Investigation of COVID-19 infection in patients with polymyalgia rheumatica during the predominance of the omicron variant
Edited by Zhiyu Wang and Lishao Guo.
Abstract
Background
Polymyalgia rheumatica (PMR) is an inflammatory disease that affects the older adult population. The aim of this study was to investigate the risk and prognosis associated with the coronavirus disease 2019 (COVID-19) infection among patients diagnosed with PMR during the predominance of the Omicron variant.
Methods
In this retrospective study, we included a cohort of patients with PMR who met the 2012 European League Against Rheumatism/American College of Rheumatology classification criteria or the 1982 PMR diagnostic criteria and tracked their progress over time. The diagnosis of COVID-19 was based on the clinical manifestations and laboratory tests. We collected demographic information, PMR disease activity, treatment data, and clinical data related to COVID-19.
Results
In total, 101 patients diagnosed with PMR were enrolled. Most patients with PMR exhibited low disease activity. Of the total cohort, 81 patients (80.2%) were categorized as individuals diagnosed with COVID-19, while the remaining 20 (19.8%) were not diagnosed with COVID-19. Among the patients with PMR diagnosed with COVID-19, 65 (80.2%) exhibited the presence of the COVID-19 antigen, while 16 (19.8%) tested positive for COVID-19 RNA. Most COVID-19 patients with PMR were classified as having mild disease (72, 88.9%). Two cases were identified within the confirmed infected group, resulting in a recurrence rate of 2.5% (2/81). Conversely, no relapses were observed in the non-infected group (0/20). In our multivariate logistic regression analysis, we found that pre-COVID-19 PMR disease activity was an independent risk factor for COVID-19 infection (odds ratio = 30.00, 95% confidence interval: 2.137–421.117, p = 0.012).
Conclusion
The increased susceptibility to COVID-19 may be influenced by the pre-existing disease activity of PMR.
Key points
-
The pre-existing disease activity of polymyalgia rheumatica was an independent risk factor for COVID-19 in these patients.
-
After contracting COVID-19, patients with polymyalgia rheumatica experienced a low proportion.
1 INTRODUCTION
Polymyalgia rheumatica (PMR) is an inflammatory disease characterized by pain and morning stiffness in the neck, shoulder, and pelvic girdles. It is often accompanied by elevated acute-phase reactants and may manifest as systemic symptoms such as fatigue, fever, and weight loss. PMR significantly impairs daily functioning and diminishes the quality of life. The incidence of PMR is age-related, with a higher occurrence in individuals older than 50 years, whereas it is rare in individuals younger than 50 years. Differentiating between PMR and the onset of rheumatoid arthritis in older adults based on clinical manifestations can sometimes be challenging. However, the underlying pathogenesis of these two diseases is distinct.1, 2 Although there is no comprehensive global data on PMR incidence, various studies have reported a prevalence rate ranging from 0.37% to 1.53%.3 Unfortunately, epidemiological data regarding PMR in China are lacking.
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, more than 700 million infections have been reported worldwide.4 According to a retrospective cohort study utilizing data from the COVID-19 Global Rheumatology Alliance physician registry, individuals diagnosed with PMR exhibited greater susceptibility to severe COVID-19 outcomes and experienced higher mortality rates than patients with rheumatoid arthritis.5 Until December 2022, China had significantly limited COVID-19 cases owing to stringent quarantine and control measures. However, after the easing of the control measures, there was a noticeable rise in the number of reported COVID-19 cases. Significant global attention has been paid to the subsequent surge in infections, particularly in patients undergoing PMR. Although the pathogenesis of PMR is not fully understood, it is believed to be influenced by a range of factors, including genetics, infection, injury, and immunity. Activation of the secondary immune system triggered by COVID-19 can affect PMR development.6 Additionally, the chronic inflammatory state and immunosuppressive treatments used in the management of PMR may influence the risk and outcomes of COVID-19 in patients with this condition.
As the COVID-19 pandemic persists, there has been growing interest among researchers and clinicians in understanding the connection between rheumatic diseases and COVID-19. This study aimed to examine the incidence and outcomes of the Omicron pandemic in patients with PMR and the influence of the pandemic on PMR management. By investigating the infection risk and prognosis of COVID-19 in patients with PMR, we aimed to gain valuable insights into the impact of COVID-19 on individuals with PMR and enhance our understanding of this complex relationship.
2 METHODS
2.1 Study design and participants
This study was a single-center, retrospective, cross-sectional observational analysis conducted using an inventory survey of COVID-19 infections among patients diagnosed with PMR. All participants were patients diagnosed with PMR at the First Affiliated Hospital of Zhejiang University School of Medicine between January 1, 2022, and February 19, 2023. The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (No. IIT20200070C-R1). Informed consent was obtained from all participants.
2.2 Data collection
All patients diagnosed with PMR met the 2012 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria7 or the 1982 PMR diagnostic criteria.8 This study aimed to analyze various aspects of the patients under investigation, including their demographic characteristics, comorbidities, current disease activity, treatment strategies, and presence of COVID-19 infection. For patients diagnosed with COVID-19, information was gathered regarding pathogen diagnosis, disease severity, treatments administered, treatment outcomes, and the occurrence of PMR disease relapse. All relevant data were extracted from medical records spanning the period between January 1, 2022, and February 19, 2023.
Rheumatologists evaluated the stages of PMR before and after the COVID-19 infection. For patients with acute-phase reactant data within 1 month before the COVID-19 pandemic, the PMR activity score (PMR-AS) was used to assess disease activity. PMR-AS values < 7 indicated low disease activity, 7–17 indicated medium disease activity, >17 indicated high PMR activity, and 0–1.5 indicated remission.9, 10 The clinical diagnosis of COVID-19 followed the guidelines outlined in the 10th edition of the diagnosis and treatment protocol for COVID-19.11 These guidelines consider clinical symptoms and relevant pathogenic or serological examination results. COVID-19 severity was classified according to an approved protocol.
2.3 Outcomes
The primary objective of this study was to assess the rate of COVID-19 infection and its severity among patients with PMR. As a secondary objective, we examined the alteration in disease activity in patients with PMR following COVID-19 infection, which required rheumatologists to differentiate between symptoms related to PMR and those induced by COVID-19.
2.4 Statistical analysis
Statistical analysis was performed using SPSS Statistics 26.0 software (IBM Corp). The demographic characteristics and laboratory data of the participants were analyzed. The Kolmogorov–Smirnov test was used to assess the normal distribution of the measurement data. Normally distributed data were presented as mean ± standard deviation, and group comparisons were made using a t-test. For nonnormally distributed data, the median (p25, p75) was reported, and the Mann–Whitney U-test was used for group comparisons. Categorical data between the groups were compared using the chi-square test or Fisher's test. Relevant risk factors were screened using logistic univariate regression analysis, and variables with statistical significance were included in logistic multivariate regression analysis. A p-value < 0.05 was considered statistically significant.
3 RESULTS
3.1 Participant characteristics
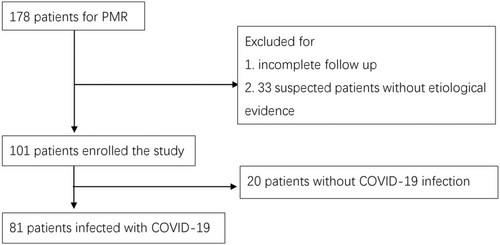
A total of 178 patients diagnosed with PMR were included in this study. However, after excluding those with incomplete follow-up and 33 patients with PMR suspected of being infected with COVID-19 but without definitive evidence, the final investigation included 101 patients with a mean age of 66.98 ± 8.04 years. In the final cohort, 81 patients (80.2%) were found to be infected with COVID-19 (Figure 1). Thirty-two patients (31.7%) had comorbidities, and the prevalence between the two cohorts had no significant difference (p = 0.324; Table 1). Among the PMR patients, 25 individuals (24.5%) had hypertension, two (2.0%) had cardiovascular diseases (excluding hypertension), 10 (9.8%) had diabetes mellitus, and nine (8.8%) had respiratory diseases such as interstitial pneumonia and bronchitis.
| Characteristics | Total (n = 101) | Noninfection (n = 20) | Confirmed infection (n = 81) | p-Value |
|---|---|---|---|---|
| Age (years) | 66.98 ± 8.04 | 67.20 ± 8.15 | 66.86 ± 8.03 | 0.868 |
| Gender | 1.000 | |||
| Female | 81 (79.4) | 16 (80.0) | 64 (79.0) | |
| Male | 21 (20.6) | 4 (20.0) | 17 (21.0) | |
| Height (cm) | 161.33 ± 7.55 | 156.83 ± 5.71 | 163.13 ± 7.59 | 0.084 |
| Weight (kg) | 59.41 ± 9.61 | 51.97 ± 6.23 | 62.21 ± 9.27 | 0.022 |
| BMI (kg/m2) | 22.51 ± 2.92 | 21.12 ± 2.19 | 23.07 ± 3.06 | 0.127 |
| Comorbidities | 32 (31.7) | 4 (20.0) | 28 (34.6) | 0.324 |
| Hypertension | 25 (24.5) | 2 (10.0) | 23 (28.4) | 0.156 |
| Diabetes mellitus | 10 (9.8) | 3 (15.0) | 7 (8.6) | 0.664 |
| CVD except for hypertension | 2 (2.0) | 0 | 2 (2.5) | 1.000 |
| Respiratory diseases | 9 (8.8) | 2 (10.0) | 7 (8.6) | 1.000 |
- Note: Data are expressed as n (%) or mean ± standard deviation.
- Abbreviations: BMI, body mass index; CVD, cardiovascular disease.
Table 1 provides a comprehensive overview of the patient characteristics. In the cohort, 81 individuals (79.4%) were female, and it was observed that patients infected with COVID-19 tended to have a higher average weight compared to those without COVID-19 (62.21 ± 9.27 kg vs. 51.97 ± 6.23 kg, p = 0.022).
3.2 COVID-19 infection status
Of the 101 patients, 81 (80.2%) were infected with COVID-19. Among the infected patients, 65 (80.2%) were diagnosed using the antigen test COVID-19, while 16 (19.8%) were diagnosed using the RNA test for COVID-19. Most patients (72, 88.9%) experienced mild symptoms, nine patients (11.1%) experienced moderate symptoms, and none progressed to a severe or critical stage. The most commonly reported symptoms were fever (72, 88.9%), cough (73, 90.1%), and sore throat (1, 1.2%). Fever was typically brief, lasting 1–2 days, whereas coughing persisted for a longer duration of 2–4 weeks. Among the patients who underwent chest imaging (44 patients), pneumonia was observed in nine patients. In terms of therapy, most patients (n = 77, 95.1%) opted for symptomatic treatment, such as the use of ice or nonsteroidal anti-inflammatory drugs (NSAIDs) for cooling relief, and four (4.9%) patients used glucocorticoids such as methylprednisolone. Most patients experienced relief through home rest or outpatient treatment, with only three patients requiring hospitalization.
3.3 Association between patients with PMR and COVID-19 infection
In the context of the association between PMR and COVID-19, it was noted that 84 (83.2%) patients with PMR were receiving treatment with low dosage of glucocorticoids like prednisolone or methylprednisolone (7.26 ± 4.56 mg/day prednisolone equivalent), while 13 (12.9%) were utilizing targeted synthetic disease-modifying anti-rheumatic drugs (tsDMARDs) such as tofacitinib. Before contracting COVID-19, all patients were in remission or had low disease activity. Eleven (10.9%) were in remission, whereas the remaining 90 (89.1%) had low disease activity. In general, patients with PMR with low disease activity were more susceptible to COVID-19 than those in remission (p = 0.007). Among 101 patients, two (2.0%) experienced relapse following COVID-19 infection (Table 2). Both cases were identified within the confirmed infected group, resulting in a recurrence rate of 2.5% (2/81). Conversely, no relapses were observed in the non-infected group (0/20). One patient discontinued glucocorticoid treatment, while another required an increased glucocorticoid dosage.
| Items | Total (n = 101) | Noninfection (n = 20) | Confirmed infection (n = 81) | p-Value |
|---|---|---|---|---|
| Treatment about PMR | 0.848 | |||
| Glucocorticoid | 84 | 16 | 68 | |
| tsDMARDs | 13 | 3 | 10 | |
| NSAIDs | 2 | 1 | 1 | |
| Others | 2 | 0 | 2 | |
| Pre-COVID-19 PMR's disease activity | 0.007 | |||
| Moderate, high, or severe | 0 | 0 | 0 | |
| Low | 90 | 13 | 74 | |
| Remission | 11 | 7 | 7 | |
| Post-COVID-19 PMR's disease stage | 0.794 | |||
| Relapse | 2 | 0 | 2 | |
| Decrease the dosage of GCs | 1 | 0 | 1 | |
| Increase the dosage of GCs | 1 | 0 | 1 | |
- Abbreviations: COVID-19, coronavirus disease 2019; GCs, glucocorticoids; NSAIDs, nonsteroidal anti-inflammatory drugs; PMR, polymyalgia rheumatica; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs.
Incorporating age, gender, height, weight, body mass index (BMI), comorbidities, PMR treatment, and pre-existing disease activity of PMR before COVID-19 infection, the univariate logistic regression analysis revealed significant associations between COVID-19 infection and four parameters, including age (p = 0.049), weight (p = 0.030), diabetes mellitus (p = 0.022), and pre-COVID-19 PMR's disease activity (p = 0.004). Multivariate logistic regression analysis of the aforementioned factors revealed that pre-COVID-19 PMR disease activity was an independent risk factor for COVID-19 infection (odds ratio [OR] = 30.00, 95% confidence interval [CI]: 2.14–421.12, p = 0.012).
4 DISCUSSION
This single-center study focused on patients with PMR during the COVID-19 pandemic in December 2022. Unlike previous studies that have primarily focused on assessing the influence of rheumatic diseases on COVID-19 severity, this study uniquely investigated the risk and severity of COVID-19 in patients with PMR. The research was carried out at a time when the Omicron variant was prevalent, during the implementation of China's “zero COVID” policy. This provided an opportunity to explore the relevant clinical features of particular interest during this period.
In our study, the COVID-19 infection rate among the patients with PMR was 80.2%. A comparison with two other concurrent studies conducted in China revealed that the infection rate was 77.7% (4007/5160) in the general population12 and 84.3% (1690/2005) in the population with autoimmune inflammatory rheumatic diseases.13 Our data fall within the ranges observed in these studies. Interestingly, the infection rate among patients with PMR was higher than that observed in the general population but lower than that reported in a previous study.13 This disparity could potentially be attributed to the geographical locations where the studies were conducted; the latter study was conducted in Beijing, whereas ours was conducted in Zhejiang, situated in the north and south of China, respectively. Differences in COVID-19 strains, climatic conditions, and population densities between these regions may have influenced the observed infection rates.
In our study, we found that patients with PMR had a lower likelihood of developing viral pneumonia, as only nine patients progressed to this condition. Additionally, the non-hospitalization rate of patients with PMR in our study was 96.3%. Most patients (88.9%) experienced only mild symptoms. These findings differ from those of previous studies14 that reported a higher probability of moderate-to-severe viral pneumonia in patients with rheumatic diseases. For instance, Sattui et al.15 demonstrated that patients with primary systemic vasculitis or PMR had higher odds of experiencing worse COVID-19 outcomes if they had high or severe disease activity as opposed to remission or low disease activity. In their study, the non-hospitalization rate was 57.9% (187 out of 323). However, in our study, pre-COVID-19 disease activity in patients with PMR was either low or in remission. One possible explanation for these divergent findings is the presence of relatively milder COVID-19 strains, particularly the omicron variant, which was prevalent during the study period. Most previous studies were conducted when wild-type strains were predominant. In the era of “Long COVID,” the effects of different COVID strains require further research. Additionally, the scarcity of medical resources during the pandemic may have influenced the limited availability of comprehensive imaging tests for patients, potentially affecting our ability to identify instances of viral pneumonia.
Most individuals initially experienced common symptoms such as fever, cough, and sore throat. However, the fever typically resolved relatively quickly, lasting only 1–2 days, whereas coughing persisted for a longer duration, for approximately 2–4 weeks. These clinical features are consistent with those typically observed in Omicron infections. Overall, our study suggests that patients with PMR with low or remission disease activity may have a more favorable outcome when infected with COVID-19, particularly considering the milder variant strain prevailing during our research and the limitations of comprehensive diagnostic testing during the pandemic.
Our study revealed that pre-COVID-19 disease activity in the PMR was an independent risk factor for contracting COVID-19. Individuals in remission are less susceptible to COVID-19 than those with lower disease activity. During the pandemic, the majority of individuals were infected with COVID-19, and the likelihood of exposure to it was comparable for patients with PMR. Previous studies have indicated a direct link between infection risk and disease activity in patients with various autoimmune diseases such as SLE16 and ANCA-associated vasculitis.17 A study involving idiopathic inflammatory myopathy patients revealed a connection between higher disease activity and severe COVID-19 outcomes.18 Given the heightened risk of COVID-19 infection across cohort during the pandemic, we hypothesize that disease activity in PMR may be associated with an increased risk of COVID-19 infection. This difference in susceptibility may be attributed to the immunosuppressive effects of glucocorticoids or other immunosuppressive therapies. Moreover, differences in adherence to protective measures may contribute to varying infection rates. Patients in remission may underestimate the necessity of taking precautions, whereas those with low disease activity are likely to be more vigilant due to their underlying PMR condition.
In our study, we observed two cases in which the patients experienced a relapse of PMR along with COVID-19. Several reports have documented new onset or recurrence of PMR following COVID-19 infection19 or vaccination.20 It is speculated that the virus triggers the activation of monocytes and dendritic cells, leading to the production of pro-inflammatory cytokines and potentially causing PMR symptoms. Imbalance resulting from the overproduction of interleukin-6 during the “cytokine storm” may cause the recurrence of PMR.21 However, the pathophysiological mechanisms underlying this association remain unclear.
However, this study has several limitations that should be acknowledged. First, this was a single-center study conducted in a specific location, which may have restricted the applicability of the results to a broader population. Second, the sample size in this study was relatively small, which increases the potential for bias. To gain a broader and more reliable understanding of the relationship between PMR and COVID-19, future prospective studies should incorporate a multicenter approach and include a larger sample size. This enables a more comprehensive examination of PMR within the context of COVID-19.
In summary, in comparison to two other concurrent studies conducted in the general population of China, in our study, patients with PMR exhibited a relatively lower infection rate and experienced less severe cases of COVID-19. The reduced susceptibility to COVID-19 in individuals with PMR may be influenced by the pre-existing disease activity of PMR before being infected with COVID-19.
AUTHOR CONTRIBUTIONS
Xinlei Ma, Lanlan Xiao, Jinzhi Wu, Guanhua Xu, and Jin Lin participated in the main manuscript. Weiqian Chen prepared Figure 1. All authors reviewed the manuscript. Furthermore, all authors have provided their approval for the final version of the article to be published.
ACKNOWLEDGMENTS
The authors would like to thank the participants who generously gave their time to take part in this study. This work was supported by the Natural Science Foundation of China, Grant/Award Number: 82171768, the National Key Research and Development Program of China, Grant/Award Number: 2022YFC3602000, and the Zhejiang Province Excellent Young Talent Fund for Traditional Chinese Medicine, Grant/Award Number: 2022ZQ051.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (No. IIT20200070C-R1). Informed consent was obtained from all participants.
Open Research
DATA AVAILABILITY STATEMENT
The data were available upon reasonable request.