[3-5] or as sensing layer in chemiresistors[6] for the detection of gaseous ethanol,[7, 8] CO,[9, 10] NO2,[11-13] H2,[14] H2S,[15] O2,[11] O3[16] or NH3.[17] This property, in combination with a high transparency in the visible light spectrum, renders ZnO to be a so called transparent conductive oxide (TCO)[18] and enables its usage as a buffer layer in solar cells.[19-21] At the same time crystalline ZnO has a high density of 5.61 g cm−3 [22] and can function as a gas barrier layer (GBL), thereby protecting moisture and air sensitive layers in a potential application.[23]
For many of the above-mentioned applications, pinhole-free, dense, uniform, and conformal thin films with a defined thickness over complex substrate geometries are needed, preferably in a broad range of deposition temperatures. Atomic layer deposition (ALD) can fulfill all the mentioned criteria by using sequential self-limiting surface reactions of a gaseous precursor and a coreactant with the solid surface, separated by inert gas purges, in order to deposit the desired material with atomic scale precision.[24-26] Since ALD is a chemical vapor phase technique, process conditions and thin film properties can be fine-tuned with the right precursor choice. Thus, precursors with different volatilities and reactivities toward different coreactants are of utmost importance.[27]
Although there is a large body of work published on the ALD of ZnO, there is only a limited number of ALD precursors reported. Starting from only inorganic precursors, Kopalko et al. used elemental zinc (Zn) in combination with oxygen (O2) at high deposition temperatures of 480 °C, but only observed island growth instead of closed films.[28] Switching to zinc(II) chloride ([ZnCl2]) with O2 or H2O as coreactant, compact films could be obtained at deposition temperatures between 450 and 550 °C.[28, 29] However, no composition of the layers was reported which is of relevance when halide based precursors can induce some impurities into the films as observed for aluminum oxide.[30] Going away from the halide based precursors, the metalorganic zinc precursor zinc acetate [Zn(CH3COO)2] in combination with water enabled the growth of ZnO at lower temperatures (280–360 °C).[28, 31-33] Recent efforts to lower the deposition temperatures was achieved using the bis[4-((2-ethoxyethyl)imino)-pent-2-en-2-olato]zinc(II) ([Zn(eeki)2]) using the β-ketoiminates class of precursors with H2O at temperatures as low as 175 °C.[34] However, further reduction in deposition temperature was possible till date by using the extremely reactive and pyrophoric zinc alkyls namely dimethyl zinc ([ZnMe2], DMZ) and diethyl zinc ([ZnEt2], DEZ) among which, DEZ is by far the most commonly used precursor.[35] Owing to its high reactivity, it is the most explored Zn precursor and has been used in combination with H2O,[36] O2,[37] O3,[38] or N2O[39] as coreactants. Furthermore, DEZ was used in plasma-enhanced (PE)ALD processes with oxygen or water plasma, especially for very low deposition temperatures down to room temperature.[40-47] ALD processes employing DEZ/water as precursors are predominantly described as “true” ALD processes in the individual publications, showing the typical ALD characteristics saturation, linearity and a defined ALD window. However, what is most striking from reported reviews is that the ALD window spreads over a broad range of possible ALD deposition temperatures (25–300 °C) and is, therefore, not as consistent as is often described.[35] Muneshwar et al. investigated this phenomenon and found DEZ to partly decompose, thereby adding a non-ALD component to the processes, for deposition temperatures above Tdep > 60 °C.[48]
Taking the instability of DEZ into consideration, there is basically no precursor available until now which facilitates only ALD-like growth in a deposition temperature range between 60–150 °C. This motivated us to choose and investigate a stabilized nonpyrophoric Zn precursor that can fill that gap. Recently we successfully demonstrated that intramolecular stabilized aluminum compounds can potentially substitute trimethyl aluminum (TMA), which is virtually the DEZ equivalent of aluminum precursors.[49, 50] This approach was adapted toward zinc compounds which is the focus of this study for ALD applications. Thus, bis-3-(N,N-dimethylamino)propyl zinc(II) ([Zn(DMP)2], BDMPZ) was synthesized by introducing the 3-(N,N-dimethylamino)propyl (DMP) ligand which stabilizes the Zn center via a dative bond of the amine,[51, 52] and thoroughly analyzed the compound in terms of its thermal properties. Encouraged by the promising physico-chemical properties of BDMPZ, a PEALD process with O2 plasma was developed for ZnO on Si(100) in the aimed temperature region. Following detailed characterization of the resulting ZnO thin films, they were applied in chemiresistors and tested for gas sensing of CO, NO2, and NH3. Herein, we report the appealing PEALD process for ZnO followed by the successful application of ZnO for gas barrier and sensing applications.
2 Results and Discussion
The design of ALD precursors has always been a challenge wherein one needs to find an optimal compromise between volatility, thermal stability and reactivity of the compound. In our study, the aim was to tune the properties of DEZ in terms of reducing the pyrophoric nature. The strategy employed was to increase the stability of the zinc compound, thereby reducing the reactivity to a certain extent where it becomes nonpyrophoric but at the same time not compromising much on the volatility of the compound within the targeted low temperature region below 100 °C. The approach of intramolecular stabilization turned out to be an efficient way to achieve these goals. While DEZ is only a 14 electron complex, rendering it to be electronically undersaturated, extremely reactive and chemically unstable, the substitution of the ethyl groups in DEZ with the 3-(N,N-dimethylamino)propyl (DMP) ligand results in an intramolecular stabilization via an electron donor effect of the DMP ligands amine group via a dative bond to the Zn center. This results in the chemically stable 18 electron complex [Zn(DMP)2] (BDMPZ).
2.1 Precursor Synthesis, Characterization, and Evaluation
The compound BDMPZ is synthesized, based on the procedure described earlier,[51] via a salt metathesis reaction between the Grignard reagent 3-(N,N-dimethylamino)propyl magnesium chloride with zinc chloride in THF and is schematically depicted as an inset in Figure 1. After the reaction went into completion, BDMPZ could be isolated after extraction in pentane, to remove the precipitated MgCl2. In the next step, the compound was sublimed at temperatures as low as 60 °C which is a first indication that the compound is volatile.
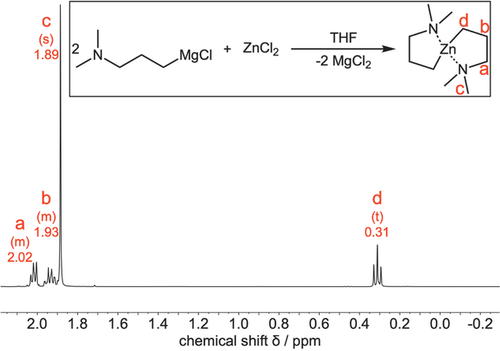
1H-nuclear magnetic resonance (NMR) spectroscopy performed on the sublimed BDMPZ in benzene-d6 resulted in a spectrum with assigned peaks as shown in Figure 1. The peak a at δ = 2.02 ppm with an integral of 4 protons is the most de-shielded peak and thus, can be assigned to the protons of the CH2-group adjacent to the nitrogen atom of the amine-group. The multiplet b at δ = 1.93 ppm with also 4 protons is induced by the CH2-group in the middle of the propyl chain, while the singlet c with 12 protons at δ = 1.89 ppm can be assigned to the protons of the methyl groups at the amine functionality. Because of the shielding effect of the metal center, the triplet d with 4 protons can be found upfield shifted at δ = 0.31 ppm and is caused by the CH2-group of the propyl chain bonded to the Zn center. Apart from a 13C-satellite signal of peak c at δ = 1.72 ppm, no other peaks are visible than the ones described. Thus, the spectrum shows only the expected peaks for BDMPZ which reveals the formation of the target compound with a high spectroscopic purity.
The identity of the compound was further validated by electron-impact mass spectrometry (EI-MS) as well. Selected m/z peaks are listed in Table 1 with a possible assigned fragment and the mass spectrum of BDMPZ is shown in the Supporting Information (Figure S1, Supporting Information). Most importantly, it is striking that the molecular peak can be detected at m/z = 236.2 with a low intensity of only 1.6% which is not surprising under the harsh EI-MS conditions. The other peaks can be clearly assigned to the cleavage of a DMP ligand at m/z = 150 (7.1%) from the compound and to the free standing DMP ligand itself at m/z = 86.1 (6.9%). The peak with the 100% intensity at m/z = 58.1 can be assigned to [Me2NCH2]+, which presumably occurs after the cleavage of one ethene molecule from the DMP ligand. It should be noted that peaks higher than the molecular peak cannot be found in the spectrum which is a clear indication that BDMPZ is monomeric in the gas phase.
| Fragment | m/z | Intensity [%] |
|---|---|---|
| M+ | 236.2 | 1.6 |
| M+ – DMP | 150.0 | 7.1 |
| DMP+ | 86.1 | 6.9 |
| DMP+ – C2H4 | 58.1 | 100.0 |
| Precursor characteristic | Value | |
|---|---|---|
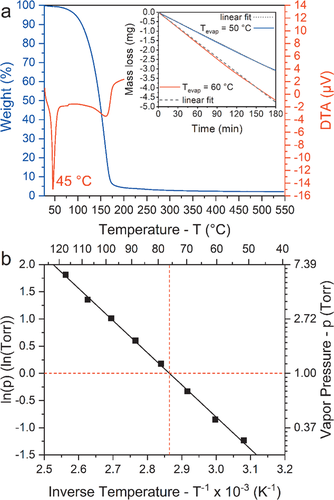
| Melting point Tmp [°C] | 45 | |
| Evaporation rate revap [µg min−1 cm−2] | Tevap = 50 °C88.46 | Tevap = 60 °C134.86 |
| Temperature of 1 Torr vapor pressure T1Torr [°C] | 76 | |
| Enthalpy of vaporization ΔHvap [kJ mol−1] | 49.2 | |
Summarizing the physico-chemical properties, it can be stated that the stabilization via a chelate complex was successful resulting in a volatile compound that is nonpyrophoric and is reactive to air. All these attributes make it appealing for ALD application. In addition, the high solubility in solvents such as pentane, hexane, toluene, and benzene is a promising feature for a usage as precursor for solution based thin film techniques. The good volatility and thermal stability of the compound also renders it highly suitable for chemical vapor deposition (CVD) techniques in a wide temperature range.
2.2 PEALD Process Development
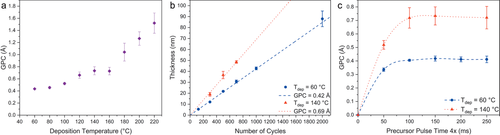
As one of the primary goals was to develop an ALD process employing BDMPZ within the temperature range between Tdep = 60–150 °C and encouraged by the physico-chemical properties of BDMPZ, we chose PEALD as the plasma enables low deposition temperatures. During the thin film depositions, the precursor temperature was maintained at Tevap = 55 °C and the optimized pulse/purge sequence is shown in Figure S2 in the Supporting Information. If not stated otherwise, for all depositions this sequence was used. The typical ALD-characteristics in terms of the deposition temperature dependency of the growth rate, thickness dependence as function versus the applied number of cycles as well as saturation of the precursor on Si(100) are shown in Figure 3a–c, respectively. First, the influence of the deposition temperature on the growth per cycle (GPC) was investigated and is depicted in Figure 3a. Three different growth regions can be identified: At deposition temperatures of Tdep ≤ 100 °C, a growth rate around 0.4 Å cycle−1 was found which increases to 0.7 Å cycle−1 in the second growth region between Tdep = 120–160 °C. At deposition temperatures of Tdep ≥ 180 °C, the growth rate is increasing drastically with increasing deposition temperatures, indicating precursor decomposition above 180 °C and thus, leading to a CVD-contribution. To investigate the growth in the first and second region, the linearity (Figure 3b) and the saturation (Figure 3c) criterion was tested exemplarily for the deposition temperatures Tdep = 60 °C and Tdep = 140 °C to confirm the ALD-like growth. As can be seen for both deposition temperatures, the thickness increases linearly with the number of applied cycles. The GPC was calculated with a linear fit through the data points and was found to be 0.42 and 0.69 Å at Tdep = 60 °C and Tdep = 140 °C, respectively. Furthermore, the precursor is undergoing a self-limiting reaction as proven by the saturation curve which shows a constant GPC for precursor pulse lengths longer than 4 × 100 ms with a 100 ms gap in between the precursor pulses at deposition temperatures of Tdep = 60 °C (blue curve) and Tdep = 140 °C (red curve). The influence of the plasma pulse time on the growth rate was investigated as well and is depicted in Figure S3 in the Supporting Information. As can be seen for all plasma pulse times ranging between tPlasma = 50–400 ms no significant difference in the growth rate could be observed. At this point it should be noted that the quality of the thin films is affected by the plasma pulse time as described later in the discussion of the thin film analysis part. However, for both growth regions the typical ALD characteristics could be shown. Hence, the process can be described to be a “true” ALD process in the temperature range of Tdep = 60–140 °C. Compared to other reported PEALD processes employing DEZ, this is certainly an advantage as many reports do not show a saturating behavior of DEZ and just state “true” ALD behavior.[42, 45-47] However, the growth rates of the DEZ/O2-plasma processes are higher, in the range between 1.5–2 Å cycle−1 for low deposition temperatures (below Tdep ≤ 100 °C)[40, 44, 46, 47] and 2–2.7 Å cycle−1 for higher deposition temperatures (above Tdep > 100 °C).[43-46] The increased growth rates for processes using DEZ can be accounted not only because of the very high reactivity of the precursor, but partly also due to the decomposition, which suggests CVD type contribution for processes at higher temperatures (Tdep > 60 °C). On the other hand, this is a convincing case study where a compromise made between reactivity and stability in BDMPZ results in lower growth rates which could have been influenced by its lower reactivity and by the higher stability.
2.3 Thin Film Analysis
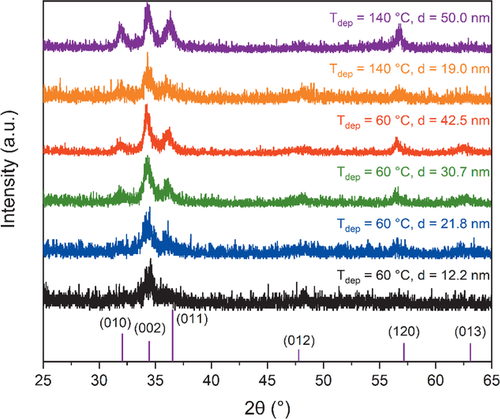
The film characteristics of the PEALD layers employing BDMPZ and O2-plasma, were analyzed in terms of crystallinity, topography and composition. From grazing incidence X-ray diffraction (GIXRD) experiments with an incident angle of ω = 1°, the crystallinity was investigated. As depicted in Figure 4 the zinc oxide films deposited at Tdep = 60 °C and Tdep = 140 °C are polycrystalline, with an increasing crystallinity for increasing film thicknesses. The reflexes from the zincite phase were detected with the strongest reflex appearing at 2θ = 34.5° which can be assigned to the (002) lattice plane. This is even seen for very thin films (d = 12.2 nm) deposited at Tdep = 60 °C, indicating a preferential orientation along the c-axis. Interestingly, there are some reports, observing a preferential orientation along the a-axis, corresponding to the (010) lattice plane, for low deposition temperatures (Tdep < 100 °C) which changes to a preferential orientation along the (002) lattice plane for higher temperatures (Tdep > 100 °C).[40, 43, 44] As this is not the case for the ZnO thin films grown from BDMPZ, this study shows that the precursor also has an influence on the crystallization. However, the orientation is dependent on a variety of parameters such as the deposition temperature, the used substrate or the process, thermal or plasma with different plasma parameters, making it difficult to assign only one parameter for a difference in the orientation.[35, 40] Interestingly, the crystallinity seems to be comparable for equivalently thick films independent of the deposition temperature, as shown for the d ≈ 20 nm thick films deposited at Tdep = 60 °C and Tdep = 140 °C which shows a high energy input into the film during growth from the O2-plasma. Apart from the (002) reflex, the (010), (011) and (120) reflexes at 2θ = 32°, 2θ = 36.5°, and 2θ = 57.2°, respectively, could be observed for films with a thickness above d ≥ 30 nm.
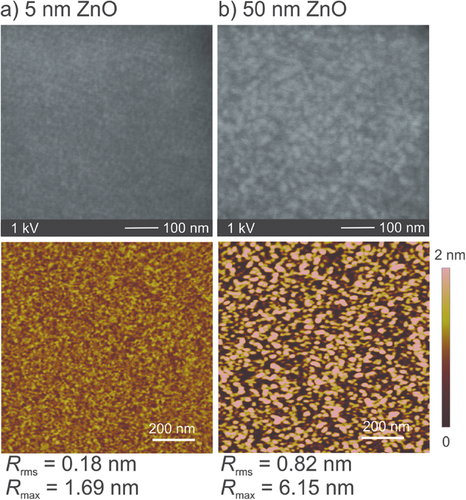
The topography of the films was investigated with scanning electron microscopy (SEM) and atomic force microscopy (AFM) for a very thin (d = 5 nm) and a thicker film (d = 50 nm) deposited at Tdep = 60 °C (Figure 5). For the 5 nm film, no conspicuous features can be seen which is confirmed by the topography from the AFM analysis where a smooth surface with a root-mean-square roughness (Rrms) of Rrms = 0.18 nm was measured with a maximum height of Rmax = 1.69 nm. These values are in the range of the underlying Si(100) substrate. In the SEM image of the ZnO film with a thickness of 50 nm, a structured surface can be seen which can be seen as hillocks distributed evenly over the whole surface. The roughness was calculated to be Rrms = 0.82 nm with a maximum height of Rmax = 6.15 nm, which is in accordance to a more structured surface. This feature can be attributed to the crystallites that evolve as a result of the increase in crystallinity for thicker films as discussed above in the XRD analysis. Similar analyses were performed for layers deposited at Tdep = 140 °C and the images are shown in Figure S4 in the Supporting Information. It is seen at higher deposition temperatures the surface is even more nanostructured with hillocks over the whole surface with some additional significantly pronounced structures with a maximum height of Rmax = 19.4 nm, which could be explained by an increased thickness at higher deposition temperatures. The RMS roughness was determined to be Rrms = 1.18 nm which is higher than for the samples deposited at Tdep = 60 °C, as expected taking the larger structures into account. All these values lie in the same range as reported for ZnO thin films deposited from DEZ by PEALD.[45]
The composition of the thin films deposited under different process conditions was determined using Rutherford backscattering spectrometry (RBS) in combination with nuclear reaction analysis (NRA) and the results are summarized in Table 3. Initially, the influence of the O2-plasma pulse time (tPlasma) was investigated for plasma pulse times between tPlasma = 50–300 ms. A clear trend of a decreasing carbon contamination and O/Zn ratio with an increasing plasma pulse time can be observed. Especially for short plasma pulse times of tPlasma = 50 ms, the reaction of the plasma species with the adsorbed surface species seems to be incomplete, resulting in a high carbon (14.2 at.%) and nitrogen (4.1 at.%) incorporation into the film. This is most likely due to the partly oxidized ligand fragments as indicated by the high oxygen to zinc ratio (zinc deficiency compared to stoichiometric ZnO) of O/Zn = 2.11. The difference from the plasma pulse times of tPlasma = 150 ms and tPlasma = 300 ms is not that significant with low carbon impurities of 1.7 at.% or undetectable carbon impurities, respectively. The O/Zn ratios indicate nearly stoichiometric ZnO thin films within the given overall error. This was confirmed for a variation in the film thickness between d = 5–100 nm, too. Here, it is noteworthy that besides low carbon and nitrogen contaminations of maximum 1.7 and 3.8 at.%, respectively, the O/Zn ratio is decreasing from O/Zn = 1.10 over O/Zn = 1.07 to O/Zn = 1.03 with higher thicknesses (5.0, 21.6, and 100 nm) which shows the influence of adventitious surface impurities, that have a smaller contribution in thicker films as RBS is measuring the composition over the whole film thickness. However, these very close values need to be considered with care as the estimated relative error value of ±2% limits the conclusion to see an indication of this trend. Interestingly, no clear trends could be observed for a variation in the deposition temperature as the maximum carbon impurity was found to be 1.7 at.% and the highest nitrogen contamination was 1.9 at.%. The O/Zn ratios are all in the same range between 1.02 and 1.06 which again confirms the high energy input and reactivity of the plasma for plasma pulse times of tPlasma ≥ 150 ms, enabling the deposition of stoichiometric ZnO thin films over the whole temperature range.
| Parameter varied | Tdep [°C] | tPlasma [ms] | D [nm] | Composition [at.%]a) | Ratiob) O/Zn | |||
|---|---|---|---|---|---|---|---|---|
| C | N | O | Zn | |||||
| Plasma Pulse Time | 606060 | 50150300 | 24.121.624.7 | 14.21.70.0 | 4.10.80.9 | 55.450.350.6 | 26.347.148.5 | 2.111.071.04 |
| Thickness | 6060 | 150150 | 100.05.0 | 0.20.0 | 0.53.8 | 50.450.3 | 49.045.9 | 1.031.10 |
| Deposition Temperature | 80100120140160180200220 | 150150150150150150150150 | 22.826.133.136.736.452.163.376.1 | 0.00.20.00.70.41.20.51.2 | 1.40.41.90.60.00.40.30.0 | 50.551.050.650.750.950.150.050.3 | 48.148.347.648.048.748.349.148.5 | 1.051.061.061.061.051.041.021.04 |
- a )For all concentration values an error of ±2 at.% can be considered
- b )The O/Zn ratios have a relative error of ±2% and an overall normalization error of ±4%.
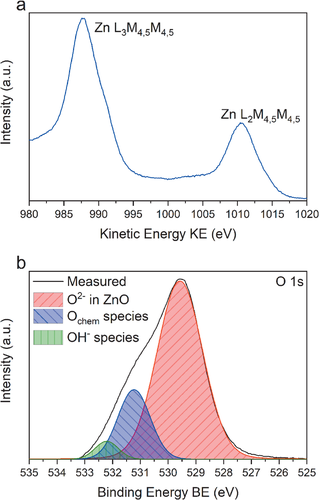
For application of ZnO in chemical sensors, the surface of the thin film plays a major role. Hence, synchrotron-based X-ray photoelectron spectroscopy (XPS) on a 50 nm thick sample deposited at Tdep = 60 °C was performed in order to obtain an insight into the binding situation on the surface of the ZnO layers with a very high precision as synchrotron X-rays naturally provide a much better resolution in comparison to the conventional laboratory based Al Kα or Mg Kα sources. From a first glance on the survey spectrum, only peaks originating from Zn, O, and C are seen as shown in Figure S5 in the Supporting Information. However, a minor contribution of the N 1s peak overlaying with the C KLL Auger peak around BE = 400 eV cannot be completely ruled out as evident from the data from the NRA measurements. As the surface was not cleaned via sputtering before the measurement, the peak in the C 1s core level spectrum at BE = 284.8 eV can be assigned to adventitious carbon and is shown in Figure S5 in the Supporting Information. Peaks for carbon-oxygen bonds like alcohols or carbonyls could not be detected and the broad peak at higher energies was assigned to a π bond shake-up satellite.[57] In the XPS spectra of ZnO samples, the differentiation between Zn2+ in fully oxidized ZnO and metallic Zn0 is challenging for the Zn 2p peak, because the peaks are only separated by ≈0.4 eV,[58, 59] rendering it unsuitable for a quantitative peak analysis. Thus, the high-resolution X-ray excited L3M4,5M4,5 Auger peak of zinc (Figure 6a) was used for that purpose. In photoelectron spectroscopy, the L3M4,5M4,5 Auger peak for metallic Zn should arise around KE = 992–995 eV and for oxidized zinc around KE = 987–989 eV, the difference of which is sufficient to quantify the degree of oxidation.[58, 60, 61] In this study only one peak (excited with an X-ray photon energy of 1150 eV) in the area of the L3M4,5M4,5 Auger peak of zinc was detected at a kinetic energy of KE = 988.3 eV. This value matches with the fully oxidized Zn2+ peak position from the already mentioned literature values for synchrotron XPS analysis of ZnO[58, 60, 61] while metallic Zn0 was not detected. The O1s core level peak was excited with an X-ray photon energy of BE = 660 eV and is shown in Figure 6b. The peak shows the contribution of three species positioned at BE = 529.1 eV, BE = 531.24 eV, and BE = 532.21 eV and could be assigned to the lattice O2− in ZnO, to chemisorbed oxygen species such as and to hydroxides, respectively.[62] Quantification of the oxygen peak shows that it consists of 75.74% lattice oxygen, 20.43% of chemisorbed oxygen species and 3.82% of hydroxides. Taking into account that only Zn2+ is detectable in the film, together with the presence of hydroxides and chemisorbed oxygen, the data is in good agreement with the RBS data, indicating a slightly oxygen rich O/Zn ratio of O/Zn = 1.02–1.06, even if XPS is giving a surface sensitive picture of the film composition.
2.4 Functional Properties
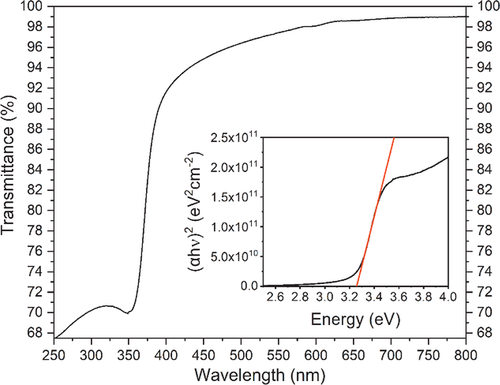
For potential application of the ZnO layers for example as TCO, the optical properties on quartz glass were investigated. As depicted in Figure 7, the 50 nm thick ZnO film deposited at 60 °C on quartz glass is highly transparent with a transmittance of ≥95% in the visible region between λ = 450–800 nm (average ≈98%). Above λ ≥ 580 nm, the transparency is even higher with transmittance ≥98%. These values are slightly better than for those reported for ZnO thin films deposited via PEALD at deposition temperatures below Tdep = 100 °C,[34, 41] confirming the high quality of our films. While the ZnO thin film has a high transparency in the visible spectrum, it shows a strong absorption band at λ = 350–380 nm which is known to be the characteristic band-to-band transition in ZnO.[63-65] From this absorption, the band gap was estimated to be Eg = 3.3 eV by calculating the term (αhν)2 for a direct allowed band gap in a Tauc-plot[66] (Figure 7, inset), which is in accordance to literature values for ZnO thin films.[2, 64, 65] Thus, the optical properties are indeed suitable for the application of these ZnO layers as TCO.
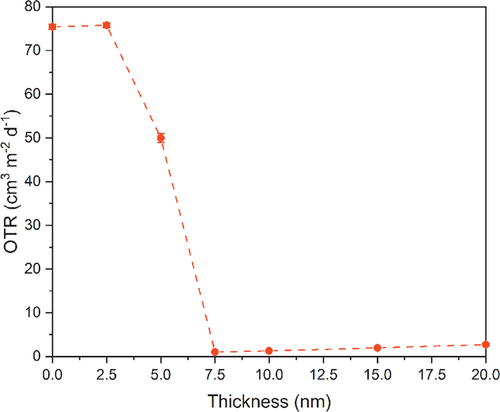
To demonstrate that the newly developed PEALD process for ZnO on Si can also be used for depositions on flexible substrates such as polymers, we deposited ZnO with different thicknesses on polyethylene terephthalate (PET) substrates and measured the oxygen transmission rate (OTR) (Figure 8). While a 2.5 nm thick film does not show any barrier performance with an OTR in the range of the bare PET (OTR = 75.4 cm3 m−2 d−1), indicating a discontinuous film, at a thickness of only 7.5 nm the films seem to be completely closed and show a high barrier performance with an OTR = 1.0 cm3 m−2 d−1 which is an improvement factor of 98.7%. Interestingly, this thin film already has the best OTR value and is slightly increasing for thicker films to an OTR = 2.7 cm3 m−2 d−1 for 20 nm thick films. One possible explanation for this trend might be an increased amount of oxygen diffusion paths due to the higher crystallinity of the thin films at higher thicknesses resulting in a more nanostructured surface. Another probable reasoning, might be the intrinsic film stress which for example changes from compressive stress, thereby closing possible defects, to tensile stress or a reduced compressive stress as already shown for SiO2 barrier layers of different thicknesses in our earlier studies.[67] Of course, for the ZnO layers obtained herein, this needs to be proven and is currently under investigation. However, the barrier performance is in the same range as the OTR of equivalently thick Al2O3 layers on PET, as shown earlier for the same polymer substrates,[49, 50] rendering the ZnO layers to be applicable as transparent gas barrier layers (GBLs).
2.5 Application in Gas Sensors
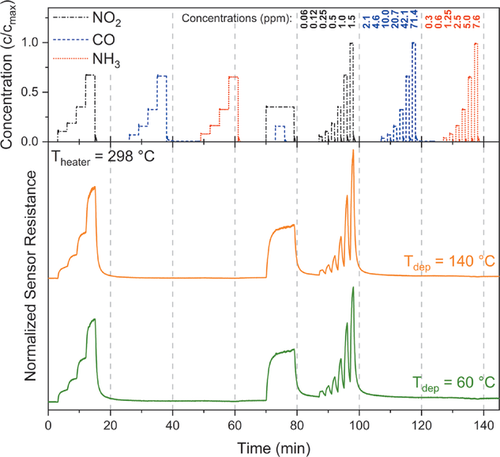
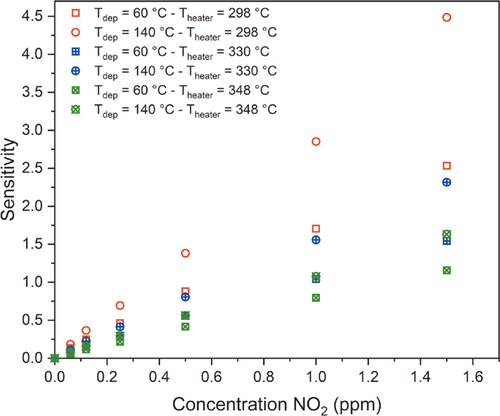
Encouraged by the XPS studies, showing a high amount of chemisorbed oxygen species, which are known to give functionality to gas sensors,[68] we deposited 50 nm thick ZnO onto industrially approved sensor chips at deposition temperatures of Tdep = 60 °C and Tdep = 140 °C. As a proof-of-concept, the sensitivity of the ZnO films towards NO2, NH3, and CO at heater temperatures of 298, 330, and 348 °C of the sensor chip was investigated. For the measurements, the resistance of the coated chip was recorded while running a defined gas measurement profile, employing gas pulses between 1–10 min, as depicted in Figure 9 (top). The response of the sensor, which is the measured resistance, at a heater temperature of 298 °C is shown in Figure 9 (bottom) exemplarily while the other sensor tests at heater temperatures of 330 and 348 °C are illustrated in Figure S6, Supporting Information. What is noteworthy, is that the films show an increase in the resistance for the oxidative gas NO2 in concentrations between 0.06 and 1.5 ppm, indicating a typical n-type semi-conducting behavior. Furthermore, they are sensitive towards NO2 with a high selectivity without any cross-sensitivity toward the other, reductive gases namely NH3 (0.3–7.6 ppm) and CO (2.1–71.4 ppm), used in this study. This is a remarkable advantage as cross-sensitivities are often a problem for commercially available sensors, as indicated by the used reference sensor for NO2 at a heater temperature of 330 °C (Figure S6, Supporting Information), which is based on metal oxides and shows a small cross-sensitivity toward NH3 as well. Interestingly, other reports could show a sensitivity on ZnO toward NH3 and CO but are incomparable as for NH3 detection, ZnO nanoparticles on a TFT-based sensor element[17] was used while for CO, ZnO was combined with SnO2 or was measured in a humid atmosphere.[9, 10] Following the sensor response towards NO2, in comparison to the commercial reference sensor at Theater = 330 °C (Figure S6, Supporting Information) it is striking that the resistance of the PEALD coated sensors can follow the NO2 concentration quite precisely with a great reversibility.
3 Conclusion
As a potential alternative to the industrially used Zn precursor DEZ, we successfully synthesized a nonpyrophoric and volatile zinc alkylamine precursor, BDMPZ [Zn(DMP)2]. An enhanced stability accompanied by only reactive Zn–C and Zn–N bonds in this monomeric complex is achieved by intramolecular stabilization by a dative bond from the amine group to the zinc center. Thermal evaluation confirmed the high volatility at low evaporation temperatures as proven by sufficient vaporization of BDMPZ at Tevap = 50 °C even under ambient pressure. This, in combination with a melting point of only 45 °C, render this compound to be an extremely promising precursor for ALD. Subsequently a new PEALD process for ZnO employing BDMPZ with an oxygen plasma was developed. Polycrystalline, smooth and stoichiometric ZnO thin films were grown in a deposition temperature range between 60 and 160 °C. Self-limiting behavior with growth rates of 0.42 and 0.69 Å cycle−1 at deposition temperatures of 60 and 140 °C, respectively, was proven, showing that the new BDMPZ process can fill the deposition temperature gap that was opened by the proof that DEZ is already decomposing at deposition temperatures above 60 °C.[48] Furthermore, a high transparency of ≈98% of the ZnO films on quartz was shown. An improvement in the gas barrier performance of 98.7% for a 7.5 nm thick film on PET substrates at deposition temperatures as low as 60 °C was obtained, which shows the suitability of these layers for GBLs. Finally, by coating ZnO on sensor chips, a high sensitivity toward NO2 without cross-sensitivities against NH3 and CO was demonstrated in a working gas sensor. In summary, BDMPZ is an excellent candidate to substitute DEZ in the low temperature range and the developed O2-plasma process facilitates deposition of high quality ZnO films that can be easily adapted to a various industry relevant applications. Currently, the development of a water-assisted thermal ALD process employing BDMPZ as well as the application of the ZnO films as channel material in TFTs are under investigation.
4 Experimental Section
Precursor Synthesis
Precursor synthesis was performed in an Ar atmosphere using standard Schlenk-techniques. THF and n-hexane were purified and dried using a MBraun solvent purification system (MBraun SPS). All the compounds including ZnCl2 (abcr, 98%, anhydrous) were stored, handled and prepared for analysis under an argon atmosphere (Air Liquide, 99.999%) in a glove box (MBraun, Labmaster 120).
Bis-(N,N-dimethylamino)propyl zinc (II), [Zn(DMP)2], BDMPZ
The synthesis of BDMPZ was performed by slightly modifying the procedure reported earlier.[51] A solution of ZnCl2 (3.47 g, 25,5 mmol) in 50 mL THF was cooled to 0 °C and added dropwise to a cooled (0 °C) solution of 3-(N,N-dimethylamino)propyl magnesium chloride (51 mmol, 0.911 mol L−1) in THF that was synthesized as described in literature.[69] The resulting suspension was allowed to warm up and was refluxed over night at 80 °C and afterwards it was stirred at room temperature. The THF was removed under reduced pressure and the colorless solid was extracted in 120 mL pentane. The suspension was filtered over a celite pad and pentane was removed under reduced pressure. The colorless solid was sublimed at 60 °C resulting in 3.8 g (16 mmol) of the spectroscopically pure BDMPZ (yield: 62%).
1H NMR (400 MHz, Benzene-d6) δ [ppm] = 2.06–1.99 (m, 4H, [(CH3)2NCH2CH2CH2]2 Zn), 1.98–1.90 (m, 4H, [(CH3)2NCH2CH2CH2]2 Zn), 1.89 (s, 12H, [(CH3)2NCH2CH2CH2]2 Zn), 0.31 (t, J = 7.1, 4H, [(CH3)2NCH2CH2CH2]2 Zn); EI-MS (70 eV, 80 °C): m/z (%) = 236.2 (1.6) [M+], 150.0 (7.1) [M+-DMP], 86.1 (6.8) [DMP+], 58.1 (100) [(CH2)NMe2+]; Elemental Analysis C10H24N2Zn, calculated: C 50.53%, H 10.18%, N 11.79%, found: C 49.43%, H 9.72%, N 14.87%.
Precursor Characterization
1H-nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AV III 400 spectrometer. All spectra were referenced to internal solvent proton signals and corrected to the tetramethylsilane (TMS) standard value of δ = 0.00 ppm. The recorded NMR spectra were analyzed using MestReNova software. Electron impact ionization mass spectrometry (EI-MS) was performed at 70 eV using a VG Instruments Autospec instrument. CHNS elemental analysis (EA) was performed using a vario Micro cube from Elementar Analysensysteme. Simultaneous thermogravimetric and differential thermal analysis (TG/DTA) studies, as well as isothermal studies were carried out by using a Seiko TG/DTA 6200/SII at ambient pressure (sample size 10 mg). For TGA, a heating rate of 5 K min−1 and a nitrogen (AirLiquide, 99.999%) flow rate of 300 mL min−1 was used. The vapor pressure was determined by using a stepped isothermal TG within a temperature range of 50–150 °C while each temperature in 10 °C steps was kept constant for 10 min.
Thin Film Deposition
Plasma enhanced atomic layer deposition (PEALD) of ZnO was performed in a custom-built shower head PEALD reactor using a 13.56 MHz ECWR oxygen plasma. The precursor BDMPZ was filled in a stainless-steel cartridge maintained at 55 °C evaporation temperature and was pulsed without carrier gas. For purging, argon (AirLiquide, 99.999%) with a flow of 25 sccm and for the plasma, oxygen (AirLiquide, 99.995%) with a flow of 25 sccm was used. If not further specified, for all depositions, the plasma power was set to 200 W while the reflected power was adjusted within an area of 35–45 W. The base pressure of the reactor was 5 × 10−4 mbar. Following pulse/purge sequence was used (see also Figure S2 in the Supporting Information): 5 × 150 ms precursor pulse separated by a 100 ms gap. At a total time of 1000 ms, a 250 ms argon purge was applied followed by 250 ms applied vacuum without purging gas. Then, oxygen was pulsed into the chamber for 500 ms in which the plasma was ignited for 150 ms. After 500 ms of applied vacuum, again 250 ms argon purging followed by 250 ms inactive purging was performed to complete one cycle. The process temperature was varied between 60 and 220 °C.
For the process optimization Si(100) wafers (Si-mat) were used without further cleaning. For the OTR measurements PET (23 µm, Hostaphan RD23, Mitsubishi, Wiesbaden, Germany) was used as substrate in the PEALD experiments without any prior surface treatment.
Thin Film Characterization
The thickness of the thin films was investigated by X-ray reflectivity (XRR) measurements using a Bruker D8 Discover XRD (Cu Kα radiation (1.5418 Å)). It was used in the θ–2θ locked coupled mode and 2θ was varied between 0.1° and 3° with a step size of 0.01. Grazing incidence XRD (GIXRD) was performed on ZnO layers using an X'Pert Panalytical diffractometer with the films held at an incident angle of ω = 1 °, while the detector (2Θ) scanned from 25°–60°. The topography of the ZnO films was characterized by means of scanning electron microscopy (SEM, LEO Gemini 982) and atomic-force microscopy (AFM, Digital Instruments, Nanoscope V). Rutherford backscattering spectrometry (RBS) was executed with a 4He+ ion beam of 2.0 MeV. With a high sensitivity to O as well as to C and N contaminations, nuclear reaction analysis (NRA) was performed by using an ion beam of 1.0 MeV deuterons. The SIMNRA program was employed for RBS and NRA raw data processing and analysis.[70] The XPS was carried out at the MATLINE beamline at the ASTRID synchrotron in Denmark. The instrument work function was calibrated and the spectra were referenced to the metallic Au 4f7/2 peak at 84.00 eV. Fermi level corrections were applied if necessary. The instrument pressure was 5 × 10−10 Torr during the measurements. The spectra were measured for a 0.7 × 1 mm area with an electron analyzer directed to the surface normal. The pass energy values ranged between 10 and 100 eV. The X-ray photon energies utilized were 660 and 1150 eV for the oxygen and zinc determination, respectively. All samples were charged neutralized before measuring and not sputter cleaned prior to the measurement. The spectra were analyzed using the KolXPD software. For the peak analysis, a convolution of Gaussian and Lorentzian line shapes was used and a standard Shirley background was subtracted. Oxygen transmission rates (OTR) were measured with a MOCON X-TRAN 2/61 device (Mocon Inc., Minneapolis, USA) using the carrier gas method at 23 °C and 0% humidity at the AEPT institute at RUB. The application of the ZnO thin films for gas sensing was performed by depositing 50 nm thin ZnO layers onto the commonly used substrate material, which is a 0.5 mm thick aluminum oxide ceramic. In order to reach the sensitivity level of the semiconducting metal oxides, the materials need to be heated. Hence, a Pt heater is deposited and patterned via laser structuring, directly into the aluminum oxide substrates. The sensing layers can then be deposited right onto the Pt–heater and thus the needed temperature can be set by the sensor electronics. After the deposition of the ZnO layers onto the substrates, the sensor elements were integrated in the industrially used sensor electronics and sensor cover, developed by paragon GmbH & Co KGaA. The gas sensitive materials are placed in the front part of the sensor housing, which is covered by a gas permeable membrane. The sensors are placed and fixed with the front part in the gas channel, where the gas concentration between 0.06 and 1.50 ppm is regulated using a mass flow controller (Figure S8, Supporting Information). An even distribution of gases in the whole gas channel is ensured. As the response to the gas profile, the sensor resistivity is detected and processed by the sensor electronics.
Acknowledgements
The authors are grateful for funding and support from the European Funds for Regional Development (EFRE-0800672-FunALD). The authors (L.M., A.D., F.M., P.A.) thank the Deutsche Forschungsgemeinschaft (DFG) for partly funding this work within the SFB-TR 87 Collaborative Research Center. L.M. thanks the Stiftung der deutschen Wirtschaft (sdw) within the Klaus Murmann fellowship for supporting his Ph.D. project. E.C. is grateful to ASTRID Synchrotron at Aarhus University for beamline time at MATLINE.
Conflict of Interest
The authors declare no conflict of interest.




