Human CD141+ dendritic cells: A unique tolerogenic subset inducing immune tolerance in systemic lupus erythematosus patients
Xiaodong Qin, Yujiao Wang, Yi Chen contributed equally to this work.
Edited by Zhiyu Wang.
Abstract
Background
Tolerogenic dendritic cells (DCs) are promising for cell-based treatment for systemic lupus erythematosus (SLE). We previously showed that tolerogenic CD1c+ DCs play an important role in mesenchymal stem cell transplantation treatment of SLE. We further investigated the immunoregulatory effects of CD141+ DCs on lupus pathogenesis.
Methods
A total of 12 SLE patients and 12 age- and sex-matched healthy subjects were included in the Affiliated Drum Tower Hospital of Nanjing University Medical School from January 2019 to January 2021. The percentage of peripheral blood CD141+ DCs and their functional expressions of costimulatory markers (CD40, CD80, CD83, and CD86) were compared between patients with SLE and healthy controls. Moreover, the tolerogenic properties of CD141+ and CD1c+ DCs were compared following stimulation with lipopolysaccharide (LPS) or SLE serum.
Results
Our research revealed that the presence of peripheral CD141+ DCs decreased in SLE patients compared with controls (1.03% ± 0.40% vs. 1.44% ± 0.36%, p < 0.05), which was negatively correlated with the SLE disease activity index score (R2 = 0.5547, p = 0.0055). Moreover, CD141+ DCs in patients with SLE exhibited distinct tolerogenic properties by decreasing the mean fluorescence intensity (MFI) of CD40, CD80, and CD86 when compared with controls (2447.00 ± 1386.14 vs. 11,357.25 ± 4581.05, p < 0.001; 1528.00 ± 876.61 vs. 14,181.42 ± 7599.33, p < 0.001; 17,409.42 ± 8180.98 vs. 33,614.25 ± 7408.69, p < 0.001). Furthermore, CD141+ DCs exhibited superior tolerogenicity when compared to CD1c+ DCs in SLE, by reducing MFI of CD86 (93,526.58 ± 51,763.00 vs. 169,552.00 ± 51,225.74, p < 0.01 by LPS stimulation; 107,837.40 ± 26,124.76 vs. 145,450.90 ± 50,436.69, p < 0.05 by SLE serum stimulation), and reducing production of tumor necrosis factor α (8.83% ± 3.51% vs. 13.94% ± 2.87%, p < 0.05 by LPS stimulation; 10.12% ± 2.34% vs. 14.39% ± 2.70%, p < 0.05 by SLE serum stimulation).
Conclusions
CD141+ DCs play a more significant role than the commonly studied CD1c+ DCs in ameliorating immune dysfunction and maintaining immune homeostasis in SLE. Our findings contribute to a better understanding of the immunoregulatory mechanisms underlying SLE and pave the way for novel therapeutic approaches targeting CD141+ DCs.
Key points
-
The number of circulating CD141+ dendritic cells (DCs) decreased in systemic lupus erythematosus (SLE).
-
CD141+ DCs in SLE exhibited distinct tolerogenic properties by decreasing the expression of CD40, CD80, and CD86.
-
CD141+ DCs exhibited superior tolerogenicity compared to CD1c+ DCs in SLE.
-
Cellular therapy involving the use of tolerogenic CD141+ DCs in vivo may represent new therapeutic approaches for SLE treatment.
1 INTRODUCTION
Dendritic cells (DCs) are specialized antigen-presenting cells that play crucial roles in initiating and directing immune responses.1, 2 Despite their relatively small numbers, DCs are essential for controlling both innate and adaptive immune responses. In humans, DCs could be divided into two subsets: plasmacytoid DC (pDC, blood dendritic cell antigens [BDCA]-2) and conventional DC (cDC). Furthermore, cDCs were observed as two unique populations characterized by the expression of CD1c (BDCA-1) and CD141 (BDCA-3).3, 4 cDCs exhibit two distinct states: activated cDCs secrete interleukin-12 (IL-12) and tumor necrosis factor α (TNF-α), priming T cell responses; while in steady-state conditions, the cDCs secrete lower levels of pro-inflammatory cytokines and higher levels of anti-inflammatory cytokines. This steady-state phenotype was associated with their tolerogenic properties.5, 6
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the immune system attacking healthy tissues throughout the body. The abnormal activation of the immune system leads to exaggerated T cell or B cell responses and loss of immune tolerance against self-antigens.7 The pivotal role of DCs in immune regulation investigating the mechanisms and applications of DCs in SLE treatment has gained considerable attention in recent years. Previous studies have mainly focused on pDCs and CD1c+ DCs in the context of SLE,8-10 but the contribution of CD141+ DCs to SLE pathogenesis remains largely unknown. Although direct evidence linking circulating CD141+ DCs to immune tolerance induction is currently lacking, several lines of evidence link them with immunoregulatory ability.11-14 Hence, the purpose of this study was to investigate the immunoregulatory role of CD141+ DCs in the pathogenesis of lupus. We previously identified that CD1c+ DCs were tolerogenic DCs, which played important roles in mesenchymal stem cell transplantation (MSCT) treatment for SLE.10 Here, we have further determined the immune tolerance of CD141+ DCs in SLE and compared the tolerogenic properties of CD141+ DCs and CD1c+ DCs to provide a new therapeutic target for lupus patients.
2 METHODS
2.1 Research subject
From January 2019 to January 2021, SLE patients were consecutively enrolled in the Affiliated Drum Tower Hospital of Nanjing University Medical School. All patients met the revised SLE criteria established by the American College of Rheumatology in 1997.15 Healthy volunteers were enrolled into the control group. All patients received conventional therapies, including glucocorticoids, with or without immunosuppressive treatment. The study received approval from the Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School (Ethics number: 2016-027-01) and was carried out in accordance with the principles outlined in the 1989 Declaration of Helsinki. A total of 12 SLE patients and 12 age- and sex-matched healthy controls (HC) were included in the study.
2.2 Isolation and culture of peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated from patients with SLE and HCs by density gradient centrifugation on Ficoll (Amersham Biosciences, Piscataway, USA). The isolated PBMCs were suspended in PBS containing 1% bovine serum albumin (BSA) and 0.1% sodium azide.
2.3 Antibodies and reagents
The following anti-human antibodies were utilized: fluorescein isothiocyanate-conjugated hematopoietic lineage cocktail (eBioscience), phycoerythrin (PE)-conjugated CD1c (eBioscience), CD141 (BD Biosciences, San Jose, USA), allophycocyanin (APC)-conjugated CD11c (eBioscience), PE-Cy7-conjugated human leukocyte antigen-DR (HLA-DR) (eBioscience), BV711-conjugated CD40 (BioLegend, San Diego, California, USA), PE-Dazzle594-conjugated CD80 (BioLegend), TNF-α (BioLegend), APC-Cy7-conjugated CD83 (BioLegend) and CD1c (BioLegend), BV605-conjugated CD86 (BioLegend), BV421-conjugated IL-12 (BD Biosciences), and peridinin-chlorophyll-protein (PerCP)-Cy5.5-conjugated CD11c (BioLegend). Additionally, isotype-matched control antibodies (mouse immunoglobulin G1κ [IgG1κ], mouse IgG2a, mouse IgG2b, Rat IgG1κ) were sourced from eBioscience.
2.4 Flow cytometry analysis
To investigate the changes in surface antigen expression (CD40, CD80, CD83, and CD86), PBMCs from patients with SLE (n = 12) were stimulated with 1 μg/mL lipopolysaccharide (LPS) (Sigma, St. Louis, USA) or 100 μL serum obtained from one SLE patient (No. 1 in Supporting Information: Table S1) and incubated at 37°C for 6 h. To detect the changes in intracellular cytokines (TNF-α and IL-12), PBMCs need permeabilization and only 5 out of 12 patients had enough PBMCs for detection. PBMCs from 5 SLE patients (No. 2, 3, 6, 9, 11 in Supporting Information: Table S1) were stimulated with 1 μg/mL LPS or 100 μL serum obtained from one SLE patient (No. 1 in Supporting Information: Table S1) at 37°C for 6 h, in the presence of 10 μg/mL brefeldin A (Enzo, New York, USA), 20 ng/mL phorbol-12-myristate-13-acetate (Enzo), and 1 μg/mL ionomycin (Enzo) during the final 4 h of stimulation.
For flow cytometry analysis, PBMCs were resuspended in PBS supplemented using 1% BSA and 0.1% sodium azide. To stain the cell surface antigens, PBMCs were incubated on ice for 15 min, with monoclonal antibodies indicated previously, or their respective isotype-control antibodies. To stain intracellular cytokines, PBMCs were permeabilized and fixed before incubation with monoclonal antibodies indicated before, or their isotype-control antibodies for 30 min on ice. The stained cells were analyzed using an LSR Fortessa cytometer (BD Biosciences), and the data were analyzed using FlowJo 10.0 software (Treestar, Ashland, OR, USA).
2.5 Statistical analysis
Statistical analysis was performed using SPSS 22.0 software and GraphPad Prism 4.3. Parametric data were analyzed using t-test or chi-square test, while nonparametric data were analyzed using the Mann−Whitney U-test. A significance level of p < 0.05 was considered statistically significant in this study.
3 RESULTS
3.1 Demographic characteristics
The average age of SLE patients and HCs was 36.8 ± 9.5 years and 33.3 ± 8.1 years, respectively. The sex ratio (female/male) was 9/3 for both the SLE patients and controls. The average SLE disease activity index (SLEDAI) score among the patients was 12.58 ± 3.96, with a range of 8.00–20.00. Detailed demographic characteristics of the subjects can be found in Supporting Information: Table S1.
3.2 CD141+ DCs decrease in SLE
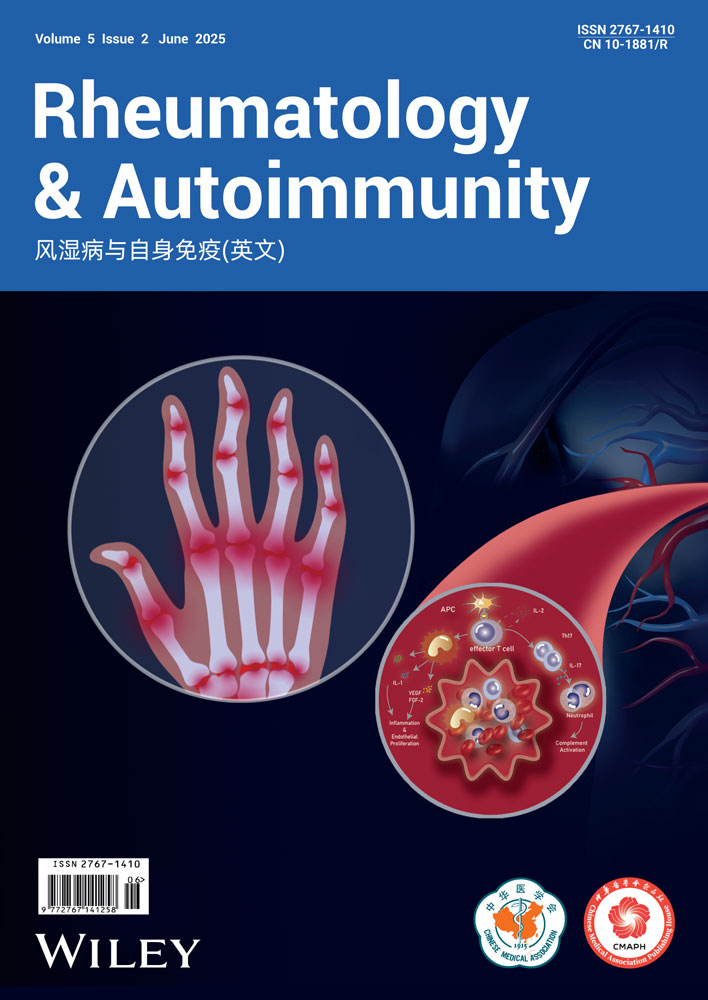
We first conducted an analysis of peripheral blood CD141+ DCs in patients with SLE using flow cytometry. The CD141+ DCs were identified as Lin (CD3/19/56/14)−HLA-DR+CD11c+CD141+ (Figure 1A for the gating strategy). The findings revealed a significant decrease in the number of CD141+ DCs in patients with SLE compared with controls (1.03% ± 0.40% vs. 1.44% ± 0.36%, p < 0.05) (Figure 1B,C). Additionally, a negative correlation was observed between the number of CD141+ DCs and the SLEDAI score (R2 = 0.5547, p = 0.0055) (Figure 1D). The results indicate that CD141+ DCs might be associated with the regulation of SLE pathogenesis.
3.3 CD141+ DCs sustained tolerogenic properties in SLE compared with HC
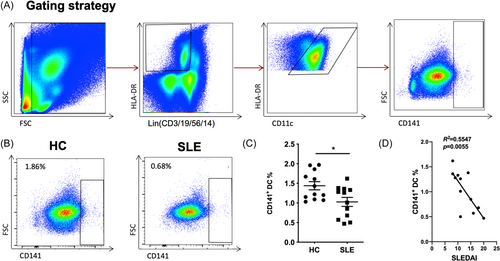
In assessment of the impact of the internal inflammatory environment characteristic of lupus patients on the function of CD141+ DCs, we conducted a comparative analysis of these cells between patients with SLE and HCs. This analysis specifically focused on the expression of functional costimulatory markers, namely, CD40, CD80, CD83, and CD86. PBMCs were cultured from a group of SLE patients (n = 12) as well as from a control group of healthy individuals (n = 12). We quantified the mean fluorescence intensity (MFI) of the CD40, CD80, CD83, and CD86 on CD141+ DCs and compared the values between the two groups. Our findings revealed significantly lower expression levels of CD40, CD80, and CD86 in SLE patients compared to the HCs (2447.00 ± 1386.14 vs. 11,357.25 ± 4581.05, p < 0.001; 1528.00 ± 876.61 vs. 14,181.42 ± 7599.33, p < 0.001; 17,409.42 ± 8180.98 vs. 33,614.25 ± 7408.69, p < 0.001), suggesting a sustained tolerogenic phenotype of CD141+ DCs in SLE (Figure 2).
3.4 CD141+ DCs showed more tolerogenic properties compared with CD1c+ DCs in SLE
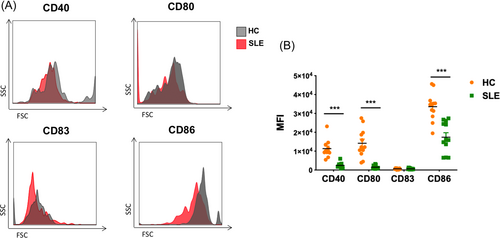
We previously identified CD1c+ DCs as a subset of tolerogenic DCs in SLE,10 and we further found CD141+ DCs sustained tolerogenic phenotype in SLE. Therefore, we compared the tolerogenic properties of the two subsets of cDCs. SLE PBMCs (n = 12) were stimulated with LPS or SLE serum. Before stimulation, there were no significant differences in costimulatory molecule expression between CD141+ DCs and CD1c+ DCs (Figure 3A). After LPS stimulation for 6 h, the expression of CD86 was significantly reduced in the CD141+ DCs compared with CD1c+ DCs (93,526.58 ± 51,763.00 vs. 169,552.00 ± 51,225.74, p < 0.01) (Figure 3B). After SLE serum stimulation for 6 h, the expression levels of CD40, CD80, and CD86 were significantly lower in the CD141+ DCs than that in CD1c+ DCs (2942.17 ± 1140.95 vs. 6877.50 ± 4263.52, p < 0.01; 2412.83.00 ± 2763.03 vs. 9308.50 ± 8926.82, p < 0.05; 107,837.40 ± 26,124.76 vs. 145,450.90 ± 50,436.69, p < 0.05) (Figure 3C). Moreover, cytokine production including TNF-α and IL-12 was detected after LPS or SLE serum stimulation in SLE PBMCs (n = 5). The TNF-α produced by CD141+ DCs were significantly lower than that produced by CD1c+ DCs after LPS stimulation (8.83% ± 3.51% vs. 13.94% ± 2.87%, p < 0.05) or SLE serum stimulation (10.12% ± 2.34% vs. 14.39% ± 2.70%, p < 0.05), whereas the level of IL-12 showed no significant differences between the two DC subsets after LPS or SLE serum stimulation (Figure 3D,E). Collectively, our data demonstrated that in the lupus microenvironment, CD141+ DCs showed more tolerogenic properties compared with CD1c+ DCs.
4 DISCUSSION
Tolerogenic DCs play critical roles in promoting immune tolerance and resolving ongoing immune responses. These specialized cells have the ability to reduce pro-inflammatory cytokine production and increase anti-inflammatory activity. Cytokine production, enhancing the expression of inhibitory molecules, promotes the generation of regulatory T cells (Tregs), thereby facilitating immune tolerance.16-18 Recently, tolerogenic DCs have been used as potential cell-based therapy, showing promise in the treatment of various autoimmune diseases, including type I diabetes, rheumatoid arthritis, multiple sclerosis, and Crohn's disease.19-23 However, despite these advancements, there is limited information available regarding the application of tolerogenic DCs in the treatment of SLE. This may be attributed to a partial understanding of the mechanisms underlying the immunoregulatory effects of tolerogenic DCs in the context of SLE.
Pre-cDCs in humans consisted of two distinct populations of precursors, which were precommitted to becoming either CD1c+ or CD141+ DCs.24, 25 In our previous study, CD1c+ DCs were tolerogenic in SLE. Furthermore, we explored the potential immunoregulatory role of CD141+ DCs in the pathogenesis of SLE.
CD141+ DCs are present in various human tissues, such as lymph nodes, bone marrow, tonsils, and blood samples. Previously, CD141+ DCs have been known for their high expression of toll-like receptor 3, production of IL-12p70 and interferon-β (IFN-β), and their ability to induce Th1 cell responses, surpassing that of CD1c+ DCs in this regard.26 Recently, more and more evidence has suggested that CD141+ DCs might have tolerogenic phenotypes in several conditions. Chu et al.11 identified a unique population of immunoregulatory tissue-resident DCs known as CD141+ dermal dendritic cells (CD141+ DDCs), in human skin. These cells expressed CD141 and CD14 and constantly secreted IL-10, showing characteristics associated with immune tolerance. Researchers have found that Vitamin D3 could enhance the specific phenotypic and functional properties of CD141+ DDCs derived from human blood DCs, which had potential clinical use owing to their capacity to induce immune tolerance. Comi et al.12 discovered a population of tolerogenic DCs in humans coexpressing CD141 and CD163 and produced IL-10. These CD14+CD16+CD141+ CD163+ cells were isolated from the peripheral blood of healthy individuals and displayed spontaneous production of IL-10. Upon activation, they produced limited levels of IL-12. This finding further supports the presence of a tolerogenic CD141+ DC subset. Sachamitr et al.13 demonstrated that human-induced pluripotency stem cells can differentiate into tolerogenic DCs and exhibit similar characteristics to the CD141+ subset. This highlights the potential of utilizing stem cell-derived DCs in therapeutic applications for immune tolerance induction. Tomita et al.14 discovered that the tolerogenic CD141+ DCs could undergo conversion to immunogenic DCs in the tumor environment. This suggests that the tumor microenvironment can modulate the functional properties of CD141+ DCs, shifting them from a tolerogenic to an immunogenic state.
Herein, our study demonstrated that the number of CD141+ DCs in PBMCs of SLE patients was lower compared to HCs. Additionally, the number of CD141+ DCs was negatively correlated with the SLEDAI score, indicating the important role of this DC subset in the pathogenesis of SLE. Furthermore, our findings indicated that CD141+ DCs in patients with lupus exhibited tolerogenic properties, as evidenced by reduced expression of CD40, CD80, and CD86 levels compared to HCs. Stimulation with LPS, CD141+ DCs displayed a greater tolerogenic response than CD1c+ DCs in SLE, characterized by decreased expression of CD86 and reduced production of TNF-α. Moreover, when exposed to SLE serum, CD141+ DCs exhibited a greater decrease in costimulatory molecule expression (CD40, CD80, and CD86) compared to CD1c+ DCs, indicating that CD141+ DCs performed more tolerogenic functions in the inflammatory microenvironment of lupus. The reason for the differences between LPS stimulation and SLE serum stimulation might be associated with pro-inflammatory cytokines in the SLE serum, such as high levels of IFN-α. It was reported that DC differentiation would be induced by IFN-α from SLE serum.27 We would further investigate which cytokine in the SLE serum induces CD141+ DCs to be more tolerogenic in future work. These findings suggest that CD141+ DCs may play a more significant role than the commonly studied CD1c+ DCs in regard to ameliorating immune dysfunction and maintaining immune homeostasis in SLE. This observation could potentially contribute to the development of effective treatments for SLE. Cellular therapy involving the use of CD141+ DCs and the induction of tolerogenic CD141+ DCs in vivo may represent new therapeutic approaches for SLE treatment.
Limitations of this study include the following: (1) The CD141+ DCs are difficult to isolate from PBMCs due to their relatively low number. We want to try to isolate them and investigate their tolerogenic properties directly in future work. (2) The mechanism of how CD141+ DCs differentiate into tolerogenic DCs remains unknown and needs to be further investigated.
In summary, we found that CD141+ DCs tolerated phenotype in SLE patients, and showed more tolerogenic properties compared with CD1c+ DCs, which could be the therapeutic target for SLE patients.
AUTHOR CONTRIBUTIONS
Xiaodong Qin: Conducted the experiments; analyzed the data; wrote the paper. Yujiao Wang: Conducted the experiments. Yi Chen: Collected the blood sample and clinical data. Dong Xie: Collected clinical data. Xinran Yuan: Designed the research and revised the manuscript.
ACKNOWLEDGMENTS
We thank Professor Lingyun Sun for helping with the study design and all the patients and healthy controls for kindly participating in this research. This work was supported by grants from the National Natural Science Foundation of China, Grant/Award Numbers: 81901649, 82002260; Nanjing Postdoctoral Research Funding, Grant/Award Number: 308241.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All of the experimental procedures were performed in compliance with the National Institutes of Health Guidelines and with approval from the ethics committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School (Ethics number: 2016-027-01). All participating subjects provided informed consent for the collection of peripheral blood samples.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.