Temperature dependence of activation energies for self-diffusion of water and of alkali ions in aqueous electrolyte solutions. A model for ion selective behavior of biological cells
Abstract
Samoilov's kinetic hydration model, based on short-range interactions for aqueous electrolyte solutions, is critically examined. The treatment suffers from a basic assumption that the activation energy for self-diffusion of ion in water (E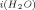 ) is equivalent to the activation energy for self-diffusion of water from near an ion (E
) is equivalent to the activation energy for self-diffusion of water from near an ion (E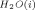 ). The change in activation energy for self-diffusion of water (ΔE) in the presence of an ion is also obtained under that assumption. A simple approach is outlined, using experimental diffusion data at different temperatures, which shows that (1) E
). The change in activation energy for self-diffusion of water (ΔE) in the presence of an ion is also obtained under that assumption. A simple approach is outlined, using experimental diffusion data at different temperatures, which shows that (1) E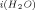 is not equal to E
is not equal to E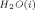 except at 12°C for Li+, 28°C for Na+, and 44°C for K+, and (2) ΔE is obtained directly without Samoilov's assumption. The present approach shows that “positive hydrators” and “negative hydrators” interchange their roles along the temperature scale in terms of the sign of ΔE. This property of change in the sign of ΔE with temperature for a given ion may serve as a model to the more complex ion selective behavior of biological cells.
except at 12°C for Li+, 28°C for Na+, and 44°C for K+, and (2) ΔE is obtained directly without Samoilov's assumption. The present approach shows that “positive hydrators” and “negative hydrators” interchange their roles along the temperature scale in terms of the sign of ΔE. This property of change in the sign of ΔE with temperature for a given ion may serve as a model to the more complex ion selective behavior of biological cells.




