Upregulation of iNOS and phosphorylated eNOS in the implantation-induced blastocysts of mice
Abstract
Purpose
This study aimed to examine expressions of iNOS and phosphorylated eNOS (p-eNOS) in implantation-induced blastocysts. We also examined the upstream of p-eNOS.
Methods
To address the protein expressions in implantation-induced blastocysts, we performed immunohistochemical analysis using a delayed implantation mouse model. Immunostaining for iNOS, p-eNOS, and p-Akt was done. To address the relationship between p-eNOS and p-Akt, activated blastocysts were treated with an Akt inhibitor, MK-2206.
Results
iNOS expression was at low levels in dormant blastocysts, whereas the expression was significantly increased in the activated blastocysts. Double staining of p-eNOS and p-Akt in individual blastocysts showed colocalization of p-eNOS and p-Akt of the trophectoderm. p-eNOS and p-Akt expressions were at low levels in dormant blastocysts, whereas both of them were significantly increased in the activated blastocysts. Both dormant and activated blastocysts showed significant positive correlations between p-eNOS and p-Akt. MK-2206 treatment for activated blastocysts showed that blastocysts with lower p-Akt had significantly lower p-eNOS levels.
Conclusions
iNOS and p-eNOS, Ca2+ independent NOS, are upregulated by E2 in the blastocysts during implantation activation. Furthermore, p-eNOS is upregulated in implantation-induced blastocysts downstream of p-Akt.
1 INTRODUCTION
Successful implantation and pregnancy depend on the cellular and molecular crosstalk between the uterus and the embryo, and the intricate succession of gene-cell interactions must be executed within an optimal time frame.1-5 L-arginine (Arg) is essential during pregnancy for conceptus growth and development as a precursor to the synthesis of molecules (e.g. nitric oxide, polyamines, and creatine) with cell signaling and metabolic functions.6 Dietary Arg supplementation during gestation has been effective in improving embryonic survival and conceptus development in many species, including mice, rats, pigs, sheep, and humans.6 Arg can be metabolized by nitric oxide synthase (NOS) synthase to nitric oxide (NO).7 NO is a major vasodilator and contributes to utero-placental blood flow.8-10 Furthermore, it has been suggested that NO production by the implanting mouse trophoblast possibly participates in vasodilatation and angiogenesis, and cytotoxic mechanisms involved in the intense phagocytosis of injured maternal cells during the implantation process.11
For successful implantation, the blastocyst must attain implantation competency in the receptive uterus.1-4 Highly coordinated cellular and molecular events directed by ovarian steroid hormones estrogen (E2) and progesterone (P4) produce a receptive uterine environment to support implantation.1-4, 12 The blastocyst also functions as an active unit in this process, with its own molecular cell growth and differentiation program.3, 4 E2 is essential for on-time uterine receptivity and blastocyst activation in mice. Indeed, ovariectomy before pre-implantation E2 secretion on the morning of Day 4 (Day 1 = vaginal plug) results in implantation failure, initiating a state of blastocyst dormancy.13 This condition, referred to as delayed implantation, can be maintained by continued P4 treatment. Conversely, injecting P4-primed mice with E2 rapidly results in implantation, with blastocyst activation.13, 14 The delayed implantation model is a powerful tool for defining the molecular signaling components that direct blastocyst activation or dormancy.3, 4 Using this model, researchers have aimed to understand how the blastocyst is programmed for implantation by studying numerous genes and signal pathways by which this is achieved.15-20
During pregnancy, NO generated by NOS from Arg contributes to utero-placental blood flow.10 NOS has been identified as three isoforms, including type I or neuronal form (nNOS), type II or inducible form (iNOS), and type III or endothelial form (eNOS).21 NOS activity is dependent on calmodulin (CaM). CaM regulates NOS enzymatic activity by docking to the oxygenase domain, thereby constraining and regulating the accessible conformations of the CaM-NOS complex to control the rates of electron transfer between the reductase and oxygenase domains.22 eNOS and nNOS bind CaM reversibly so that their activity depends on the calcium (Ca2+) concentration in the environment and hence constantly available for activation by Ca2+-CaM.22
Although iNOS contain a putative CaM binding domain, its activity is Ca2+-independent.5 Meanwhile, independent of elevations in intracellular calcium, permitting eNOS phosphorylation and activation at resting Ca2+ levels promote NO release through a PI3K-Akt-dependent mechanism.23 A previous study has suggested that NO production from iNOS and eNOS by the mouse trophoblast possibly participates in vasodilatation and angiogenesis during the post-implantation process.11 Furthermore, several studies reported the importance of the PI3K pathway in pre-implantation embryo survival.24, 25 Therefore, Ca2+ independent NOS, iNOS and phosphorylated eNOS (p-eNOS) might regulate blastocyst functions during the peri-implantation period.
This study aimed to examine expressions of iNOS and phosphorylated eNOS (p-eNOS) in implantation-induced blastocysts. We examined the distribution of iNOS and p-eNOS in activated blastocysts in mice using the delayed implantation model and in vitro culture system supplemented with the p-Akt inhibitor MK-2206. Our results showed that both iNOS and p-eNOS are expressed in implantation-induced blastocysts. Using an Akt inhibitor, MK-2206, we also found that p-eNOS is upregulated in implantation-induced blastocysts downstream of p-Akt.
2 MATERIALS AND METHODS
2.1 Animals and treatments
Experimental procedures in this study were performed per the instructions of the Guide for the Care and Use of Laboratory Animals published by Utsunomiya University. Adult ICR mice were purchased from Japan SLC and bred in our animal care facility. We mated 3- to 5-month-old virgin females with fertile males to induce pregnancy (Day 1 = vaginal plug). Delayed implantation was induced according to previously described methods.15-18 In brief, mice were ovariectomized on the morning (8:00–9:00 h) of Day 4 of pregnancy and maintained on daily injections of P4 (2 mg/mouse; Sigma) from Days 5 to 7. To obtain activated blastocysts, P4-primed delayed implantation, pregnant mice were injected with estradiol-17β (E2; 25 ng/mouse; Sigma). Dormant blastocysts were collected 23 h after the last P4 injection, whereas activated blastocysts were collected 23 h after the E2 injections.
2.2 Immunohistochemical analysis
Immunohistochemistry was performed as described previously15, 17, 18, 26 with slight modifications. Embryos were fixed in 3.7% formaldehyde in Ca2+- and Mg2+-free Dulbecco's phosphate-buffered saline (D-PBS) at room temperature for 30 min, permeabilized in 0.25% Tween-20 in D-PBS for 5 min and then incubated overnight at 4°C with specific antibodies for iNOS, p-eNOS (Ser 1177) (both from Santa Cruz Biotechnology), or p-Akt (Ser 473) (Merck). After several washes, the embryos were incubated with a secondary antibody labeled with fluorescein isothiocyanate (FITC) (MP Biomedicals) or Alexa Fluor 594 (Thermo Fisher Scientific) to visualize the specific antigens. Nuclei were labeled with 5 μg/mL of Hoechst 33342 (bisBenzimide H33342 trihydrochloride, Thermo Fisher Scientific) for 30 min at room temperature. The embryos were mounted on Glass Bottom Dish (Matsunami Glass Ind., Ltd.) and viewed with a TCS SP8 confocal scanning laser microscope (Leica). Fluorescent intensities were quantified using Leica Application Suite X.
2.3 Culture of blastocysts with the Akt inhibitor
Embryos were collected in KSOM medium without phenol red (KSOM-P) and cultured in the same medium in four-well multidishes (Thermo Fisher Scientific) at 37°C in a humidified atmosphere of 5% CO2 in the air.18 Akt inhibitor MK-2206 (Cayman Chemical) was dissolved in dimethyl sulfoxide (DMSO) (Sigma). Activated blastocysts were cultured in KSOM-P with 1 μM or 10 μM MK-2206 for 1 h. DMSO was added as a control, and all cultures contained 0.1% DMSO.
2.4 Statistical analysis
Statistical analyses were performed using JMP version 13.0 (SAS Institute Inc.). Protein expression levels are evaluated as relative values with the average fluorescence intensities of dormant as 1. Data are shown as mean ± SEM. Student's t-test was used to evaluate differences between dormant and activated blastocysts. The relationships between protein expressions and cell numbers were determined by Pearson correlation coefficient and linear regression analysis. Correlation between p-Akt and p-eNOS was also determined by Pearson correlation coefficient and linear regression analysis. The Steel–Dwass all pairs test was used for multiple comparison tests to evaluate the effect of MK-2206. Fischer's exact probability test was used to evaluate the percentile of blastocysts with low p-Akt and p-eNOS levels.
3 RESULTS
3.1 iNOS upregulation in implantation-induced (activated) blastocysts
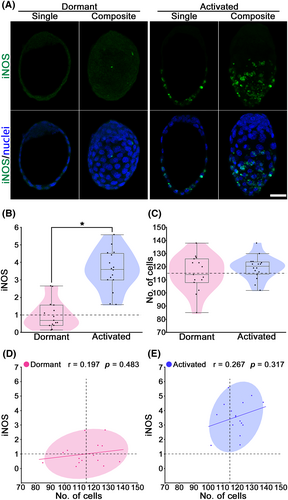
The immunohistochemical analysis detected low iNOS expression levels in dormant blastocysts, whereas higher iNOS were expressed in the mural trophectoderm (TE) of activated blastocysts (Figure 1A,B). iNOS expression (mean ± SEM) was significantly increased in the activated blastocysts (3.56 ± 0.28; p < 0.05) as compared to dormant blastocysts (1.00 ± 0.21) (Figure 1B). Meanwhile, cell numbers (mean ± SEM) in blastocysts were not different between the dormant (115.0 ± 3.48) and activated (119.3 ± 2.17) blastocysts (p > 0.05; Figure 1C). The relationship between iNOS level and cell numbers was determined by Pearson correlation coefficient and linear regression analysis (Figure 1D,E). Neither dormant nor activated indicated significant correlations between iNOS level and cell numbers (p > 0.05). These results suggest that iNOS expression is upregulated in implantation-induced blastocysts independently of cell proliferation.
3.2 p-eNOS upregulation in activated blastocysts with the concurrent p-Akt expression
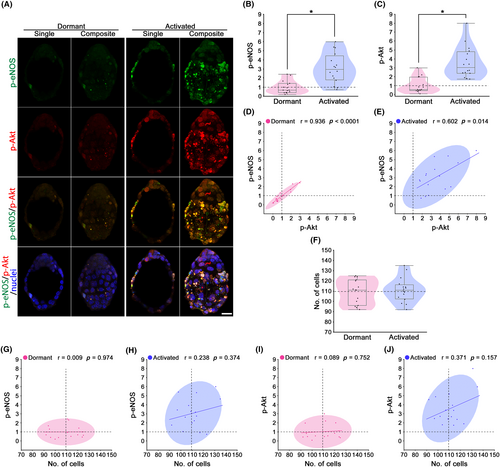
The immunohistochemical analysis detected low p-eNOS expression levels in dormant blastocysts, whereas higher p-eNOS were expressed in the TE of activated blastocysts (Figure 2A,B). p-eNOS expression (mean ± SEM) was significantly increased in the activated blastocysts (3.07 ± 0.41; p < 0.05) as compared to the control (1.00 ± 0.18) (Figure 2B). eNOS is one of the downstream Akt substrates that induces eNOS phosphorylation.27 To determine the upstream of p-eNOS, double staining of p-eNOS and p-Akt was performed in individual blastocysts (Figure 2A). p-Akt staining showed low expression levels in dormant blastocysts, while higher expression levels were detected in the TE of activated blastocysts (Figure 2A). Moreover, yellow/orange regions show colocalization of green (p-eNOS) and red (p-Akt), i.e., the possible interaction between p-eNOS and p-Akt in activated blastocysts (Figure 2A). p-Akt expression (mean ± SEM) was significantly increased in the activated blastocysts (3.58 ± 0.44; p < 0.05) as compared to the control (1.00 ± 0.22) (Figure 2C). The relationship between p-eNOS and p-Akt level was determined by Pearson correlation coefficient and linear regression analysis (Figure 2D,E). Both dormant and activated blastocysts showed significant positive correlations (p < 0.05; Figure 2D,E). Meanwhile, cell numbers (mean ± SEM) in blastocysts were not different between the dormant (109.5 ± 3.09) and activated (111.1 ± 2.93) blastocysts (p > 0.05; Figure 2F). The relationship between p-eNOS level and cell numbers was determined by Pearson correlation coefficient and linear regression analysis (Figure 1G,H). Neither dormant nor activated indicated significant correlations between p-eNOS level and cell numbers (p > 0.05). The relationship between p-Akt level and cell numbers was determined by Pearson correlation coefficient and linear regression analysis (Figure 1I,J). Neither dormant nor activated indicated significant correlations between p-Akt level and cell numbers (p > 0.05). These results suggest that upregulated p-eNOS expression is possibly associated with p-Akt in implantation-induced blastocysts independently of cell proliferation.
3.3 p-eNOS is upregulated in activated blastocysts downstream of p-Akt
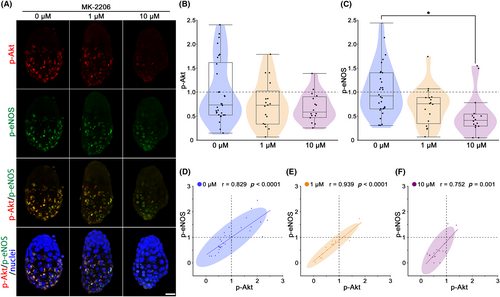
As shown in Figure 2, p-eNOS and p-Akt expressions were induced in activated blastocysts concurrently. To clarify the relation between p-eNOS and p-Akt, we analyzed blastocysts treated with Akt inhibitor MK-2206. Immunohistochemical analysis revealed lower p-Akt and p-eNOS expressions in MK-2206 treated blastocysts (Figure 3A). p-Akt levels (mean ± SEM) tended to decrease in MK-2206 treatment at 1 μM (0.75 ± 0.11) and 10 μM (0.68 ± 0.08) as compared to control (0 μM; 1.00 ± 0.12) (Figure 3B). p-eNOS levels (mean ± SEM) was tended to decrease at 1 μM (0.67 ± 0.10) and significantly decreased at 10 μM (0.48 ± 0.11; p < 0.05) compared to control (0 μM; 1.00 ± 0.10) (Figure 3C). The relationship between p-Akt and p-eNOS was determined by Pearson correlation coefficient and linear regression analysis (Figure 3D–F). All treatments (control (0 μM), 1 μM, and 10 μM) showed significant strong positive correlations, and blastocysts with lower p-Akt had lower p-eNOS (p < 0.05; Figure 3D–F). Fischer's exact probability test indicated that the percentile of blastocysts with both p-Akt and p-eNOS levels in the range below 1.00 tended to increase at 1 μM (76.5% (13/17)) and significantly increased at 10 μM (87.5% (14/16); p < 0.05) compared to control (50.0% (15/30)) (Figure 3D–F).
4 DISCUSSION
Previous work by our group indicated that the E2-responsive target gene was up-regulated in activated blastocysts during the peri-implantation period using a delayed implantation model.3, 4, 15, 17, 18 During the post-implantation process, the production of NO from iNOS and eNOS by the mouse trophoblasts has been suggested that the possible participation of these molecules in uterine vasodilatation and angiogenesis.11 Our present study shows that iNOS and p-eNOS are E2 targets in blastocysts during implantation activation. Furthermore, p-eNOS is upregulated downstream of p-Akt in activated blastocysts.
eNOS and iNOS are expressed in early pre-implantation embryos.28 Furthermore, it has been suggested that adequate NO levels play a crucial role in embryogenesis, including blastocyst hatching.28-33 The differences in the required amount of NO and the sensitivity to cytotoxicity of NO in each developmental stage of embryos suggest that the NO/NOS system is tightly regulated in a developmental stage-specific manner.30 Indeed, the addition of an NO donor inhibits embryo development, whereas inhibition of NOS also adversely affects embryonic development in mice.28-32 Namely, the role of the NO/NOS system and its regulation remains controversial.
Using delayed implantation model, we found that Ca2+ independent NOS, iNOS and p-eNOS, are upregulated by E2 in the blastocysts during activation of implantation in mice. Both NOSs are highly expressed in the TE at 23 h after E2 injection. The attachment reaction between the blastocyst and uterus occurs 24 h after the E2 injection in delayed implantation in mice.3, 4 In contrast, in normal pregnancy in mice, the attachment reaction is initiated around 2400 h on Day 4 of pregnancy (Day 1 = vaginal plug).1, 3, 4 During the peri-implantation period of normal pregnancy in mice, very low iNOS was expressed in blastocysts at pre-attachment (Day 4 16:00), whereas iNOS expression was remarkably increased at post-attachment (Day 5 5:00) and decreased later on (Day 5 10:00).34 These results suggest that the expression of iNOS is upregulated by E2, but its expression is limited during the peri-attachment period. Therefore, NO derived from iNOS in mouse trophoblasts may not participate in uterine vasodilatation or angiogenesis during the early post-implantation period.
It has been reported that eNOS can be found in the trophoblast, the cells that adhere to and penetrate the uterine endometrium at implantation.11, 28, 35 Furthermore, the eNOS-knockout mouse model showed that the number of embryos reaching implantation between days 6.5 and 8.5 after the vaginal plug was lower in eNOS-knockout mice than in wild-type mice.36 The roles of NO and NOS, specifically eNOS, have been shown to be involved in implantation and embryo development.11, 28, 35 Embryo nutrition before completion of the placenta is achieved by the phagocytic activity of the trophoblast.37, 38 Moreover, eNOS induces vasodilatation, angiogenesis, and phagocytosis of the maternal cells during implantation.11 These findings and our present results suggest that NO produced by eNOS mainly functions after blastocyst attachment to maintain pregnancy.
Meanwhile, p-eNOS was expressed in trophoblast cells and hypoxic conditions showed an increase in p-eNOS.39 Although physiological uterine hypoxia during implantation may contribute to embryo implantation through hypoxia inducible factor (HIF), factors other than hypoxia, such as embryonic stimuli, may be associated with HIF induction.40 Hif1a and Hif2a were shown to be strongly expressed in the mouse uterus during embryo implantation and HIFα is functionally critical for successful embryo implantation.40, 41 Furthermore, stromal Hif2a is critical for embryo invasion and PI3K-AKT pathway activation in the implanting embryos.40 This study revealed that p-Akt is upstream of p-eNOS in implantation-induced blastocysts. The PI3K-Akt pathway plays an important role in the survival of pre-implantation embryos.24, 25 Therefore, the PI3K-Akt pathway is also associated with p-eNOS in blastocysts as the embryonic survival signal during the peri-attachment period.
In this study, the cell numbers in implantation-induced blastocysts by E2 were not different from those in dormant blastocysts, while iNOS and p-eNOS levels were upregulated in implantation-induced blastocysts. Furthermore, the cell number was not related to iNOS or p-eNOS. These results suggest that these NOSs are not associated with cell proliferation during peri-attachment, but with embryonic survival signals via the PI3K-Akt pathway. Meanwhile, in vitro, the cell number was reduced in Day 4 blastocysts cultured with the NOS inhibitor L-NAME, whereas NOS inhibition did not affect blastocyst development.31 Furthermore, the NOS inhibitor increased the oxygen consumption, whereas Arg decreased it. NO may limit oxygen consumption at the blastocyst stage at the level of mitochondrial cytochrome c oxidase, and support developmental competence.31 In contrast to the pre-implantation development to the blastocyst stage, implantation-induced and dormant blastocysts are fully developed during pre-implantation in vivo and reach the peri-attachment period, i.e., fully developed blastocysts with adequate cell numbers. Because low oxygen tension promotes the blastocyst implantation rate,42 NO produced by iNOS and/or p-eNOS may decrease oxygen consumption, resulting in trophoblast differentiation and implantation ability.
Trophoblast outgrowth is a reliable marker of trophoblast differentiation and migration.43, 44 Inhibition of NO by the NOS inhibitor L-NAME resulted in the inhibition of blastocyst outgrowth.30 Arg is metabolized to NO by NOS and contributes to trophoblast outgrowth induction.45 Meanwhile, addition of a low concentration of NO donor SNP (10−7 M) enhanced trophoblast outgrowth, whereas a high concentration of SNP (10−3 M) completely inhibited blastocyst attachment and spreading.30 Therefore, it is possible that elevated NOSs in activated blastocysts produce appropriate NO, which functions blastocyst attachment to the uterine luminal epithelium and/or trophoblast migration during the post-attachment period.
While iNOS expression can be induced by cytokines and other agents in almost any cell type in various types of inflammatory diseases, eNOS is mostly expressed in endothelial cells as physiological vasodilators.46 Therefore, it is possible that E2 mediated upregulation of iNOS and eNOS is associated with inflammation and vasodilation, respectively, in a spatiotemporal manner.
The delayed implantation mouse model is a powerful approach for studying and manipulating the triggers of embryo implantation.15-20 Embryonic diapause is a temporary arrest of embryo development and is characterized by delayed implantation in the uterus.47-51 The incidence of embryonic diapause is widespread and has been reported to occur in nearly 100 mammals in seven different taxonomic orders.52, 53 Delayed implantation does not normally occur in certain species including sheep, hamsters, rabbits, guinea pigs, or pigs.49 Meanwhile, an interspecies embryo transfer study found that embryos from non-diapausing sheep endured dormancy when transferred to delayed-implantation mouse uteri.47 When these dormant blastocysts were transferred back into the donor sheep uterus, they underwent activation and implantation with the birth of apparently normal lambs.47 These findings demonstrate the flexibility of blastocyst survival and implantation competence and will be of increasing importance for the study of embryonic diapause in elucidating the molecular mechanisms of blastocyst dormancy in the acquisition of implantation competence. Embryonic diapause studies will become increasingly important for elucidating the molecular mechanisms underlying the acquisition of implantation competence from blastocyst dormancy.
In conclusion, the present study indicated that Ca2+ independent NOS, iNOS and p-eNOS, are upregulated by E2 in implantation-induced blastocysts in mice. Furthermore, p-Akt is upstream of p-eNOS in implantation-induced blastocysts, suggesting that the embryonic survival signal PI3K-Akt pathway is also associated with p-eNOS in blastocysts during peri-attachment to the uterine luminal epithelium. Meanwhile, neither iNOS nor p-eNOS are associated with cell numbers in mouse blastocysts during peri-attachment, suggesting that they are not important for cell proliferation during the transition from the dormant to the activated phase of the blastocyst.
ACKNOWLEDGMENTS
This work was supported, in part, by JSPS KAKENHI Grant Numbers 25450390, 18 K05936, and 21 K05903 (H.M).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ANIMAL STUDY
All animal experiments described here were approved by the Animal Experimentation Committee at Utsunomiya University and were performed per the instructions in the Guide for the Care and Use of Laboratory Animals published by Utsunomiya University.
HUMAN RIGHTS
This article does not contain any studies with human subjects performed by any of the authors.




