Crystal structure of the mouse p53 core domain in zinc-free state
INTRODUCTION
p53 is a sequence-specific transcription factor that maintains the genetic integrity of an organism as a tumor suppressor.1 p53 comprises three domains: the N-terminal transactivation domain, the DNA-binding core domain, and the C-terminal tetramerization domain.2 The core domain of p53 is directly involved in DNA binding, thereby mutations within this domain result in a loss in the function of p53 and the development of a variety of human cancers, due either to impaired DNA binding or to the instability of the protein.1 The crystal structure of the core domain of p53 in complex with DNA provided a structural basis for our understanding of cancerous p53 mutations.3
p53 is a zinc-finger protein, in which three cysteines and a hisidine contribute to the ligation of a zinc ion. Therefore, the zinc-depletion or the oxidation of thiol groups or both abolish the DNA-binding activity of p53 in vitro and in intact cells.4, 5 In addition, the supplement of reducing agents with zinc ions restores the DNA binding activity of p53.6 In the light of other transcription factors, of which activities are modulated by redox states,7 these results suggest the possibility of a redox control of p53. These zinc- or redox state-mediated functional regulations have significant physiological implications within the cells. For example, low concentrations of intracellular zinc disrupt the binding of p53 to DNA and cause impairments in subsequent DNA repair.8 In addition, thioredoxin reductase, thioredoxin, and redox factor-1 have been demonstrated to control the p53 activity via regulating the redox state of p53.9
Although the crystal structures of the p53 DNA binding domain were well studied,3, 10-12 the structural changes impart to p53 under metal-free or oxidation conditions and its relevance to the functional regulation have yet to be fully characterized. Here we reported the crystal structure of mouse p53 core domain in zinc-free state with oxidized thiols at a resolution of 1.5 Å and compared with the crystal structures of the zinc-bearing mouse p53 core domains10-12 for a better understanding of the manner by which metal ions and redox states regulate the sequence-specific DNA binding activity of p53.
MATERIALS AND METHODS
The amplified DNA encoding for the DNA binding core domain of mouse p53 (residues 92–292) was digested using NdeI and HindIII (New England Biolabs) and inserted into pET-22b(+) (Novagen). The transformed E. coli BL21(DE3) cells were grown in LB medium at 37°C until the absorbance at 600 nm reached 0.5–0.6. The expression of the p53 core domain was induced by adding 0.5 mM isopropyl-1-thio-β-D-galactopyranoside and 100 μM zinc acetate. The cells were grown overnight at 15°C, and harvested via 10 min of centrifugation at 4600g. The expressed protein was purified as described by Zhao et al.10 Crystallization trials were conducted at 4°C via the microbatch method. The best crystals were grown over a period of several days in the mixture containing 2 μL of the protein solution (10 mg/mL) and 2 μL of the reservoir solution, containing 100 mM Tris-HCl (pH 8.5), 25%(v/v) ethanol, and 50 mM MgCl2. For cryoprotection, 1 μL of 100% ethanol was added to the drop and the crystals were frozen in a cold nitrogen stream.
Diffraction experiments were performed at beam line 6B of Pohang Light Source, Korea, and at NW12 of Photon Factory, Japan. The 1.5 Å resolution X-ray diffraction data were collected using an ADSC Quantum 210 detector at beam line NW12, Photon Factory, Japan, and indexed, integrated, and scaled using HKL200013 with overall Rsym of 7.2%. The crystal belongs to the P212121 space group, with the unit-cell parameters: a = 45.46 Å, b = 60.04 Å, and c = 70.51 Å. The structure was solved by molecular replacement using the EPMR program,14 with the crystal structure of the mouse p53 core domain as a template Protein Data Bank (PDB code 1HU8)10. The model was refined with energy-minimization, simulated-annealing, and B-factor refinement procedures, using the program CNS.15 The 2Fo−Fc map suggested that residues 237–245 were misplaced. After the omission of these residues, the model was refined using the same procedures again with the CNS program. These residues were later manually positioned, using the graphics program O.16 After several refinement cycles including introduction of water molecules, the refinement was finished with an R factor of 21.3% and an R-free of 23.6%. The final model contains 1546 protein atoms in 196 residues (92–287) and 321 water molecules (Table I). PROCHECK17 was employed to evaluate the quality of the final model and to validate the secondary structures. All of the figures were generated using PyMOL.18 LSQKAB program was used to superimpose PDB files and to calculate the rmsds.19 The atomic coordinates and structure factors were deposited in the PDB ID code 2P52.
| Data-collection statistics | |
|---|---|
| Wavelength (Å) | 1.0000 |
| Space group | P212121 |
| Unit cell (Å) | a = 45.46, b = 60.04, c = 70.51 |
| Resolution (Å) | 30−1.5 |
| Unique reflectionsa | 60182 (5988) |
| Completeness (%) | 98.4 (100) |
| Redundancy | 6.12 |
| Rsym (%)b | 7.2 (30.5) |
| I/σ (I) | 29.0 (4.15) |
| Refinement statistics | |
| Resolution (Å) | 30.0−1.5 |
| Reflections (working) | 29740 |
| Reflections (test) | 1554 |
| Rwork/Rfree (%)c | 21.3/23.6 |
| Number of protein atoms | 1546 |
| Number of waters | 321 |
| Average B factor (Å2) | 17.71 |
| Model quality: r.m.s. deviation from idealityd | |
| Bond lengths (Å) | 0.0048 |
| Bond angles (°) | 1.3 |
| Ramachandran plot (%)d | |
| Most favored regions | 90.3 |
| Additional allowed regions | 9.7 |
| Generously allowed regions | 0.0 |
- a Numbers in parentheses indicate the statistics for the last resolution shell.
- b Rsym = ∑|Ii−〈I 〉|/∑I × 100, in which Ii is the intensity of the ith observation and 〈I 〉 is the mean intensity of the reflections. The values are for unmerged Friedel pairs.
- c Rwork and Rfree= ∑||Fobs|−|Fcalc||/∑|Fobs|. 5% of the reflections were excluded for the calculation of Rfree.
- d The percentage Ramachandran outliers was calculated using PROCHECK.17
RESULTS AND DISCUSSION
The crystal structure of the mouse p53 DNA binding core domain was determined at a resolution of 1.5 Å in zinc-free state with oxidized thiols (Table I). The overall fold is found to be virtually identical to the zinc-bearing p53 core domains [Fig. 1(A)].3, 11, 12 The hydrophobic core is composed of the nine β strands that formed a sandwich of two antiparallel β-sheets. Two small strands, S2 and S2′ and C-terminus of S10 strand form an additional small sheet. Two α-helices (H1 and H2), three large loops on the DNA-binding face (L1, L2, and L3), and the other small loops decorate around the three β-sheets [Fig. 1(A)].
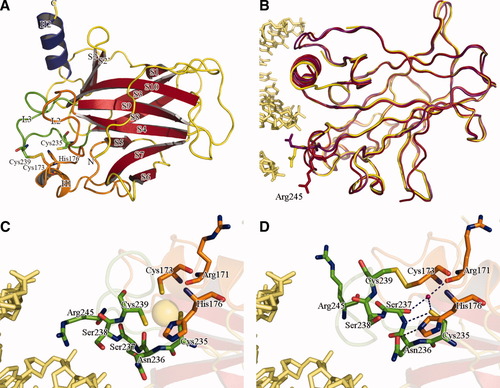
Structural comparison of the zinc-free and the zinc-bearing p53s. (A) Overall structure of the mouse p53 core domain in the absence of the zinc ion. The helices, strands, and loops are colored in blue, red, and yellow, respectively. Each secondary structure is labeled, as are the N- and C-termini. The residues involved in zinc-chelation (Cys173, His176, Cys235, and Cys239) are drawn in stick models and labeled. The L2 and L3 loops are shown in orange and green. Each residue in the loop is also colored in the same scheme. (B) Mainchain traces of the zinc-free mouse p53 (red), the zinc-bearing mouse p5312 (blue, PDB ID 2IOI), and the zinc-bearing mouse p53 bound to DNA11 (yellow, PDB ID 2GEQ). DNA (light-yellow) and Arg residues are depicted in stick models. (C) The ribbon diagram of the mouse p53 in complex with the zinc ion (light-yellow ball),12 using the same color schemes as in Figure 1(A). The residues in the zinc binding region and DNA are drawn in stick models and labeled. (D) The ribbon diagram of the zinc-free mouse p53 in the same orientation and the same color schemes as in Figure 1(C). The disulfide bond between Cys173 and Cys239 is drawn in a stick model. The hydrogen bonds between Ser237 N and Cys235 SG, Asn236 OD1 and His176 NE2 among Ser237 OG, Cys235 SG, and Arg171 O through water, which stabilize the L3 loop, are drawn as dotted lines.
However, the zinc-free p53 core domain exhibits substantial structural alterations when compared with the core domains of the zinc-bearing mouse p53s,11, 12 especially in the L3 loops, by which p53 binds to the minor groove of DNA [Fig. 1(B)]. The zinc-free p53 core domain can be superimposed on the zinc-bearing p53 DNA binding domain12 (PDB code 2IOI) with rmsd of 1.283 Å for 184 Cα atoms (residues 100–283). But they are overlapped with rmsd of 0.536 Å for 175 Cα atoms when the L3 loop (residues 237–245) is excluded. When the same superposition is performed with the zinc-bearing p53 DNA binding domain bound to the DNA11 (PDB code 2GEQ; chain A), rmsds are 1.338 Å for 179 Cα atoms and 0.568 Å for 170 Cα atoms without L3 loop, respectively. In comparison, rmsds between two zinc-bearing p53 core domains bound to DNA11 and in DNA-free state12 are 0.284 Å for 179 Cα atoms and 0.284 Å for 170 Cα atoms, respectively. These data suggest that the lack of a bound zinc-ion induces a significant displacement of the L3 loop.
As the zinc ion is coordinated between Cys235 and Cys239 on the L3 loop, and Cys173 and His176 on the L2 loop [Fig. 1(C)], the L3 loop adopts an altered conformation when the ligated zinc ion is released and the cysteine residues are oxidized. In this altered conformation, additional disulfide and hydrogen bonds are employed to stabilize the L3 loop [Fig. 1(D)]. The oxidization of thiol groups of Cys176 and Cys242 results in the formation of a disulfide bond, which holds the L3 loop close to the L2 loop, and new hydrogen bonds between Ser237 N and Cys235 SG, Asn236 OD1 and His176 NE2, and Cys235 SG and Water119 that in turn interact with Ser237 OG and Arg171 O [Fig. 1(D)]. As the result of the conformation change of the L3 loop accompanied with new disulfide and hydrogen bonds, Arg245 is pulled away from the minor groove of DNA [Fig. 1(B,D)].
Arg245 in mouse p53 and its human counterpart (Arg248), which perform a crucial function in sequence-specific DNA recognition, are directly involved in DNA recognition.3, 11 Regardless of the presence of DNA, the conformations of the L3 loops of zinc-bound p53s are similar, thereby the positions of Arg245 are within reach of the minor groove of DNA [Fig. 1(B,C)].11, 12 However, in the current structure, Arg245 is moved away from the DNA and thus does not overlap with the corresponding residues in the zinc-bearing p53s [Fig. 1(B,D)]. Therefore, it is clear that the differential location of Arg245 in the zinc-free mouse p53 can be attributed to the lack of the ligated zinc ion and the oxidation of the zinc-ligating cysteine residues. Thus, the observed reduction of the transcriptional activity of p53 associated with the zinc-depletion or the oxidation4, 5, 8 is due to the conformational change of the L3 loop and the movement of the Arg residue away from the minor groove of DNA, as the Arg residue is crucial to sequence-specific DNA recognition.
In conclusion, the crystal structure of the p53 core domain determined in our study elucidates the consequences of the zinc release and the oxidation of zinc-binding cysteine residues. The impairments in subsequent DNA binding activity can be structurally interpreted by the remarkable differences in the location of Arg245, and in the conformation of the L3 loop. This structure provides us with an atomic-level perspective with regard to the functional regulation of p53 by the zinc ion or by altered redox states.
Acknowledgements
We thank H. W. Lee for providing the full mouse p53 gene, Dr. K. Zhao for the helpful discussions during the purification process, and thank beam line scientists at beam line 6B of PAL and NW12 of PF for assistance during data collection.




