Protocol for a randomized controlled trial of electroacupuncture in the management of patients with axial spondyloarthritis in Singapore (E-AcuSpA)
Abstract
Background
Despite therapeutic advances, treatment of patients with axial spondyloarthritis (axSpA) continue to pose as a challenge as many do not respond well to conventional Western medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and biologic disease-modifying antirheumatic drugs (bDMARDs). Hence, acupuncture is a possible alternative. Some studies found electroacupuncture to be better than manual acupuncture, though no trials have been conducted in patients with axSpA. This clinical trial aims to evaluate the clinical efficacy, safety, and cost-effectiveness of electroacupuncture compared to manual acupuncture for patients with axSpA.
Methods/Design
This randomized controlled trial will recruit 100 patients diagnosed with axSpA, who have active disease despite NSAIDs and bDMARDs. Eligible patients will be randomized to receive either electroacupuncture or manual acupuncture in a 1:1 ratio. All participants will receive standard rheumatologic care in addition to 20 acupuncture sessions. The mean difference in Bath Ankylosing Spondylitis Disease Activity Index score between the 2 groups over 12 weeks will serve as the primary outcome. Secondary outcomes include improvements in other clinical, quality of life, and economic outcomes over 24 weeks. All adverse events will be recorded.
Discussion
Results from this trial may provide evidence regarding the clinical effectiveness, safety, and cost-effectiveness of electroacupuncture compared to manual acupuncture for patients with axSpA, and guide implementation into clinical practice. Limitations of this trial include the lack of patient blinding, use of a repeated measures design, and possible variation in acupuncture technique amongst the various Traditional Chinese Medicine practitioners.
Highlights
-
There are currently a lack of randomised controlled trials comparing the efficacy between manual acupuncture and electroacupuncture.
-
This is a protocol for a randomised controlled trial comparing the efficacy between manual and electroacupuncture in patients with axial spondyloarthritis with inadequate response to conventional western medications.
-
The mean difference in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score between the two groups over 12 weeks will serve as the primary outcome.
Abbreviations
-
- AE
-
- adverse event
-
- ASAS
-
- Assessment of SpondyloArthritis International Society
-
- ASAS HI
-
- Assessment of SpondyloArthritis international Society Health Index
-
- ASQoL
-
- Ankylosing Spondylitis Quality of Life
-
- axSpA
-
- axial spondyloarthritis
-
- BASDAI
-
- Bath Ankylosing Spondylitis Disease Activity Index
-
- BASFI
-
- Bath Ankylosing Spondylitis Functional Index
-
- BAS-G
-
- Bath Ankylosing Spondylitis Global score
-
- bDMARDs
-
- biologic disease-modifying antirheumatic drugs
-
- CEA
-
- cost-effectiveness analyses
-
- EQ-5D-5L
-
- EuroQol 5 Dimension 5 Level
-
- ICER
-
- incremental cost-effectiveness ratio
-
- NSAIDs
-
- nonsteroidal anti-inflammatory drugs
-
- PROMs
-
- patient-reported outcome measures
-
- QALY
-
- quality-adjusted life years
-
- QoL
-
- quality of life
-
- SAE
-
- serious adverse event
-
- STCMI
-
- Singapore Thong Chai Medical Institution
-
- TCM
-
- Traditional Chinese Medicine
-
- VAS
-
- visual analog scale
1 INTRODUCTION
Axial spondyloarthritis (axSpA) is a chronic inflammatory condition that primarily affects the axial skeleton and sacroiliac joints, and is characterized by inflammatory back pain and stiffness.1, 2 Goals of treatment include suppressing inflammation, so as to minimize symptoms, and preserve physical function.3 Despite advances in the treatment of axSpA, there is an urgent need for more treatment modalities as currently approved therapies for axSpA include nonsteroidal anti-inflammatory drugs (NSAIDs) for treatment naïve patients and biologic disease-modifying antirheumatic drugs (bDMARDs) for patients with inadequate response to NSAIDs, and are effective for only about 35%4 and 60%5 patients, respectively. Furthermore, patients who initially respond to bDMARDs can develop diminished response over time due to immunogenicity.6 Patients often turn to complementary and alternative medicine due to unsatisfactory treatment effects, poor tolerability, high costs of bDMARDs, adverse effects, or simply due to the fear of adverse effects associated with western medication use.7, 8
Acupuncture, based in Traditional Chinese Medicine (TCM), can be effective in alleviating chronic pain, in reducing levels of inflammation, and is also a relatively low-cost intervention.9-14 Furthermore, adverse events (AEs) are often minor and transient15, 16 and complications can be avoided if it is performed by trained practitioners.17, 18 Manual acupuncture and electroacupuncture are two variations of acupuncture treatment, with the latter supplying an electric current to stimulate the acupoints. One advantage of electroacupuncture is its ability to produce a more continuous and quantifiable stimulation as compared to manual acupuncture,19 and some studies have shown that the use of electroacupuncture results in better treatment effects than manual acupuncture.20, 21 However, none of these studies were of patients with axSpA.
Therefore, we aim to evaluate the clinical effectiveness, safety, and cost-effectiveness of electroacupuncture, as compared to manual acupuncture, in the treatment of patients with axSpA. We hypothesize that electroacupuncture will result in better control of symptoms and disease activity in patients with axSpA, again as compared to manual acupuncture, over 12 weeks, as well as in greater improvements in other clinical, quality of life (QoL), and economic outcomes. We also hypothesize that safety is no different between the two acupuncture treatments.
2 METHODS/DESIGN
2.1 Study design and setting
This is a single-center, two-arm, randomized controlled trial. Eligible patients will be randomized to receive electroacupuncture or manual acupuncture via random permuted blocks with varying block size, assuming a 1:1 ratio. One hundred participants in total will be recruited from the Department of Rheumatology and Immunology in Singapore General Hospital. Acupuncture procedures will be conducted at Singapore Thong Chai Medical Institution (STCMI). Protocol for this trial (version 2.1, dated February 1, 2021) is guided by the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)22 and Standards for Reporting Interventions in Clinical Trials of Acupuncture guidelines (Tables S1 and S2).23 This trial has been registered on ClinicalTrials. gov (NCT04519866).
2.2 Participants
We aim to recruit patients diagnosed with axSpA, who have spinal pain and active disease despite conventional therapy. Patients will be eligible if they are above 21 years of age, have axSpA diagnosed according to the 2009 Assessment of Spondyloarthritis International Society (ASAS) criteria, active disease based on Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score ≥ 4, and have failed two sequential NSAIDs (including COX-2 inhibitors) at maximal tolerated doses for ≥4 weeks in total or failed bDMARDs (e.g., tumor necrosis factor-α inhibitors, interleukin-17 inhibitors). Patients on concomitant bDMARDs or conventional synthetic disease-modifying antirheumatic drugs (e.g., methotrexate, sulfasalazine, leflunomide) at study entry must be on the drug for ≥12 weeks and at a stable dose for ≥4 weeks before randomization. Patients on systemic corticosteroids have to be on a stable dose of ≤10 mg/day of prednisolone or equivalent for ≥2 weeks before randomization.
Patients will be excluded if they are pregnant or breastfeeding, have bleeding disorders, blood-borne communicable diseases (e.g., hepatitis B, hepatitis C, human immunodeficiency virus), implantable electrical device (e.g., pacemakers) or suffer from impaired skin sensation or serious skin lesions along the vertebrae.
2.3 Recruitment, randomization, and blinding
The attending rheumatologist will obtain informed consent from eligible patients. Patients will then be referred to a research coordinator who will assign them to receive either electroacupuncture or manual acupuncture based on the randomization list pregenerated by a biostatistician using the ralloc command of STATA (version 16.0).
The randomization list will be kept by the biostatistician and research coordinator until the end of the study to ensure allocation concealment. The attending rheumatologist will be blinded to treatment allocation for the first 12 weeks and participants will be instructed not to disclose the allocation. Unblinding before week 12 will only occur if knowledge of treatment allocation is critical for further management, such as in the event of a serious AE. The trial statistician will be blinded as to treatment for the entire duration of the study.
2.4 Data collection and management
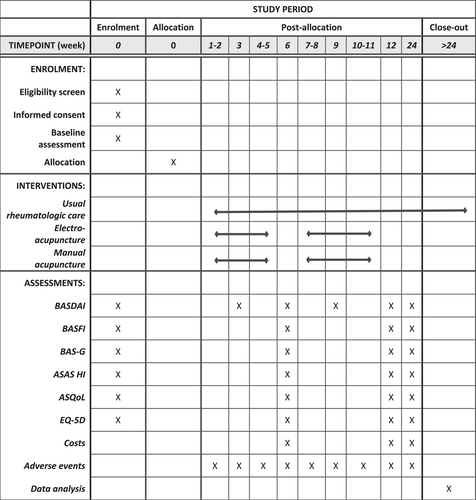
All participants will complete a series of validated patient-reported outcome measures (PROMs) as part of data collection at baseline, weeks 3, 6, 9, 12, and 24 (Figure 1). Patient demographics, clinical data, and medical history will be obtained at baseline.
Research data will be entered into SingHealth Research Electronic Data Capture, a secure application for the management of research-related data. Before data analysis, data validation and range checks will be implemented. Softcopy research data will be password-protected and hardcopy documents will be kept in a locked cabinet.
2.5 Standard rheumatologic care
All patients will receive standard rheumatologic care for axSpA, which comprises medications (e.g., NSAIDs, bDMARDs), physiotherapy, and regular monitoring of other complications related to axSpA (e.g., cardiovascular, metabolic, and gastrointestinal comorbidities). The attending rheumatologist will see each patient at weeks 12 and 24 (Figure 1). During each visit, he/she will monitor the patient's disease activity using PROMs (which patients complete before their outpatient attendances) and prescribe the full range of medications as per treatment guidelines. Investigations (e.g., diagnostic imaging, laboratory tests) will be ordered if necessary. Extra visits will be arranged if required, such as for titration of medications, management of disease flares, or monitoring of complications. Patients will be requested not to seek other TCM treatment or alternative therapies for the first 24 weeks of the study. The research coordinator will enquire about any use of these therapies during the data collection encounters (Figure 1).
2.6 TCM care
TCM practitioners in STCMI will administer either electroacupuncture or manual acupuncture based on the randomized allocation. There will be a total of 20 acupuncture sessions (or two courses of treatment). Each course of treatment consists of 10 acupuncture sessions held over 5 weeks. Patients will take a 1-week break between each course of acupuncture (Figure 1).
Other interventions to be carried out by the TCM practitioners include diagnosis of patients' TCM syndromes based on their clinical presentation, assessment of patients' disease activity, and counseling of patients. In the event of any adverse reactions, the TCM practitioners will inform the attending rheumatologist.
2.7 Selection of acupoints
To reflect clinical practice, we have structured a semi-standardized acupuncture protocol, where additional acupoints can be selected based on the patient's syndrome and symptoms in addition to the main acupoints (Table 1).
| Syndrome/symptoms | Acupoints | |
|---|---|---|
| Syndrome | Blockage due to damp cold syndrome | Yaoyangguan (DU3) |
| Yinlingquan (SP9) | ||
| Blockage due to damp heat syndrome | Quchi (LI11) | |
| Yinlingquan (SP9) | ||
| Blockage due to stagnated blood | Sanyinjiao (SP6) | |
| Geshu (BL17) | ||
| Deficiency in liver and kidney | Ganshu (BL18) | |
| Sanyinjiao (SP6) | ||
| Yang deficiency in kidneys and Du meridian | Mingmen (DU4) | |
| Yaoyangguan (DU3) | ||
| Symptoms | Neck pain | Fengchi (GB20) |
| Jianjing (GB21) | ||
| Dazhui (DU14) | ||
| Thoracic pain | Dazhu (BL11) | |
| Shenzhu (DU12) | ||
| Zhiyang (DU9) | ||
| Lumbar pain | Dachangshu (BL25) | |
| Yaoyan (EX-B7) | ||
| Weizhong (BL40) | ||
| Sacrum pain | Ciliao (BL32) | |
| Knee pain | Yinlingquan (SP9) | |
| Yanglingquan (GB34) | ||
| Weizhong (BL40) | ||
| Ankle pain | Taixi (KI3) | |
| Kunlun (BL60) | ||
| Qiuxu (GB40) |
Jingjin points, also known as “positive reaction points” along meridian sinews will serve as the main acupoints in this study. Under normal circumstances, meridian sinews provide support for muscles, bones, and joint structures to facilitate movement. In the presence of external pathogens, trauma, overuse, or strains, lesions form in the area, and if unmanaged, will result in nodules, adhesions, and calcifications.24, 25 These pathological changes obstruct the flow of qi and blood, hence leading to pain and impaired range of motion,26 and are also palpable over the skin as “positive reaction points.”
Main acupoints include Jingjin points along the Bladder Meridian of Foot Taiyang, such as Shenshuci, as well as Jingjin points at transverse processes of L2–L4, iliac crest, and spinous processes of S1–S4. Acupoints along the Bladder Meridian of Foot Taiyang are commonly chosen to treat conditions related to the spine and back due to its route through the paraspinal muscles.27 As Jingjin points are obtained by palpating the area over the skin for any “positive reaction points,” acupoints can be either unilateral or bilateral, depending on the presence of the points. The number of needles used will hence vary across patients and during each session.
2.8 Manual acupuncture (control group)
Treatment will be administered using sterile disposable acupuncture needles (Huan Qiu), with diameters ranging from 0.25 to 0.30 mm and lengths ranging from 25 to 75 mm. The area of the acupoint will be sterilized before needle insertion. Depending on the acupoint, needles will then be inserted to a depth of 10–50 mm. Rotating manipulation and/or lifting-thrusting manipulation will then be done to achieve de qi, normally described as an aching, numb, tingling, heavy sensation, or distension in the area.28 Needles will be left for 30 min, and the TCM practitioners will repeat needle manipulation every 10 min.
2.9 Electroacupuncture (intervention group)
For patients randomized to the electroacupuncture group, paired electrodes of the electroacupuncture device (ES-160; Ito Co., Ltd.) will be connected to 1–3 pairs of acupoints after de qi is elicited. The paired electrodes will be connected to acupoints on the same side of the body and will not cross the spine to prevent electrical impulses from reaching the heart. Electrical stimulation at regions near the chest and carotid sinus will also not be allowed.
Before the device is switched on, all adjustment knobs of the electroacupuncture unit will be set to 0 (no current). Electrical stimulation will be delivered through a dense-sparse wave (3 Hz/10 Hz). The intensity of the current will be increased to a maximum of 5, depending on the tolerance of each patient. Current will be increased gradually to prevent sudden muscle spasms and shock. Current will be applied to the acupoints from superior to inferior, medial to lateral. For patients who repeatedly report weak or no sensation, the device will be switched off for 1–2 min and restarted. Needles will similarly be left for 30 min, but regular needle manipulation will not be required.
2.10 Outcome measurement
Outcome measures and time of assessment are summarized in Figure 1 and Table 2.
| Outcomes | Study period | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 3 | Week 6 | Week 9 | Week 12 | Week 24 | |
| Primary outcome | ||||||
| BASDAI | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Secondary outcomes | ||||||
| Clinical outcomes | ||||||
| BASDAI | ✓ | ✓ | ||||
| BASFI | ✓ | ✓ | ✓ | ✓ | ||
| BAS-G | ✓ | ✓ | ✓ | ✓ | ||
| ASAS HI | ✓ | ✓ | ✓ | ✓ | ||
| Quality of life outcomes | ||||||
| ASQoL | ✓ | ✓ | ✓ | ✓ | ||
| EQ-5D-5L | ✓ | ✓ | ✓ | ✓ | ||
| Economic outcomes | ||||||
| Costs | ✓ | ✓ | ✓ | |||
- Abbreviations: ASAS HI, Assessment of Spondyloarthritis International Society Health Index; ASQoL, Ankylosing Spondylitis Quality of Life; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BAS-G: Bath Ankylosing Spondylitis Global Score; EQ-5D-5L, EuroQol 5 Dimension 5 Level.
The outcome measures selected were guided by the endorsed ASAS/Outcome Measures in Rheumatology (OMERACT core sets.29 All PROMs used have been validated across various countries, including Singapore.30-41 We have selected week 3 as the first time-point as it would be after the first five sessions of acupuncture. Pain-relieving effects of electroacupuncture are expected to be faster than manual acupuncture and would be more evident at this point. Weeks 6 and 12 are selected because participants would have completed their 10 and 20 sessions of acupuncture respectively, and most acupuncture trials have assessed treatment outcomes at this time point.42-44 Secondary outcomes up to week 24 were selected to assess long-term effectiveness of electroacupuncture, which are of practical relevance to patients.
2.11 Primary outcome
The mean difference in BASDAI score between the two groups over 12 weeks will be the primary outcome. BASDAI45, 46 is used to assess axSpA disease activity based on the severity of fatigue, spinal and extra-spinal joint pain, localized tenderness, and morning stiffness. The 0–10 numerical rating scale will be used, with higher values indicating more active disease. This was selected to capture the pain-relieving effects of both modes of acupuncture.
2.12 Secondary clinical outcomes
Secondary clinical outcomes will include the mean difference in BASDAI score between the two groups over 24 weeks, as well as the mean differences in Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Patient Global Score (BAS-G) and Assessment of Spondyloarthritis International Society Health Index (ASAS HI) scores between the two groups over 24 weeks.
BASFI, BAS-G, and ASAS HI are disease-specific PROMs. BASFI47 measures patients' functional status and scores range from 0 to 10, with higher scores indicating worse function. BAS-G48 is a visual analog scale (VAS), that provides a global assessment of well-being. Scores range from 0 to 100, with higher scores implying a greater impact of the disease on the patients' well-being. ASAS HI49, 50 aims to assess health status in patients with spondyloarthritis by measuring function, disability, and health. It consists of 17 items with dichotomous response options (“I agree” and “I do not agree”) and scores range from 0 to 17, with lower scores indicating better health status.
2.13 Secondary QoL outcomes
The mean differences in Ankylosing Spondylitis Quality of Life (ASQoL) and EuroQoL 5-dimension 5-level scale (EQ-5D-5L) scores between the two groups over 24 weeks will serve as the secondary QoL outcomes.
ASQoL51 is a self-administered, patient-derived, and disease-specific measure of QoL for AS, which consists of 18 items with dichotomous response options (“yes” or “no”). Total scores range from 0 to 18, with higher scores indicating worse QoL. The EQ-5D-5L52 comprises a descriptive system and a VAS. The former assesses five dimensions that represent different aspects of health, namely mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, each with five possible answers (“no,” “slight,” “moderate,” “severe,” and “unable”/“extreme”). EQ-5D-5L utility scores will be computed, with 0 representing death and 1 representing full health, using time trade-off valuations from the Singaporean value set.53 The VAS provides a quantitative measure of the patient's perception of their overall health and extends from 0 (“worst imaginable health state”) to 100 (“best imaginable health state”).
2.14 Secondary economic outcome
The mean differences in total costs between the two groups over 24 weeks will serve as the secondary economic outcome for this study.
Healthcare costs include costs for treatment-related and nonrelated to axSpA, such as costs of consultation at the rheumatology department, physiotherapy department, other outpatient clinics, emergency department, primary care clinics, clinics offering other complementary and alternative treatment, costs of diagnostic imaging, laboratory tests, medication, treatment procedures, assistive devices and costs of inpatient hospitalization. Nonhealthcare costs include costs of traveling, work productivity loss (as assessed using the Work Productivity and Impairment54 questionnaire). All information required will be obtained through questionnaires over 24 weeks, supplemented by a review of medical records and billing information available from the electronic databases.
2.15 Process measurement
Before trial commencement, all study team members will attend a briefing to familiarize themselves with study-related procedures. TCM practitioners who will be administering acupuncture have been registered with Singapore TCM Practitioners' Board and have had prior experience in clinical trials related to acupuncture. To minimize variability, all TCM practitioners will undergo acupuncture training sessions before study commencement to ensure their competency in performing the acupuncture. During each acupuncture session, the TCM practitioner will complete a checklist to ensure standardization of treatment (Tables S3 and S4). Patient compliance will be assessed through the number of acupuncture sessions attended.
2.16 Safety
An AE refers to any untoward event or medical occurrence that does not necessarily have a causal relationship with the intervention or medical device. A serious adverse event (SAE) is any untoward medical occurrence which results in or contributes to death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in or contributes to persistent or significant disability/incapacity, congenital anomaly/birth defect, the transmission of a communicable disease, or in such other events as may be prescribed.
AEs will be monitored and recorded throughout the study. Preplanned hospitalizations will not be recorded as an AE. Acupuncture-related AEs are defined as untoward events that resulted from acupuncture treatment and occurred between the 1st and 20th acupuncture sessions. We have adapted AEs of interest from systematic reviews,18, 55 such as broken needles, fainting during acupuncture, and local infections at the site of acupuncture.
The attending rheumatologist and study team members will perform data and safety monitoring annually till follow-ups for all participants recruited into the trial have been completed. All AEs will be monitored for safety. An independent data monitoring committee will not be set up. In the event of any SAE, the study will be stopped and managed according to local institutional guidelines and regulations.
2.17 Sample size justification
The sample size is estimated based on a repeated measures trial design with one pre- and four postintervention (3, 6, 9, and 12 weeks) measurements. Assuming a mean difference of 0.75 with a standard deviation of 2, correlation of 0.6, power of 80%, and a two-sided level of significance of 5%, a minimum sample size of 80 would be required. Further accounting for 20% attrition, a total of 100 participants will be needed, with 50 participants per arm.
2.18 Statistical analysis
All patients will be analyzed using an intention-to-treat approach. Demographic and baseline clinical characteristics will be summarized using mean and standard deviation (or median and interquartile range where appropriate) for continuous variables, and count and percentage for categorical variables. Linear mixed effect models will be implemented to determine the effect of the intervention on primary and secondary outcomes, taking into account the possible intrasubject correlation between the repeated measures. These models will be further adjusted for respective baseline covariate and other potential confounders, such as disease duration, age, and gender. The effect of the intervention on both primary and secondary outcomes will be quantified based on the mean difference and its associated 95% confidence interval. All analyses will be conducted assuming a two-sided test at the 5% level of significance. AEs that occur within the 24 week period will be presented in frequency and percentages for safety assessment.
Cost-effectiveness analyses (CEA) will be conducted for health economic evaluation. CEA will be assessed by calculating the incremental cost-effectiveness ratio (ICER) for both arms. Value of ICER will be obtained by dividing the difference in total costs by the difference in quality-adjusted life years (QALYs). Total costs will include direct healthcare costs, nonhealthcare costs and indirect costs such as loss of work productivity. Cumulative QALY for each study arm will be calculated using the area under the curve by summing the areas of the geometrical shapes obtained by linearly interpolating between utility scores at the follow-up time points, with adjustment of baseline utility value if baseline differences exist between study arms. Utility scores will be measured using EQ-5D-5L. To generate confidence intervals of the ICERs, nonparametric bootstrapping (random sampling with replacement) will be conducted. Sensitivity analysis will be conducted to evaluate the influence of uncertainties in the variables and assumptions employed on the analysis results.
2.19 Ethics and dissemination
This study has been approved by SingHealth Centralized Institutional Review Board (CIRB) (Reference number 2019/2435). Protocol modifications, AE reporting, and annual reviews will be overseen by SingHealth CIRB. SingHealth Office of Research Integrity and Compliance may perform random audits independently to ensure that regulations are met. Study participation is voluntary and can be discontinued at any time, without affecting a patient's care. All personal information of patients will be kept confidential and only shared within the study team. All study team members are required to complete a biomedical research training module offered by the Collaborative Institutional Training Initiative on human subjects' protection and data security before performing study-related procedures.
The hospital does not make any provisions to compensate study participants for research-related injury. However, compensation may be considered on a case-by-case basis for unexpected injuries due to nonnegligent causes. These costs will be covered by insurance for clinical trials.
3 DISCUSSION
This is the first study comparing the efficacy of electroacupuncture to manual acupuncture in patients with axSpA with inadequate response to NSAIDs or bDMARDs, medications currently recommended for treatment. These patients represent a group of patients in need of alternative treatment options. Results of this trial will help to determine if electroacupuncture is better than manual acupuncture in the control of symptoms and disease activity in patients with axSpA. This will add to existing knowledge and guide TCM practitioners in clinical decision-making when selecting the type of acupuncture during treatment.
Several limitations are present in this trial. First, the absence of patient blinding might lead to bias in the findings, especially since the outcome measures are based on PROMs.56 However, patient blinding would be difficult to maintain in this trial as the sensation elicited by manual acupuncture and electroacupuncture might be different. There are other features related to axSpA (e.g., uveitis, inflammatory bowel disease, etc.) which might signify disease worsening and influence treatment choices, but this trial mainly includes patients based on their level of back pain and assesses treatment efficacy based on it, which might neglect the other spectrum of the disease. This trial adopted a repeated measures design, where multiple observations are collected for an individual, which might affect the analysis of results if there are missing observations. Although efforts were made to standardize acupuncture techniques among TCM practitioners, there might still be some differences due to varying levels of experience. Lastly, acupuncture in this trial is based on Jingjin acupoints instead of the conventional meridian acupoints, which might limit the extent to which this treatment method can be implemented as TCM practitioners might not be able to perform similar acupuncture techniques without prior knowledge. However, the use of Jingjin acupoints has been shown to be more effective than conventional meridian acupoints for pain-related conditions.57
3.1 Study status
At the time of manuscript submission, participant enrollment is underway but has not been completed.
ACKNOWLEDGMENTS
This trial is funded by Reverie Rheumatology Research Fund, Spondyloarthritis: Excelling in Research for best Outcomes (SPERO) program. The funder has not taken part in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study has been approved by SingHealth CIRB (Reference number: 2019/2435). Written informed consent is required for participation. Trial registration: ClinicalTrials.gov, NCT04519866. Registered on February 24, 2021, https://clinicaltrials.gov/ct2/show/NCT04519866. Consent to publish deidentified information is included in the written informed consent process. No data has been published.
AUTHOR CONTRIBUTIONS
Warren Fong conceived and designed the study, drafted the manuscript, and solicited the funding. Yu Heng Kwan conceived and designed the study and drafted the manuscript. Su-An Quek designed the intervention for the study and drafted the manuscript. Ader Lim designed the intervention for the study. Chiah Yuen Wong designed the intervention for the study. Shin Yoong Chua designed the intervention for the study. Hui Chin Tan designed the intervention for the study. Clara Eng designed the intervention for the study. Choy Tip Tan designed the intervention for the study. Bao Qiang Dong designed the intervention for the study. Youyi Huang designed the intervention for the study. Chuen Seng Tan designed the statistical plan. Bee Choo Tai designed the statistical plan. Ting Hui Woon participated in the design and coordination of the study and drafted the manuscript. Jie Kie Phang participated in the design and coordination of the study and drafted the manuscript. Hwee Ling Koh designed the intervention for the study. Ying Ying Leung designed the study. Julian Thumboo conceived and designed the study and supervised the progress of the study. Truls Østbye conceived and designed the study and supervised the progress of the study. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data availability statement is not applicable for this study.