Exploring salivary biomarkers and swallowing perceptions in Sjogren's syndrome: A case–control feasibility study
Stacey A. Skoretz is the senior author.
Abstract
Background
The autoimmune disease Sjogren's syndrome (SS) is often characterized by salivary changes that may affect swallowing. No known study has investigated the association between salivary biomarkers and perceptions of swallowing in individuals with SS. Our objectives were to explore: (1) the operational feasibility of investigating saliva volume and composition, along with perceptions of swallowing, in those with and without SS; and (2) the relationship between saliva composition and perceptions of oral dryness, swallowing, and quality of life.
Methods
We conducted a prospective, case–control feasibility study, collecting feasibility data (recruitment rate and optimal saliva collection methods) and whole mouth saliva samples (unstimulated and stimulated). We measured total protein content and conducted sialochemical (α-amylase, cortisol, C-reactive protein [CRP], and mucins), sialometric (flow rate), and perceptual (oral dryness and swallowing-related quality of life [SWAL-QOL]) assessments. Our exploratory analyses focused on the main and fixed effects. We summarized all data descriptively, comparing: (a) outcomes between groups (t tests or Mann–Whitney U) and (b) salivary and perceptual data across participants (partial least-squares correlation [PLSC]).
Results
We enrolled 12 (N) participants (6 per group), all providing analyzable saliva. Cases had lower flow rate (p = 0.003) and higher total protein, cortisol, and CRP concentrations (p < 0.02) than controls. PLSC revealed inverse covariance between sialochemical and SWAL-QOL data across participants.
Conclusion
Our study was feasible as designed. We explored novel relationships between salivary outcomes and participant-reported perceptions, distinguishing individuals with and without SS. Our findings support further study of saliva's role in perceptions of swallowing, specifically analytes with lubricative properties.
Key points
-
Our successful feasibility study distinguished individuals with and without Sjogren's syndrome (SS) according to novel relationships between salivary properties and participant-reported perceptions of swallowing.
-
Salivary analyte profiling in SS may enable biomarker-based approaches to early identification, differential diagnosis, and bespoke treatment of dysphagia, while also informing the diagnostic process for SS and other chronic, autoimmune conditions.
1 INTRODUCTION
Sjogren's syndrome (SS) is the second most prevalent autoimmune disorder,1, 2 affecting ~35 million individuals globally.3 A disease of the exocrine system, SS impairs the lacrimal and salivary glands leading to ocular and oral dryness, reduced saliva volume and/or flow rate (hyposalivation), and an altered proteomic (protein) composition.4, 5 Inflammatory triggers may include environmental factors (e.g., virus), family history (i.e., autoimmune), and/or hormonal influences.6, 7 SS typically presents later in life (age 40+ years) with a female preponderance (90%),8 often co-occurring with other autoimmune conditions (e.g., rheumatoid arthritis).9, 10 Due to its multifactorial etiology, comorbidities, and nonspecific symptoms,8, 11 SS is often unrecognized, thereby delaying diagnosis and treatment.12, 13 Irrespective of comorbidities and diagnostic timing, xerostomia (dry mouth) and dysphagia (swallowing difficulties) often occur in SS, affecting oral health and quality of life. Due to SS disease processes, salivary changes are likely primary contributors to swallowing difficulties in this population.14
Saliva is affected by systemic (e.g., disease) and/or environmental (e.g., stressors) changes, some of which are readily detectable through salivary analysis.15, 16 For example, C-reactive protein (CRP) is a general marker of inflammation17 and cortisol is a hormone involved in the body's stress response.18, 19 Relevant to swallowing, α-amylase has enzymatic properties that support early carbohydrate digestion.15 In addition, mucins and α-amylase contribute to mucosal lubrication and protection, along with bolus cohesion and formation during swallowing.16, 20 Individuals with SS often present with hyposalivation10 and a unique salivary profile.21 Relative to healthy individuals, those with SS have saliva characterized by highly concentrated proteins,22 variable CRP concentrations,23 and lower concentrations of α-amylase.24 In effect, proteomic changes in saliva likely have implications for swallowing physiology and perception, and therefore quality of life. Collectively, salivary analytes may be used to understand an individual's health and, depending on timing of collection, may provide information regarding the current status (e.g., stress).18, 19 In some circumstances, an individual may perceive subtle changes in saliva before conventional detection and/or disease progression.25
Oral intake is intrinsically linked to quality of life.26 In those with SS, quality of life is inversely correlated with disease and dysphagia severity.14, 27 Furthermore, perceived oral dryness in SS may precede salivary protein changes that influence swallowing sensation and perception, and by extension, quality of life.28 Existing investigations of saliva and swallowing in SS largely focus on general volumetric analyses, rather than individual perceptions.25 Investigating the relationship between saliva composition and perceptions of swallowing in SS may facilitate earlier and individualized treatment measures. Volumetric and compositional differences in the saliva of those with and without SS are identified,9, 29-34 yet it remains unknown whether SS-related proteomic changes are associated with swallowing and quality of life; particularly, whether potential patterns exist between salivary analytes and participant-reported perceptions of swallowing. To date, no known study has explored the link between salivary biomarkers and perceptions of swallowing in those with SS. We hypothesized that: (1) we would be able to measure analytes and perceptions, and (2) in the absence of dysphagia, those with SS perceive changes in their swallowing secondary to an altered salivary proteome. Our primary objective was to explore the operational feasibility of determining the volume and composition of saliva in individuals with and without SS, as well as the collection of participant-reported perceptions of swallowing. Our secondary objective explored the relationship between saliva composition and participants' perceptions of oral dryness, swallowing, and quality of life.
2 METHODS
2.1 Design and participants
Following institutional ethics board approval, we conducted a prospective feasibility study using a case–control design. Feasibility studies explore novel procedures and outcome measures,35-37 extend and/or generate hypotheses,38 and inform future randomized controlled trials.39-41 Our primary feasibility aims were to determine study recruitment rate and optimal saliva collection methods. Our feasibility thresholds were: (i) a convenience sample of ≥10 participants (≥5 per group) during our 4-month recruitment period and (ii) analyzable saliva collected via at least one method. We conducted the following: sialometric and sialochemical analyses, oral mechanism examinations, and oral dryness and quality-of-life assessments. Our secondary aim explored the relationship between salivary properties and individual perceptions.
Following open call recruitment, we included adult (≥18 years) participants fluent in English. Eligible case participants were individuals diagnosed with SS. Healthy controls were age- (±5 years) and sex-matched. We excluded those with prior dysphagia, radiotherapy or surgery for head and/or neck cancer, neurological/neurodegenerative disease, smoking (within 10 years), and medication-induced hyposalivation. We collected demographic information (i.e., sex, age, date of birth, medical diagnoses, medications, and smoking history) and SS-specific variables (i.e., date of diagnosis, diagnostic test, and autoimmune comorbidities) in accordance with the international classification criteria.42
2.2 Sialometric and sialochemical analyses
We collected whole mouth saliva samples after 1500 h, to account for diurnal rhythms.43 Each matched pair provided saliva within 30 min of the collection time of their counterpart, however, on a different day. Ninety minutes before saliva collection, participants refrained from eating, drinking, chewing gum, brushing teeth, and flossing,44 and if applicable, discontinued saliva substitutes, oral moisturizers, and/or salivary replacement medications 48 h in advance.25 Adhering to established saliva sampling protocols,44-47 each participant provided three samples: two unstimulated and one stimulated. Unstimulated whole saliva (UWS) samples were collected with two devices (Salimetrics LLC): saliva collection aid (UWS-A) and SalivaBio oral swab (UWS-B). For UWS-A, participants expectorated oral fluid every 30 s into a saliva collection aid, letting it drain into an attached cryovial.44-46 UWS-B samples were collected using an oral swab placed under the tongue and, once saturated, the swab was placed into a swab storage tube.47 Stimulated whole saliva (SWS) was collected via mastication of a new swab, placed in a new storage tube once saturated.47 The endpoint for each sampling period was 5 min followed by a 2 min rest.
We conducted sialometric analyses, weighing all samples (g; converted to ml) using a laboratory balance (Mettler Toledo), and measured acidity (UWS-A only; pH indicator strips [range: 4.5–9.0]; HealthyWiser®). We then centrifuged (2190g, 10 min, 4°C) all samples and prepared aliquots stored at −25°C until sialochemical analysis. We determined aliquot volumes according to total sample volume and to limit freeze/thaw cycles.
Following enrollment period closure, we thawed all samples and conducted enzyme-linked immunosorbent assays for the following analytes: α-amylase, cortisol, CRP (Salimetrics, LLC); mucins (MUC5B, MUC7; Cloud-Clone Corp.), and total protein content (bicinchoninic acid protein assay; Thermo Fisher Scientific). We measured total protein, α-amylase, CRP, and cortisol in all samples. As mucins cannot be analyzed from swabs,47 we measured mucin concentrations in UWS-A samples only. Assays were conducted according to kit manufacturer standard protocols with specific minimum detectable concentrations (α-amylase: 3.1 U/ml, cortisol: 0.028 μg/dl, CRP: 10 pg/ml, mucins: 0.115 ng/ml); intra- and interassay coefficients of variation were <12% for all assays. We analyzed all samples simultaneously (per testing analyte) and in duplicate (volume permitting). Based on our secondary objective, we prioritized analytes with specific roles in swallowing, and therefore CRP assays were run in singlicate. For total protein, we analyzed all samples at a 2-fold dilution, re-running outputs above the upper limit of detection (ULD) at a 5-fold dilution (21-fold for 1 sample with limited volume).
2.3 Oral and quality of life assessments
According to published protocols for the (1) Oral Speech Mechanism Screening Examination-Third Edition (OSMSE-3),48 (2) Clinical Oral Dryness Score (CODS),49 and 3) SWAL-QOL (swallowing quality of life) questionnaire,50 a trained speech-language pathologist (VHL) assessed all participants. Using the OSMSE-3 to rule out oral-motor impairment, we evaluated oropharyngeal structures, breathing, and syllable repetition.48 We then assessed the appearance and adhesiveness of saliva and oral structures using the CODS.49 Participants completed the 44-item SWAL-QOL,50 with 10 quality of life domains (e.g., burden) and a dysphagia symptom domain.
2.4 Analyses
2.4.1 Descriptive analysis and data correction
We summarized all data using means (SD), medians (interquartile ranges [IQRs]), and ranges, as appropriate. To preserve participant anonymity given the specificity of SS and our small sample, we summarized select demographic information using frequencies and ranges. Sialometric, sialochemical, and salivary acidity data were summarized descriptively according to collection method and group. When control subject saliva sample volume was insufficient for analyte testing, values were replaced with the analyte-specific group mean value. For all participants, analyte-specific values that fell above the ULD were replaced with the upper limit. Outliers remaining below the ULD were Winsorized and replaced with the next highest (analyte-specific) value.51 When not replaced, analyte values were expressed per unit of total protein (MUC5B and CRP concentrations divided by total protein content).
We summarized OSMSE-3, CODS, and SWAL-QOL data consistent with published methods.48-50 Following OSMSE-3 score summation, we reported an overall pass/fail for each participant. We summarized CODS data using frequency counts for individual items and total scores for each group and the overall sample. Using the 5-point Likert ratings for each SWAL-QOL item, we summarized domain and total scores (0–100; least to most favorable) for the overall sample and each group with means. To derive each domain score, we divided summed domain-item scores by the number of items within each domain.52 We divided summed domain scores by 10, to determine total SWAL-QOL scores.50 For the symptom domain, we calculated group means for each of the 14 items/symptoms.52
2.4.2 Exploratory analyses
Given our feasibility focus, all comparisons were exploratory with the main and fixed-effect models. When normally distributed, we compared group means using an independent-samples t test. For those data not normally distributed, we used Mann–Whitney U tests. Given insufficient volumes for some unstimulated samples, we conducted multivariate analyses using SWS sample data (all participants). Accounting for differences in rating styles, we applied row-wise normalization by participant for each SWAL-QOL item before multivariate analysis. We then centered and normalized (column-wise; variance of 1) SWAL-QOL and CODS data.
Posthoc, we utilized partial least-squares correlation (PLSC) to model the relationship between patterns of sialochemical data and patterns of SWAL-QOL data. A data-driven multivariate latent-variable approach, PLSC maximizes the covariance between these data and identifies a new set of latent variables that best explain their covariance.53 Sialometric and CODS data were projected into the sialochemical–SWAL-QOL space as supplementary variables (to aid latent variable interpretation, although not part of the PLSC model). Given our small sample and few observations (N) in comparison with a large number of variables, bootstrap and permutation tests were not performed. Therefore, results represent a fixed-effect model and the interpretation cannot be generalized beyond this sample. We set statistical significance at p < 0.05, analyzed all data using IBM SPSS® Statistics 26.0 and GraphPad Prism 8.1.2, and used R Studio 1.3.1093 and R 4.0.2 with the TExPosition package54 for PLSC.
3 RESULTS
3.1 Participant recruitment and characteristics
We consented and enrolled 12 (N) participants, 6 in each group (nss, nc), exceeding our first feasibility target. We matched cases and controls for sex and age: nss = 6 (5 women), 31–68 years; nc = 6 (5 women), 31–64 years. All cases had physician-diagnosed SS (no autoimmune comorbidities) with time since diagnosis ranging from 1 to ≥20 years (mean [SD] = 7.33 [6.98] years; median [IQR] = 6 [2] years). Participant-reported SS diagnostic methods included extractable nuclear antibody blood testing, lip biopsy, salivary gland biopsy, and/or Schirmer's eye test. Two participants (a matched pair) reported a remote smoking history (ceased for 10+ years).
3.2 Saliva collection and analyses
All participants provided analyzable saliva collected via at least one method, meeting our second feasibility target. We obtained sufficient saliva volumes from all participants using the swab for the stimulated sample; however, one control and all case participants required the maximum collection duration for at least one sample. We obtained insufficient volumes to facilitate testing of some analytes for one control participant (UWS-B only) and some case participants (UWS-A and/or UWS-B). Therefore, we used only SWS sample data for PLSC.
Sialometric and acidity data differed between groups (Table 1). In general, mean/median weight and flow rate decreased over the appointment period regardless of group with stimulated rates being the lowest for controls (UWS-A > UWS-B > SWS). For one collection method (UWS-B), mean flow rate (p = 0.003) and median weight (p = 0.04) differed significantly between groups. For cases, we observed higher stimulated mean flow rates relative to unstimulated (SWS > UWS-A > UWS-B).
| Sample | Overall (N = 12) | Cases (nss = 6) | Controls (nc = 6) | pa | |||
|---|---|---|---|---|---|---|---|
| Range | Mean (SD) Median (IQR) | Range | Mean (SD) Median (IQR) | Range | Mean (SD) Median (IQR) | ||
| Weight (g) | |||||||
| UWS-A | 0.01–1.95 | 1.09 (0.57) | 0.01–1.56 | 0.84 (0.65) | 0.94–1.95 | 1.34 (0.38) | 0.14 |
| 1.19 (0.83) | 0.95 (1.20) | 1.19 (0.67) | — | ||||
| UWS-B | 0.12–1.62 | 0.99 (0.57) | 0.12–1.08 | 0.63 (0.35) | 0.24–1.62 | 1.33 (0.54) | — |
| 0.99 (1.11) | 0.65 (0.59) | 1.54 (0.42) | 0.04* | ||||
| SWS | 0.41–1.03 | 0.70 (0.19) | 0.55–1.03 | 0.72 (0.18) | 0.41–0.94 | 0.67 (0.22) | 0.70 |
| 0.65 (0.35) | 0.68 (0.27) | 0.62 (0.43) | — | ||||
| Flow rate (ml/min) | |||||||
| UWS-A | 0.00–0.81 | 0.35 (0.26) | 0.00–0.66 | 0.23 (0.24) | 0.22–0.81 | 0.48 (0.24) | 0.10 |
| 0.28 (0.50) | 0.19 (0.33) | 0.45 (0.43) | — | ||||
| UWS-B | 0.01–0.69 | 0.27 (0.22) | 0.01–0.22 | 0.10 (0.08) | 0.10–0.69 | 0.23 (0.19) | 0.003* |
| 0.20 (0.37) | 0.09 (0.17) | 0.45 (0.22) | — | ||||
| SWS | 0.10–0.52 | 0.27 (0.14) | 0.25–0.52 | 0.35 (0.11) | 0.10–0.41 | 0.20 (0.13) | — |
| 0.27 (0.26) | 0.34 (0.17) | 0.14 (0.22) | 0.13 | ||||
| Acidity (pH value) | |||||||
| UWS-A | 6.50–7.25 | 7.00 (0.24) | 6.50–7.25 | 6.92 (0.30) | 7.00–7.25 | 7.08 (0.13) | — |
| 6.88 (0.56) | |||||||
| 7.00 (0.25) | 0.40 | ||||||
| 7.00 (0.44) | |||||||
- Note: Em dash indicates no comparison according to data distribution.
- Abbreviations: IQR, interquartile range; SWS, stimulated whole saliva via swab; UWS-A, unstimulated whole saliva via saliva collection aid; UWS-B, unstimulated whole saliva via swab.
- a For normally distributed data, means were compared using an independent-samples t test; for non-normally distributed data, medians were compared using Mann–Whitney U test.
- * p < 0.05.
Sialochemical data are described according to analyte, sample, and group (Table 2). We determined total protein content (mean [SD] and median [IQR], respectively; µg/ml) across all participants according to the sample (UWS-A: 1961.90 [1222.93] and 1640.46 [1713.63]; UWS-B: 1577.48 [1349.75] and 996.90 [1181.35]; SWS: 1875.10 [397.57] and 1145.79 [1089.83]). Between groups, mean total protein content differed significantly for both UWS-A (p = 0.02) and UWS-B (p = 0.02). When compared with controls, case mean (SD) and median (IQR) concentrations for α-amylase, cortisol, and CRP were higher with greater variability across all samples. Significant differences were observed between groups for cortisol (UWS-A [mean]: p = 0.003; SWS [median]: p = 0.04), as well as median CRP concentrations (UWS-A: p = 0.01; UWS-B: p = 0.02; SWS: p = 0.002). When expressed per unit total protein, CRP concentrations (UWS-A) differed significantly between groups (p = 0.03). For all participants, MUC7 values fell below the lower limit of detection (insufficient volume for one case) and were not analyzed further.
| Sample | Overall (N = 12) | Cases (nss = 6) | Controls (nc = 6) | pa | |||
|---|---|---|---|---|---|---|---|
| Range | Mean (SD) Median (IQR) | Range | Mean (SD) Median (IQR) | Range | Mean (SD) Median (IQR) | ||
| Total protein (µg/ml) | |||||||
| UWS-Ab | 526.69–4282.53 | 1961.90 (1222.93) | 1518.30–4282.53 | 3010.05 (1241.91) | 526.69–1943.28 | 1263.14 (549.37) | 0.02* |
| 1640.46 (1713.63) | 3119.68 (2381.09) | 1286.83 (1096.67) | — | ||||
| UWS-Bc | 218.76–5043.00 | 1577.48 (1349.75) | 996.89–5043.00 | 2522.58 (1511.72) | 218.76–1410.15 | 789.90 (421.71) | 0.02* |
| 996.90 (1181.35) | 2044.05 (2245.28) | 883.47 (683.23) | — | ||||
| SWS | 766.81–5131.88 | 1875.10 (397.57) | 894.49–5131.88 | 2615.66 (1656.46) | 766.81–1758.60 | 1134.55 (335.86) | — |
| 1067.65 (371.49) | 0.13 | ||||||
| 1145.79 (1089.83) | 2332.23 (3115.51) | ||||||
| α-Amylase (U/ml) | |||||||
| UWS-Ab | 3.77–474.94 | 119.85 (133.60) | 38.21–138.42 | 176.75 (208.76) | 38.21–138.42 | 81.92 (40.76) | — |
| 72.98 (94.71) | 114.14 (375.93) | 68.72 (79.42) | 0.61 | ||||
| UWS-Bc | 7.04–275.36 | 95.85 (88.25) | 7.05–275.36 | 138.84 (118.65) | 25.26–102.01 | 60.02 (30.41) | — |
| 126.28 (234.44) | |||||||
| 67.08 (87.25) | 53.30 (55.35) | 0.33 | |||||
| SWS | 4.26–518.73 | 161.96 (136.98) | 4.26–518.73 | 192.75 (177.56) | 62.48–264.70 | 131.17 (86.47) | — |
| 133.17 (139.85) | 176.79 (231.57) | 89.71 (161.25) | 0.82 | ||||
| Cortisol (µg/dl) | |||||||
| UWS-Ab | 0.06–0.32 | 0.17 (0.09) |
0.18–0.32 | 0.25 (0.07) |
0.06–0.15 | 0.11 (0.03) |
0.003* |
| 0.14 (0.12) | 0.25 (0.13) | 0.11 (0.05) | — | ||||
| UWS-B | 0.04–0.30 | 0.16 (0.08) |
0.11–0.30 | 0.20 (0.08) |
0.04–0.22 | 0.11 (0.07) |
0.06 |
| 0.16 (0.12) | 0.19 (0.16) | 0.09 (0.13) | — | ||||
| SWS | 0.04–0.27 | 0.12 (0.07) |
0.08–0.27 | 0.16 (0.07) |
0.04–0.12 | 0.08 (0.78) |
— |
| 0.10 (0.07) | 0.15 (0.13) | 0.08 (0.07) | 0.04* |
||||
| CRP (ng/ml) | |||||||
| UWS-Ab | 0.70–21.81 | 6.38 (6.61) |
8.56–21.81 | 12.84 (6.13) |
0.70–3.00 | 2.08 (0.78) |
— |
| 2.75 (7.94) | 10.49 (10.54) | 2.17 (1.00) | 0.01* |
||||
| UWS-Bc | 0.53–11.36 | 4.61 (3.58) |
2.77–11.36 | 7.67 (3.09) |
0.53–3.00 | 2.06 (0.94) |
— |
| 2.87 (6.16) | 7.98 (4.59) | 2.18 (1.56) | 0.02* |
||||
| SWS | 0.86–13.47 | 4.50 (3.79) |
3.05–13.47 | 7.21 (3.66) |
0.86–3.00 | 1.77 (0.75) |
— |
| 3.03 (4.90) | 6.97 (5.62) | 1.64 (1.19) | 0.002* |
||||
| MUC5B (ng/ml) | |||||||
| UWS-Ad | 0.70–2.87 | 1.94 (0.64) |
1.68–2.84 | 2.37 (0.61) |
0.70–2.87 | 1.74 (0.85) |
0.30 |
| 1.68 (1.44) | 2.58 (0.58) | 1.49 (1.59) | — | ||||
- Note: Em dash indicates no comparison according to data distribution.
- Abbreviations: CRP, C-reactive protein; IQR, interquartile range; MUC5B, mucin 5B; SWS, stimulated whole saliva via swab; UWS-A, unstimulated whole saliva via saliva collection aid; UWS-B, unstimulated whole saliva via swab.
- a For normally distributed data, means were compared using an independent-samples t test; for non-normally distributed data, medians were compared using Mann–Whitney U test.
- b Bivariate analyses conducted with two missing case data values due to insufficient volume.
- c Bivariate analyses conducted with one missing case data value due to insufficient volume.
- d Bivariate analyses conducted with three missing case data values due to insufficient volume.
- * p < 0.05.
3.3 Oral assessments
All participants presented with oral-motor structure and function within normal limits and reported no prior dysphagia. Oral dryness scores (mean [SD] and median [IQR], respectively) across the sample were 1.8 (1.9) and 1.5 (3.0). When compared with controls, cases had higher mean (SD) and median (IQR) scores (nss: 2.7 [2.3] and 3.0 [5.0]; nc: 0.8 [1.0] and 0.5 [2.0]) with greater variability (nss: 0.0–5.0; nc: 0.0–2.0), although not significantly different. The most frequent feature for both groups was tongue adhesiveness (67% [nss] and 50% [nc]). Cases presented other features more frequently than controls, including glassy mucosa (67% [nss] and 0% [nc]) and dry (no pooling of) saliva (67% [nss] and 17% [nc]).
3.4 Quality of life assessment
Total SWAL-QOL scores (mean [SD] and median [IQR], respectively; Table 3) for all participants were 90.09 (12.79) and 94.00 (7.25). Total median (IQR) scores differed significantly between groups (p = 0.004) and were lower for cases (89.00 [18.00] than controls (95.50 [4.50]). Additionally, groups significantly differed (p = 0.03) in symptoms of: “thick saliva/phlegm,” “food sticking throat,” and “food sticking mouth” (Table 4).
| SWAL-QOL domain | Scores (%) | pa | |||||
|---|---|---|---|---|---|---|---|
| Overall (N = 12) | Cases (nss = 6) | Controls (nc = 6) | |||||
| Range | Median (IQR) | Range | Median (IQR) | Range | Median (IQR) | ||
| Burden | 70–100 | 100 (15.00) | 70–100 | 90 (22.50) | 100–100 | 100 (0) | 0.18 |
| Eating desire | 67–100 | 100 (0) | 67–100 | 100 (28.50) | 100–100 | 100 (0) | 0.39 |
| Eating duration | 50–100 | 100 (2.00) | 50–100 | 80.00 (50.00) | 100–100 | 100 (0) | 0.07 |
| Symptom frequencyb | 57–100 | 96.00 (22.25) | 57–100 | 81.50 (38.50) | 91–100 | 98.50 (5.25) | 0.07 |
| Food selection | 40–100 | 100 (7.50) | 40–100 | 95.00 (30.00) | 100–100 | 100 (0) | 0.18 |
| Communication | 40–100 | 100 (15.00) | 40–100 | 90.00 (30.00) | 100–100 | 100 (0) | 0.18 |
| Fear | 65–100 | 100 (12.50) | 65–100 | 90.00 (23.75) | 100–100 | 100 (0) | 0.07 |
| Mental health | 72–100 | 100 (12.00) | 72–100 | 92.00 (22.00) | 100–100 | 100 (0) | 0.18 |
| Social | 72–100 | 100 (0) | 72–100 | 100 (13.00) | 100–100 | 100 (0) | 0.40 |
| Fatigue | 27–100 | 76.50 (14.00) | 27–93 | 73.00 (31.50) | 73–100 | 87.00 (17.25) | 0.18 |
| Sleep | 20–100 | 75.00 (14.00) | 20–100 | 80.00 (50.00) | 60–100 | 75.00 (32.50) | 0.82 |
| Total score | 52–100 | 94.00 (7.25) | 52–94 | 89.00 (18.00) | 94–100 | 95.50 (4.50) | 0.004* |
| 96.50 (2.43)c | — | ||||||
| 90.09 (12.79)c | 83.67 (15.97)c | ||||||
- Note: Em dash indicates no comparison according to data distribution.
- Abbreviations: IQR, interquartile range; SWAL-QOL, swallowing-related quality of life.
- a For normally distributed data, means were compared using an independent-samples t test; for non-normally distributed data, medians were compared using Mann-Whitney U test.
- b Domain score does not contribute to SWAL-QOL score.
- c Mean (SD).
- * p < 0.05.
| SWAL-QOL symptom | Scoresa | Mean difference | pb | ||
|---|---|---|---|---|---|
| Overall (N = 12) | Cases (nss = 6) | Controls (nc = 6) | |||
| Coughing | 4.33 (0.89) | 4.33 (1.03) | 4.33 (0.82) | 0 | 1.00 |
| Choking (food) | 4.17 (1.19) | 3.50 (1.34) | 4.83 (0.41) | 1.33 | 0.06 |
| Choking (liquids) | 4.75 (0.45) | 4.67 (0.52) | 4.83 (0.41) | 0.17 | 0.55 |
| Thick saliva/phlegm | 4.00 (1.48) | 3.00 (1.55) | 5.00 (0.00) | 2.00 | 0.03* |
| Gagging | 4.67 (0.65) | 4.33 (0.82) | 5.00 (0.00) | 0.67 | 0.10 |
| Drooling | 4.83 (0.39) | 4.67 (0.52) | 5.00 (0.00) | 0.33 | 0.18 |
| Problems chewing | 4.25 (1.36) | 3.50 (1.64) | 5.00 (0.00) | 1.50 | 0.08 |
| Excess saliva/phlegm | 4.67 (0.65) | 4.33 (0.82) | 5.00 (0.00) | 0.67 | 0.10 |
| Throat clearing | 3.67 (1.37) | 3.00 (1.55) | 4.33 (0.82) | 1.33 | 0.10 |
| Food sticking (throat) | 4.17 (1.27) | 3.33 (1.34) | 5.00 (0.00) | 1.67 | 0.03* |
| Food sticking (mouth) | 4.25 (1.14) | 3.50 (1.23) | 5.00 (0.00) | 1.50 | 0.03* |
| Food/liquid out of mouth | 4.75 (0.62) | 4.50 (0.84) | 5.00 (0.00) | 0.50 | 0.20 |
| Food/liquid out of nose | 4.75 (0.45) | 4.50 (0.55) | 5.00 (0.00) | 0.50 | 0.08 |
| Coughing stuck food/liquid out of mouth | 4.50 (1.00) | 4.17 (1.33) | 4.83 (0.41) | 0.67 | 0.29 |
- Abbreviation: SWAL-QOL, swallowing-related quality of life.
- a Reported as mean (SD).
- b For normally distributed data, means were compared using an independent-samples t test.
- * p < 0.05.
3.5 PLSC analysis
PLSC identified two dimensions explaining 95% of the variance (λ1 = 75.79, τ1 = 81%; λ2 = 13.23, τ2 = 14%). On the first dimension, case participants had greater variability in SWAL-QOL ratings and salivary concentrations of total protein and α-amylase, with one case (participant 4) exhibiting the most extreme SWAL-QOL, total protein, and α-amylase measurements (Figure 1A). Nonetheless, there was clear delineation between groups along this dimension. The second dimension also revealed clear separation between cases and controls (Figure 1B), with two cases (participants 4 and 5) exhibiting SWAL-QOL ratings and cortisol concentrations similar to the control group. On the first dimension, higher ratings (no/less participant-perceived burden) on nine SWAL-QOL items primarily unrelated to bolus clearance were associated with higher total protein and α-amylase concentrations (Figure 1C and Table 5). By contrast, lower ratings (greater participant-perceived burden) on six SWAL-QOL items pertinent to bolus clearance inversely correlated with total protein and α-amylase concentrations on this dimension. Given that cortisol has no known role in swallowing, these observations regarding SWAL-QOL items and contributions to bolus clearance are supported along the second dimension. Specifically, along this dimension, lower ratings on nine SWAL-QOL items were associated with lower cortisol concentrations, whereas higher ratings on six SWAL-QOL items inversely covaried with lower cortisol concentrations (Figure 1D and Table 5).
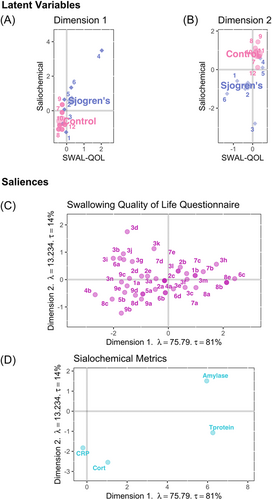
| Perceived burdena | No/Less perceived burdena | |
|---|---|---|
| SWAL-QOL Itemb | Throat clearing (3i)c | Excess saliva or phlegm (3h)c |
| Choking with food (3b)c | Food or liquid out of nose (3m)c | |
| Fear choking with food (6a)c | Fear choking with liquids (6c)c | |
| Coughing out stuck food/liquid (3n)c | Depressed by swallowing problem (7a)c | |
| Difficult to find preferred and edible foods (4b)c | Annoyed by need for careful oral intake (7b)c | |
| Swallowing problem has changed work or leisure activities (8c)c | Swallowing problem has changed role with family and friends (8e)c | |
| Most days, don't care if eat or not (2a)d | Swallowing problem limits dining out (8a)c | |
| Hard for others to understand (5a)d | Swallowing problem limits social life (8b)c | |
| Difficult to speak clearly (5b)d | Discouraged by swallowing problem (7c)c | |
| Worry about pneumonia (6b)d | Thick saliva or phlegm (3d)d | |
| Never know when might choke (6d)d | Food sticking in throat (3j)d | |
| Feel weak (9a)d | Food sticking in mouth (3k)d | |
| Trouble falling asleep (9b)d | ||
| Swallowing problem affects social enjoyment (8d)d |
- Note: Lubricative properties as indicated by higher salivary total protein and α-amylase concentrations.
- Abbreviation: SWAL-QOL, swallowing-related quality of life.
- a Perceived burden according to high (less burden) or low (greater burden) ratings for items in the SWAL-QOL symptom frequency domain.
- b Items that did not contribute: (1a) Dealing with swallowing problem is very difficult. (1b) Swallowing problem a major life distraction. (2b) Takes longer to eat than others. (2c) Rarely hungry. (2d) Lengthy mealtimes. (2e) No longer enjoy eating. (3a) Coughing. (3c) Choking with liquids. (3e) Gagging. (3f) Drooling. (3g) Problems chewing. (3l) Food/liquid dribbling out mouth. (4a) Difficult food selection. (7d) Frustrated by swallowing problem. (7e) Impatient with swallowing problem. (9c) Feel tired. (9d) Trouble staying asleep. (9e) Feel exhausted.
- c Corresponds with higher salivary total protein and α-amylase.
- d Corresponds with lower salivary cortisol.
4 DISCUSSION
Although exploratory by nature, our study was feasible as proposed. We successfully recruited individuals with SS and matched controls, exceeded our recruitment threshold, and collected analyzable saliva from all participants. Although our primary objective was not to detect minimal clinically important differences,40 we still identified differences in saliva volume and composition between those with and without SS. Furthermore, we were the first to explore the relationship between salivary biomarkers and participant-reported perceptions of swallowing in SS using a matched design. Through these explorations, we identified novel relationships between salivary and perceptual outcomes. For example, participants perceived changes in their swallowing function that aligned with analyte concentrations. Specifically, those with SS perceived greater difficulties related to oral function that covaried with higher total protein content and α-amylase concentration. Swallowing impairments are conventionally investigated when adverse health outcomes (e.g., respiratory complications) occur; however, swallowing assessments can be invasive (e.g., nasendoscopy) and expensive. Overall, this exploratory work suggests that future study of salivary biomarkers, swallowing physiology, and participant-reported perceptions of swallowing is warranted. Doing so may establish the clinical utility of noninvasive biological and/or salivary profiling. Specifically, salivary bioscience may enable detection of swallowing concerns before adverse health effects manifest, eliminating the need for and/or as part of conventional, diagnostic assessments.
The saliva collection methods we trialed were adequate for most participants, although differed according to ease, efficiency, duration, and volumes attained. All participants provided sufficient stimulated saliva volume via swab, whereas both unstimulated sampling methods resulted in lower volumes for some case participants. Regardless, ≥75% of participants provided sufficient volumes using both collection devices. Sampling method notwithstanding, mean weights and flow rates decreased over time across all participants, indicating hyposalivation.55, 56 Unstimulated flow rates fell at or below 0.1 ml/min for roughly half of case participants, with average and individual stimulated flow rates falling below 0.7 ml/min across participants. This may be related to our sample and/or design, as we did not use graphic aids for sensory stimulation, randomize collection methods, or maintain the same sampling duration across methods.
Overall, sialometric and sialochemical findings differed between groups, although we faced some challenges in our exploration of certain analytes (e.g., mucins). Saliva weight and flow rate differed significantly across groups for at least one collection method. We also observed significant differences between groups for total protein and cortisol (UWS-A) and for CRP regardless of collection method. In some case participants, we observed a threefold increase in analyte concentrations corresponding to a one-third reduction in flow rates. This inverse relationship between flow rates and protein concentration32 in cases as compared with controls aligned with differences in specific salivary analytes. pH values did not differ significantly between groups, confirming the absence of other chemical influences (i.e., acidity) on saliva composition in our study. Regarding specific analytes, mean α-amylase and cortisol concentrations were higher for cases relative to controls, with higher mean α-amylase concentrations consistent with lower mean cortisol concentrations across participants.18, 19 Given the predominant inflammatory response in SS,6 and likely influence of environmental stressors, higher cortisol and α-amylase concentrations could reflect disease-related inflammation and/or stress.57 Similarly, we observed significant group differences across samples for median concentrations of the inflammatory marker CRP.17 When investigating mucins, MUC7 could not be analyzed across participants and MUC5B concentrations did not differ significantly between groups. This may be due to collection methods, low saliva volumes, and/or selected assay kits. Altered mucin glycosylation and/or structure has been identified in SS, specifically affecting salivary rheological properties, and may therefore be a distinguishing feature in conjunction with dry mouth and perception rather than mucin concentration itself.31, 58, 59 Given the relevance of mucins, future studies should investigate mucin structure in conjunction with concentration.
We explored novel relationships between salivary and perceptual outcomes, distinguishing individuals with and without SS. As expected, participant-reported perceptions of oral dryness and quality of life varied according to group, and therefore clinical diagnosis. Moreover, analytes that theoretically contribute to swallowing were associated with perceived swallowing characteristics. Those with SS exhibited higher oral dryness scores, although not significantly different from those of controls. This may relate to recent (≤6 years) diagnosis of SS for most case participants. Those with SS also reported disease burden, reduced quality of life (related to swallowing), and more dysphagia symptoms. Our case participants had lower MUC5B and higher α-amylase concentrations, while reporting more viscous saliva, reduced salivary lubrication, globus sensation, and bolus retention. As both proteins contribute to swallowing, particularly with bolus preparation and clearance,15, 16, 60 these findings align with clinical observations. Our multivariate analysis identified an association between greater burden of disease and/or lower quality of life and more viscous, concentrated saliva with an altered proteomic composition. We observed an inverse relationship between patterns of SWAL-QOL item ratings and patterns of salivary analyte concentrations. Predominant group differentiators included higher total protein and α-amylase concentrations corresponding with lower SWAL-QOL ratings on items pertinent to bolus clearance (Dimension 1). Likewise, lower cortisol concentrations corresponded with higher SWAL-QOL ratings on items related to bolus clearance (Dimension 2), aligning with the asymmetrical responsivity of cortisol and α-amylase.18, 19 Oral dryness and flow rate data did not enrich this interpretation, punctuating the relevance of specific analyte-related data to participant-reported perceptions. Collectively, our findings highlight the value of using objective salivary profiles alongside subjective perceptions for a comprehensive representation of the effect of salivary changes on swallowing and quality of life.
Our study was exploratory in nature, and as such, had inherent limitations. Although successful in executing our proposed methods, this study was not designed for hypothesis testing, and our short recruitment period and small sample size restrict generalizability. Recruitment was community-based from one geographic location, with case participants living independently with few comorbidities. By design, some variables were not controlled, as we were investigating feasibility across a variety of saliva collection methods. Accordingly, sampling order was not randomized and we permitted variability in collection duration. Together with data correction, this may have introduced order and ceiling effects, respectively, possibly limiting the accuracy of our salivary findings and introducing bias. Despite these limitations, our study was feasible and our novel findings highlight the need for large-scale investigations. To better understand disease burden and the link between saliva and swallowing physiology, future work should incorporate salivary analyses, semistructured interviews, and swallowing instrumentation (e.g., nasendoscopy). Sialochemical analyses should focus on total protein, α-amylase, further investigation of mucins (i.e., structure), and other inflammatory markers (e.g., cytokines). Doing so may enhance the understanding of salivary changes (volume and composition) and effects on swallowing (physiology and perception) to expedite diagnosis and afford bespoke intervention for those with SS and, ultimately, other patient populations.
5 CONCLUSION
Exploring the role of salivary proteins is essential to understanding swallowing and managing dysphagia. Our study, feasible as designed, provides novel exploration of covariance between salivary analytes and perceptions of swallowing, while expanding on previous work concerning salivary changes in those with SS.9, 29-34 The observed pattern of covariance between saliva composition and participant-reported perceptions of swallowing, specifically regarding oral function, contributes to a framework upon which salivary bioscience may be integrated into swallowing research. Regardless of population, integrating a comprehensive saliva assessment with patient perspectives would facilitate customization of clinical service provision while taking quality of life into account. Routine salivary profiling in clinical practice would support treatment innovation whilst improving swallowing and quality of life for many individuals, particularly those with SS and other rheumatologic diseases.
AUTHOR CONTRIBUTIONS
All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
The authors would like to thank Wayne Yu and Dr. Tamara Bodnar for their laboratory expertise and involvement in this project, and Dr. Herve Abdi for providing statistical guidance. We are grateful to the participants who volunteered their time, saliva, and insights. They have contributed not only to this study but to future work in the area of chronic illness as well as the clinical applications of salivary bioscience and swallowing. This study was supported, in part, by a University of British Columbia (UBC) Faculty of Medicine start up grant to Dr. Skoretz and through in-kind contributions from the Weinberg Laboratory at UBC. Graphical abstract created with BioRender.com.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Clinical Research Ethics Board through the University of British Columbia.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.