NETosis: A bridge between cancer and autoimmunity
Edited by Lishao Guo.
Cancer biology recognizes inflammation as a defining characteristic. The activation of an inflammatory response toward a tumor will facilitate the onset and development of cancer, enabling tumor cells to evade immune surveillance. Cancer cells' apoptosis and necrosis create cellular debris, which leads to inflammatory responses and neutrophil recruitment. Neutrophils in the tumor microenvironment (TME) have been shown to help tumors grow and are linked to a worse prognosis in both animal models and cancer patients.
Various external factors in the TME can stimulate the differentiation of neutrophils into distinct populations due to their plasticity and heterogeneity. Tumor-associated neutrophils can be broken down into different subsets. One of these subsets is antitumor neutrophils (N1), which are identified by their expression of interleukin (IL)-12, tumor necrosis factor-α, C-C motif chemokine ligand 3 (CCL3), C-X-C motif chemokine ligand (CXCL)9, and CXCL10. These factors promote CD8+ T-cell recruitment and activation, as well as antitumor immunity. This is different from pro-tumor neutrophils (N2), which can be found by the levels of hepatocyte growth factor, oncostatin M, reactive oxygen species, reactive nitrogen species, matrix metalloproteinases and neutrophil elastase (NE). For instance, transforming growth factor-β was reported to induce protumorigenic polarization of neutrophils.1 These substances contribute to tumor angiogenesis, invasion, and metastasis.2
Tumor-associated neutrophils can further be classified as either high-density neutrophils (HDN) or low-density neutrophils (LDN). Compared with HDNs, LDNs have bigger cells, less ability to phagocytose, less ability to kill tumor cells, less ability to migrate, and less oxidative burst. LDNs demonstrate significant anti-inflammatory and tumor-promoting behavior.3 As a result, HDNs have similar functions to N1 neutrophils, whereas LDNs bear resemblance to N2 neutrophils.
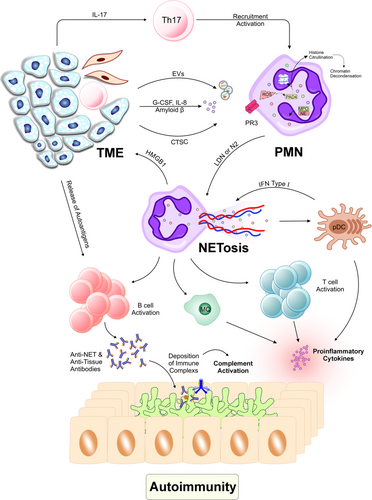
T helper (Th) 17 cells are critical in driving immune responses that can contribute to both cancer progression and inflammation. Th17 cells are a subset of CD4+ T-helper cells characterized by their secretion of pro-inflammatory cytokines, particularly IL-17, which is essential for their function. The differentiation of Th17 cells in the TME is promoted by factors such as transforming growth factor-β, IL-6, and IL-23, which are abundant in many tumors. Once differentiated, Th17 cells release IL-17, which serves as a potent chemoattractant for neutrophils, leading to their recruitment into the TME.4
In TME, amyloid β which is released by cancer-associated fibroblasts with some other factors such as IL-8, granulocyte-common stimulating factor, and tumor-derived extracellular vesicles can induce NETosis.5 Also, cathepsin C interacts with neutrophils through proteinase 3 to activate nuclear factor-kappa B signaling. This activates IL-1β, which increases IL-6 and CCL3, brings neutrophils together, and creates neutrophil extracellular traps (NETs).2 NETs are a specific structure consisting of chromatin and antimicrobial granule proteins such as NE, cathepsin G, myeloperoxidase (MPO), high-mobility group box 1 (HMGB1), peptide LL-37, defensins, DNA, and other components known as alarmins. These endogenous alarmins ought to be classified into a huge family of molecular patterns associated with damage.
NET synthesis promotes tumor growth through a positive feedback loop.6 NETs cause tumor cells to produce more HMGB1, a pro-inflammatory factor, and activate Toll-like receptor (TLR) 9-dependent pathways, which help the tumor grow and spread.7 The TLR4/9-COX2 pathway is activated by NET components binding to tumor cells, making them more resistant to death; in the case of hepatocellular cancer, DNase I and anti-inflammatory medicines can reduce metastasis.8 Additionally, NETosis-derived HMGB1 increases tumor IL-8 secretion. By connecting to tumor cell receptor for advanced glycation end-products, HMGB1 activates the nuclear factor-kappa B signaling pathway.9
Autoimmune disorders develop when immunological B and T cells mistake body proteins for their own and attack them, causing tissue and organ damage. Autoimmunity can be caused by a variety of reasons, including chemicals, infections, and genetics. NETs play a vital role in eliminating infections, but they also transport autoantigens, such as DNA and histones, to the immune system. This makes them important targets for autoimmune disorders. NETosis causes extensive cell disruption and interacts with cellular components. If this phenomenon is combined with inflammation, it can potentially stimulate the initiation and progression of autoimmunity.10
Cancer and autoimmunity crosstalk have been well-established. Cancer-induced autoimmunity is associated with the onset of several autoimmune disorders, including rheumatic diseases11, 12 and paraneoplastic cerebellar degeneration,13 among others. These disorders may be partially attributed to the autoantibodies directed against tumor antigens that are concurrently expressed in healthy tissues. Antibodies can be produced by the intracellular compounds released from apoptotic tumor cells or by mutated proteins that elicit cross-reacting antibodies to their non-mutated counterparts.14 For example, Joseph et al.15 showed that antibodies targeting a tumor-specific mutant of RNA polymerase III subunit (RPC1) cross-reacted with normal RPC1, which plays a role in the autoimmune scleroderma. In another study, Broecker et al.16 found that chemotherapy can induce self-reactive IgM targeting various organs.
Tissue damage from unregulated NETosis can result in inflammation or life-threatening diseases.17 Researchers have observed that the presence of NETs within tumors correlates with poor outcomes in cancer patients. NETs may promote tumor growth by increasing proliferation and suppressing the apoptosis of malignant cells.18 It has been shown that increased NET levels (measuring serum MPO-DNA molecules) correlate with the metastatic phenotype in colorectal cancer patients.19 Furthermore, the concentrations of circulating NE–DNA complexes escalated with the progression of breast and stomach malignancies.20, 21 Elevated concentrations of plasma biomarkers indicative of active neutrophils and NETs, including circulating cell-free DNA, NE, and citrullinated histone 3, have recently been seen in a murine model of pancreatic cancer.
In cancer patients, NETosis in the TME is a key source of anti-neutrophil antibodies (ANCAs), which can be produced by various tumor types. Mutations that emerge in cancer might be able to start an immune reaction against autoantigens, which may clarify the emergence of ANCAs.22 Progressive cancers have a network between MPO, NETs, and ANCAs. For example, MPO, a cationic molecule, binds to many negatively charged surfaces that cause the production of ANCAs. Studies have demonstrated that proteinase 3 and MPO trigger ANCAs during NETosis, leading to the activation of the complement system and damage to endothelial cells.23 On the other hand, in many SLE patients, the generation of ANCAs is associated with NETosis.24 Therefore, the production of autoantibodies has a connection to the pathogenesis of both conditions.
The exact mechanisms by which NETosis contributes to the stimulation of autoimmunity in cancer patients remain unclear. However, several potential contributing factors can be hypothesized. These include chronic inflammation, cancer immunotherapy, and tumor immunoediting, all of which may lead to the generation of autoantigens. In cancer patients, persistent NETs can stimulate other inflammatory cells, such as plasmacytoid dendritic cells, thereby promoting further NETosis and inflammation. pDCs by various receptors can identify autologous DNA and produce type I interferons and pro-inflammatory cytokines, which may drive autoinflammatory responses and immune complex-mediated autoimmune disease.25 Moreover, the generation of autoantigens, either due to mutations or as a consequence of immunotherapy, can activate neutrophils to release NETs. Collectively, these pathways may contribute to the pathogenesis of cancer-induced autoimmunity through NETosis (Figure 1).
AUTHOR CONTRIBUTIONS
Niloufar Orooji: Writing—original draft; conceptualization. Manouchehr Fadaee: Review and editing, supervision.
ACKNOWLEDGMENTS
This study was provided by Tabriz University of Medical Sciences.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not Applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not Applicable.